Abstract
Purpose
Previous research has suggested that impairments of automatic spreading activation may underlie some aphasic language deficits. This study further investigated the status of automatic spreading activation in individuals with aphasia as compared with typical adults.
Method
Participants were 21 individuals with aphasia (12 fluent, 9 non-fluent) and 31 typical adults. Reaction time data were collected on a lexical decision task with masked repetition primes, assessed at 11 different interstimulus intervals (ISIs). Masked primes were used to assess automatic spreading activation without the confound of conscious processing. The various ISIs were used to assess the time to onset, and duration, of priming effects.
Results
The control group showed maximal priming in the 200 ms ISI condition, with significant priming at a range of ISIs surrounding that peak. Participants with both fluent and non-fluent aphasia showed maximal priming effects in the 250 ms ISI condition, and primed across a smaller range of ISIs than the control group.
Conclusions
Results suggest that individuals with aphasia have slowed automatic spreading activation, and impaired maintenance of activation over time, regardless of fluency classification. These findings have implications for understanding aphasic language impairment and for development of aphasia treatments designed directly address automatic language processes.
Keywords: aphasia, anomia, automatic spreading activation, masked priming, implicit processing
Language processing is generally modeled as occurring within a network of linguistic information, with a driving force of spreading activation between elements in the network (e.g., Dell, 1986; Levelt, 1999). This spreading activation is automatic, in that it is obligatory and outside of conscious awareness and control. Previous research using priming paradigms has shown altered patterns of automatic priming effects in aphasia, including data suggesting that slowed automatic spreading activation is a potential source of lexical access impairment for non-fluent aphasia (e.g., Ferrill, Love-Geffen, & Shapiro, 2011; Love, Swinney, Walenski, & Zurif, 2008; Prather, 1994; Prather, Shapiro, Zurif, & Swinney, 1991; Prather, Zurif, Love, & Brownell, 1997; Prather, Zurif, Stern, & Rosen, 1992; Swinney, Zurif, & Nicol, 1989), while observations of typical, or too rapid, spread of activation, initially, followed by a failure to dissipate activation in a typical manner for fluent aphasia may reflect some combination of impaired inhibitory processes and/or the use of alternate processing mechanisms (Prather, 1994; Prather, et al., 1997). Regardless of the mechanisms invoked, the noted alterations in the time course of automatic priming effects likely reflect a fundamental source of impairment in aphasia and, as such, warrant further investigation.
The ability to draw confident conclusions from many of these prior studies is limited by small sample sizes, and by the use of experimental paradigms that may not adequately isolate implicit, unconscious automatic spreading activation processes. In particular, the priming paradigms used in many of these studies have allowed participants to explicitly see, and sometimes even make judgments about, prime items, potentially allowing explicit processing of the primes to influence any priming effects that are obtained. To be certain that only automatic, implicit effects are being measured, however, it is imperative that the experimental paradigm fully prevent explicit processing of the prime and only permit implicit processing.
One method for preventing explicit processing is through the use of visual masking. By combining rapid presentation of prime words with forward- and backward- visual masks, conscious processing of the prime words is interrupted, although the cognitive system still responds to the prime (Forster, Mohan, & Hector, 2003). Because conscious processing of the prime is prevented, any priming effects that are obtained using masking can safely be attributed to implicit, non-conscious processes. Effects of masked priming have been directly attributed to automatic spreading activation (Marcel, 1983), and have been shown to be fairly short-lived (Dagenbach, Carr, & Wilhelmsen, 1989; Draine & Greenwald, 1998; Ferrand, 1996; Forster & Davis, 1984; Greenwald, Draine, & Abrams, 1996). Within a spreading-activation explanation of masked priming, this short duration is attributed to the rapid degradation of activation.
Masked priming, using orthographic, phonological, morphological, semantic, and repetition primes, has been well documented in typical populations to be an effective way to investigate implicit processes while avoiding explicit confounds (e.g., Ferrand & Grainger, 1994; Forster, 2004; Forster & Davis, 1984; Morris, Grainger, & Holcomb, 2008; Perea & Gotor, 1997). Masked priming has also been used in the development of a theoretical model of semantic activation in Alzheimer’s disease (Milberg, McGlinchey-Berroth, Duncan, & Higgins, 1999), demonstrating that it is a useful tool for investigating pathological linguistic function. Application of this technique to the study of individuals with aphasia and anomia could provide important insights into the role that alterations in the timing of implicit processing play in the lexical access and language production challenges of aphasia. Understanding the contribution of implicit processing impairments in aphasia would inform models of impaired language processing and, thereby, have the potential to improve treatment and outcomes for individuals with aphasia.
One published study has used visually masked repetition primes to explore implicit processing in aphasia, with a focus on treatment effects (Avila, Lambon Ralph, Parcet, Geffner, & Gonzalez-Darder, 2001). In this single case study, a masked, cross-modal priming picture naming paradigm was used, with prime words presented briefly enough to be below the threshold of conscious perception. That is, forward and backward masked repetition primes (i.e., the printed names of the target pictures) were presented so quickly that the participant should have had no awareness of having seen them, providing an opportunity to study implicit priming effects in aphasia isolated from explicit processes. The participant in this study demonstrated greater response accuracy for words primed implicitly than for unprimed targets.
Before these findings (Avila, et al., 2001) can be broadly applied to developing anomia treatments, there are a number of questions to be addressed. Given that masked priming effects can vary depending on the duration of the interstimulus interval (Ferrand, 1996), one of the remaining questions is the most effective time interval to use between the masked prime and target. In addition to being necessary for development of clinical applications, investigation of differences in priming effects across various intervals can help further inform our understanding of the status of automatic spreading activation in aphasia, and explore similarities and differences in mechanisms of impairment for fluent and non-fluent aphasia. This study, therefore, was designed to address the time course of masked repetition priming effects in aphasia. Specifically, we investigated masked repetition priming effects at a variety of ISIs in typical adults and adults with fluent and non-fluent aphasia, all of whom exhibited anomia. Repetition primes were used to build on the line of research introduced by Avila et al. (2001). In addition, because repetition priming is stronger than other types of priming (e.g., semantic), and because masked priming effects tend to be small, we used repetition primes to increase the likelihood of eliciting measurable priming effects for this initial exploratory investigation. This information will not only refine the use of masked priming methodology for the study of aphasia, but will lay the foundation for advancing models of lexical processing deficits in aphasia, and support the development of novel treatment approaches for anomia that target implicit mechanisms directly, by providing insight into the status of automatic spreading activation processes in individuals with aphasia.
Three hypotheses were proposed for this work. First, if individuals with aphasia have slowed automatic spreading activation processes, this would be reflected in delayed activation. In this case, masked repetition priming effects on a lexical decision task would emerge only with a greater delay between the prime and target than is observed with typical adults, presumably because the spread of activation is delayed relative to typical rates of activation. Second, if individuals with aphasia have impaired maintenance of automatic spreading activation, then masked repetition priming effects on a lexical decision task would emerge at fewer interstimulus intervals than seen for typical adults, presumably because activation dissipates faster than is typical. Finally, if individuals with aphasia have impaired suppression of automatic spreading activation, then masked repetition priming effects on a lexical decision task would emerge at more interstimulus intervals than typical adults, presumably because activation dissipates more slowly than is typical. These hypotheses were tested for individuals with both fluent and non-fluent aphasia.
Method
This experiment involved a lexical decision task, with intervals between the masked primes and the lexical decision targets systematically varied, including 30, 50, 70, 100, 150, 200, 250, 500, 750, 1000, and 1500 ms intervals. The use of varied ISIs provided the opportunity to assess the duration of priming effects; that is, how long after presentation of the prime did priming effects occur for the lexical decision? This task was followed by a visibility task, which provided verification that prime items were effectively masked.
Participants
Control participants were 31 adults with no history of neurological disorders, substance abuse, or significant psychiatric disorders by self-report (range = 21-84 years old, mean = 57.1, SD = 15.26). They all scored within normal limits on the Raven’s Coloured Progressive Matrices (RCPM; Raven, 1976), and on the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001). Severe depression was ruled out with the Depression Intensity Scale Circles (DISC; Turner-Stokes, Kalmus, Hirani, & Clegg, 2005). Vision for all participants was normal or corrected to normal, as per participant report and observed performance on screening tasks. All but two control participants were right-handed and responded with their right hands in the experimental task. One of the two left-handed participants provided left-hand responses, while the other provided right-hand responses due to being accustomed to using her right hand for computer operation in general.
Participants with aphasia and/or anomia were 21 adults with a unilateral neurological lesion (range = 29-82 years old, mean = 60.95, SD = 13.28, not significantly different from the control group). All participants in this group met the same inclusion criteria as the control participants for non-linguistic, visual, and psychiatric history and function. Diagnosis of aphasia/anomia, and fluent/non-fluent designation, was made by an experienced, certified speech-language pathologist based on the Western Aphasia Battery (WAB; Kertesz, 1982) and the BNT, and/or free conversation (in the case of some participants with mild anomia). Twelve of the participants with aphasia presented with fluent aphasia, and the remaining nine presented with non-fluent aphasia. The first four subtests of the Reading Comprehension Battery for Aphasia (RCBA; LaPointe & Horner, 1979) and the Apraxia Battery for Adults (ABA; Dabul, 1979) were also given, though these measures were descriptive rather than inclusionary (individual participant profiles are provided in Appendix A, available as online supplemental material). Two participants with aphasia were left-handed and used their left hands to make responses in the experiment. Five participants with aphasia were pre-morbidly right-handed (by self-report), but used their left hands to respond due to hemiplegia. All other participants with aphasia were right-handed (by self-report) and responded with their right hands. Use of the non-dominant hand for button-press responses was considered acceptable because all calculations of priming effects were made by comparison between conditions within participants; therefore, any slowing in response times due to use of the non-dominant hand would likely be consistent across conditions. Aphasia severity ranged from mild to severe (Mean WAB Aphasia Quotient = 65.8, range = 7.4-98, SD=26.47). All participants were paid $10 per experimental session. All procedures were approved by the University of Washington Human Subjects Institutional Review Board.
Equipment
Participants were seated in a quiet room at a comfortable distance from a 20” CRT computer screen with a 100 Hz refresh rate, and wore noise-cancelling headphones (Bose QuietComfort 2) to reduce the potential for distraction by ambient noise. Stimuli were presented via E-Prime (E-Prime version 1.1.4.1, Psychology Software Tools, Pittsburgh, PA) on a personal desktop computer equipped with Windows 98. Participants responded by pressing one of two marked buttons on a serial response box (Psychology Software Tools, Pittsburgh, PA) interfaced with the computer. Reaction time data, in milliseconds, was collected by E-Prime based on the time between the onset of the visual target stimulus and the participant’s button-press response.
Stimuli
Altogether, there were 440 real-word and 440 non-word lexical decision targets in the experimental task, all between 3-8 letters in length. Additional details of the stimuli are available in Table 1. While the inclusion of closed-class words was not ideal, given evidence that these words are processed differently than open-class words (e.g., Munte, Wieringa, Weyerts, Szentkuti, Matzke et al., 2001), they were included in order to obtain enough stimuli that otherwise met the inclusion criteria. Given that this experiment involved repetition priming, and that the basic mechanism of automatic spreading activation is thought to underlie all cognitive processing even if different neural systems are engaged, it was determined that, for this initial investigation, the different word classes were not likely to substantially alter results; indeed, as will be discussed further below, they did not alter the overall patterns of priming identified.
Table 1.
Stimulus characteristics.
|
Real
Word Targets |
440 items 3-8 letters Written word frequency > 100 per million (Francis and Kucera, 1982) 377 nouns and verbs 63 adjectives/adverbs/conjunctions, prepositions and/or pronouns Morpheme count: 386 with one, 54 with two Syllable count: 239 with one, 175 with two, 22 with three, 4 with four |
|
Non-
Word Targets |
440 items 3-8 letters Pronounceable Phonotactically and orthographically legal in English |
Procedure
Participants were instructed to decide whether each string of upper-case letters that appeared on the screen constituted a real English word or not. To encourage participants to attend to the screen for the full duration of the trial, including presentation of the masked primes, they were asked to watch from the start of the visual sequence (marked by a fixation cross) so that they would be ready to respond as soon as the target word came up. No mention was made of the masked primes. Responses were made by pressing a button on the response box. Participants were encouraged to respond as fast as they could, with the goal of having a response registered before the target item turned red. Buttons on the response box were color-coded (green for yes, red for no), with the location of each response button (left or right) randomly assigned for each participant. If participants completed the protocol over more than one session, the same assignment was maintained throughout the protocol.
For individuals with aphasia, verbal instructions were simplified, if needed, and supplemented with gestures, writing, drawing, and demonstration to maximize their comprehension. For all participants, a short practice run was provided; participants could repeat the practice run as many times as they needed to feel comfortable with the task and to consistently obtain fast enough responses. The practice run used the masking sequence from the 30 ms ISI condition. After successfully completing the practice run, all participants completed the experimental lists in the same order, beginning with the shortest ISI (30 ms) and increasing sequentially through the longest ISI (1500 ms), to minimize participant awareness of the prime items.
The masked priming task consisted of eleven experimental lists of 80 trials (40 real-word and 40 non-word targets), with each list divided into four runs of 20. Half of each type of target were preceded by a repetition prime (the primed condition) and half were preceded by a string of alternating x’s and g’s that matched the target for number of characters (the unprimed condition). Assignment of targets to the primed or unprimed condition was made at random for each participant. No stimulus item was presented to a participant more than once across all conditions within this task. Each experimental list comprised a single ISI.
All visual elements for the masked priming task (masks, primes, and targets) were presented in the center of the computer screen, on a white background (see Figure 1 for schematic representation and detailed description of the masked priming sequence). If a button-press response was made during the target presentation, the target immediately disappeared from the screen and the inter-trial interval began. If no response occurred before the target interval was complete, the target item remained on the screen longer, but in red font, to signal to the participant that s/he had missed the response deadline. Participants were told that, if they saw many items turn red, it meant they were not responding fast enough and they should try to respond faster.
Figure 1.
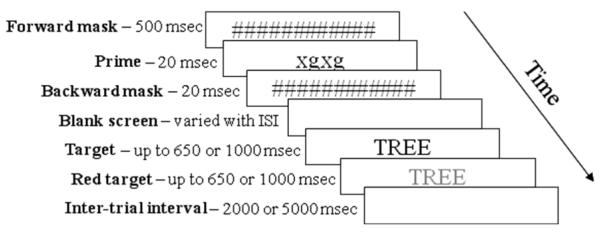
Schematic representation of the sequence of visual events for the masked priming task. The prime shown here is an xg string, used in the unprimed condition. In the primed condition, this screen would show the target word in lower-case letters. All stimuli were in 48-point, black, Arial font except for the prime item, which was 30-point font to assure that it was fully covered by the masks. For screens with two presentation times listed, the first was used for control participants and the second for participants with aphasia. Different response deadlines and inter-trial intervals for the two groups were used to balance participant abilities with appropriate, comfortable task pacing and the need for rapid responses to obtain masked priming effects (Draine and Greenwald, 1998). Analysis of outcomes for a few participants with these parameters matched demonstrated that these differences did not materially alter the patterns of performance reported here.
Visibility task for verification of effective masking
The use of masked primes in this experiment was undertaken to remove the possibility of strategic processing influencing priming effects. While masked primes are designed to interfere with conscious processing, there is known to be a large amount of individual variability in the effectiveness of masking, and the degree of information extracted from masked items by different people (Dagenbach, et al., 1989). Therefore, in order to confidently attribute any priming effects obtained in the masked priming task to strictly implicit, automatic processes, a visibility task involving lexical decision on masked items at each ISI was included to verify that the masked items were not, in fact, explicitly visible to the participants. Details of this task are available in Appendix B (available as online supplemental material), and a detailed discussion of issues surrounding this task may be found elsewhere (Silkes & Rogers, 2010). If results of this task demonstrated that the participant may have had conscious awareness of the content of prime items in a particular ISI condition, the data from that ISI condition, for that participant, were removed from analysis. This yielded the removal of a total of 16 lists in the masked priming task, distributed across 6 control participants (1 list from each of three participants, 2 lists from each of two participants, and 9 lists from one participant). None of the participants with aphasia had any ISI conditions that met the criteria for conscious visibility, so no data from this group were removed from analysis due to conscious awareness of the content of primes.
Data collection and processing
Accuracy and reaction time data from button-press responses in the masked priming task were downloaded from E-Prime. Only reaction times obtained for correct responses made by the response deadline for real-word targets were analyzed. Data were then trimmed further by eliminating any items with response times that fell greater than 3 standard deviations from the mean for each participant’s responses at that particular ISI, for that particular priming condition (e.g., primed words in the 30 ms ISI). Finally, data were removed from any runs in which it was suspected that participants may have had conscious awareness of the prime items, based on results from the visibility task. Altogether, 11,562 tokens were analyzed for control participants (out of 13,640 real-word targets; 84.8%), and 8,148 tokens were analyzed for participants with aphasia (out of 9,240 real-word targets; 88.2%).
Results
Priming effects in fluent and non-fluent aphasia
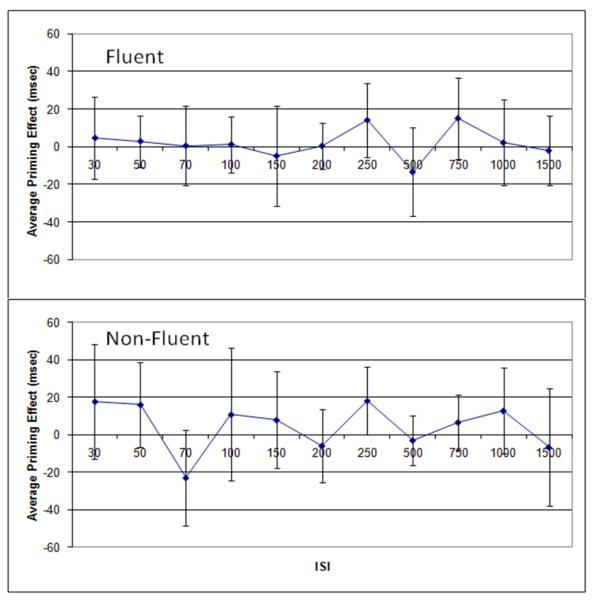
Because previous research has suggested that individuals with fluent and non-fluent aphasia present with different patterns of priming (e.g., Blumstein, Milberg, & Shrier, 1982; Hagoort, 1993; Milberg, Blumstein, & Dworetzky, 1988; Milberg, Blumstein, Giovanello, & Misiurski, 2003; Milberg & Blumstein, 1981; Prather, et al., 1997), we conducted separate analyses for these two groups. Priming effects were analyzed in a 2-way (2 prime conditions × 11 ISI conditions) repeated measures ANOVA for each group. Because the data did not meet the assumption of sphericity for either group, Greenhouse-Geisser corrections were used for all analyses. For those participants who were classified as fluent, there was a main effect of ISI that approached significance, F(4,47) = 2.241, p=.075), with no significant main effect of prime condition and no significant interaction (see Figure 2). Planned comparisons with paired t-tests at each ISI showed no significant priming effects, although a trend toward a peak in the 250 and 750 ms ISI conditions was evident. For participants who were classified as non-fluent, 2×11 repeated measures ANOVA showed no significant main effects or interaction. Planned comparisons with paired t-tests at each ISI showed a significant priming effect (uncorrected for multiple comparisons) in the 250 ms ISI condition, t(8) = 2.31, p = .05. Bonferroni correction was not applied due to concerns about missing potential patterns of interest across the wide range of ISIs in this exploratory experiment.
Figure 2.
Patterns of priming for individuals with fluent and non-fluent aphasia (top and bottom, respectively). Error bars reflect 95% confidence intervals. * reflects a priming effect significant at p<.05.
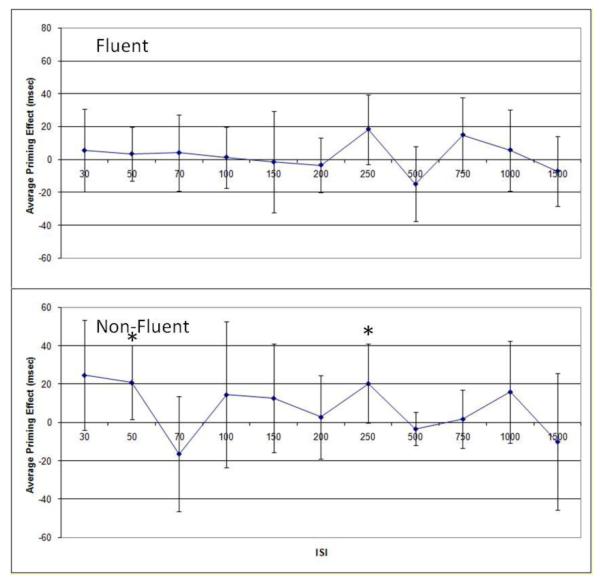
To verify that the inclusion of multimorphemic targets had no effect on the pattern of results obtained, analysis was also conducted using a more homogeneous data set, including only monomorphemic targets, with some small differences noted. A 2×11 repeated measures ANOVA was conducted for each group (see Figure 3). As with the results reported earlier, Greenhouse-Geisser correction was used due to violation of the assumption of sphericity. For those participants who were classified as fluent, there was a main effect of ISI that approached significance F(4,49) = 2.174, p = .079, with no significant main effect of prime condition and no significant interaction. Planned comparisons with paired t-tests at each ISI showed no significant priming effects, although the effect in the 250 ms ISI condition approached significance (uncorrected), t(11) = 1.91, p = .08. For participants who were classified as non-fluent, 2×11 repeated measures ANOVA showed no significant main effects or interaction. Planned comparisons with paired t-tests at each ISI showed significant priming effects (uncorrected) in the 50 ms ISI condition, t(8) = 2.51, p = .036, and the 250 ms ISI condition, t(8) = 2.29, p = .05. The effect in the 50 ms ISI condition had not been evident in the analyses of all targets.
Figure 3.
Average priming effects, for monomorphemic targets only, for individuals with fluent aphasia (top) and non-fluent aphasia (bottom). Error bars reflect 95% confidence intervals. * reflects a priming effect significant at p<.05.
Priming effects in aphasia vs. typical participants
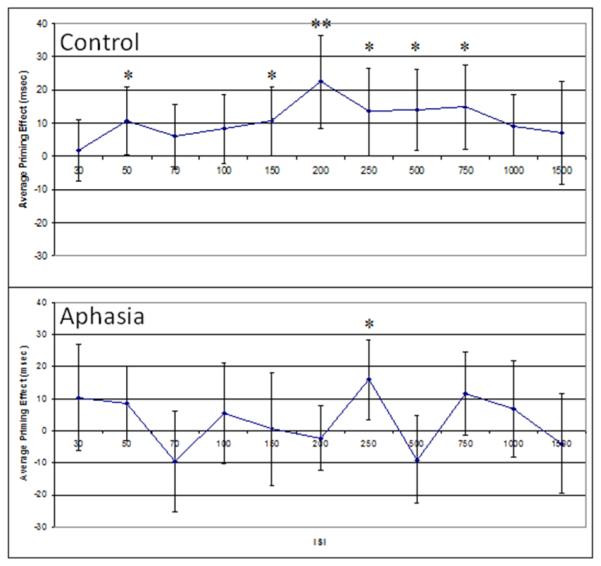
Because analysis of data from fluent vs. non-fluent participants indicated highly similar patterns of priming, with a trend toward a significant peak in the 250 ms ISI condition for fluent aphasia and significant priming effects at that interval for non-fluent aphasia, the two groups were combined for comparison with the typical control group. This analysis involved making the same comparisons of average priming effects in all ISI conditions reported above, for all participants with aphasia and for the typical control participants. Priming effects were analyzed in a 2-way (2 prime conditions × 11 ISI conditions) repeated measures ANOVA for each participant group. Because the data did not meet the assumption of sphericity for either group, Greenhouse-Geisser corrections were used for all analyses. For control participants, there was a significant main effect of prime condition (primed vs. unprimed), F(1,24) = 5.693, p = .025, a significant main effect of ISI, F(5, 129) = 2.476, p = .032, and no significant interaction (see Figure 4). For individuals with aphasia, there was a significant main effect of ISI, F(4,84) = 3.916, p = .005, but no main effect of prime condition and no significant interaction. Planned comparisons for each ISI condition were carried out using paired t-tests (participant averages compared between primed and unprimed conditions at each ISI). The control group showed statistically significant priming effects (uncorrected for multiple comparisons) in the following ISI conditions: 50 ms, t(30) = 2.138, p = .041; 150 ms, t(29) = 2.079, p = .047; 200 ms, t(29) = 3.23, p = .003); 250 ms, t(29) = 2.09, p = .045; 500 ms, t(29) = 2.35, p = .026; and 750 ms, t(29) = 2.38, p = .024. In addition, there was a marginally significant effect in the 1000 ms ISI condition, t(27) = 2.03, p = .052. The group with aphasia showed a statistically significant priming effect (uncorrected) only in the 250 ms ISI condition, t(20) = 2.65, p = .015, with a priming effect approaching significance in the 750 ms ISI condition, t(20) = 1.85, p = .078.
Figure 4.
Average priming effects (with 95% CIs) for the control group (top) and participants with aphasia (bottom). Because priming effects are difference scores (unprimed vs. primed condition), statistical significance (p<.05) is evident in error bars that do not cross zero. * indicates priming effects significant at p<.05, ** reflects priming effects significant at p<.01.
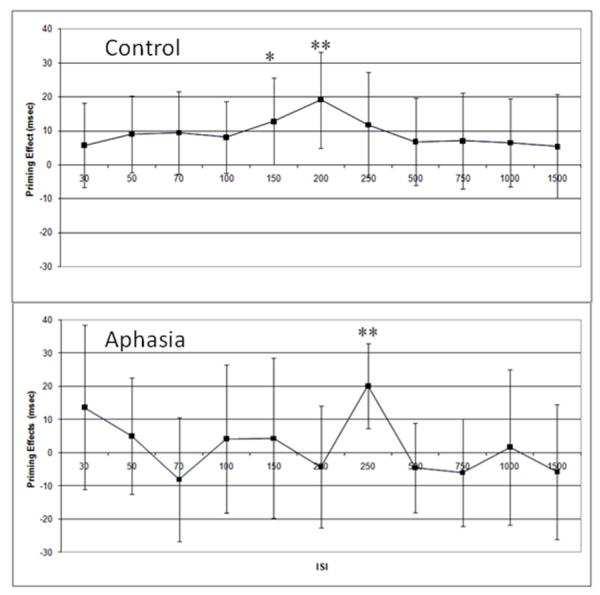
This analysis was repeated to include only monomorphemic targets. Data were analyzed using the same methods as described above for the full data set (including Greenhouse-Geisser correction). These analyses revealed that control participants showed a main effect of prime condition (primed vs. unprimed) that approached significance, F(1,24) = 3.86, p = .061, and no significant main effect of ISI, F(6,140) = 1.87, p = .091, or interaction F(7,158) = .177, p = .987 (see Figure 5). Individuals with aphasia showed a significant main effect of ISI, F(10,200) = 2.802, p = .003, but no significant main effect of prime condition, F(1,20) = .268, p = .610, or interaction, F(10,200)=.944, p=.494. Planned comparisons were then made using paired t-tests. For the control participants, significant priming effects (uncorrected) were seen in the 150 ms ISI condition, t(20) = 2.06, p = .049, and the 200 ms ISI condition, t(29) = 2.78, p = .009. For individuals with aphasia, the only significant priming effect (uncorrected) noted in this analysis was in the 250 ms ISI condition, t(20) = 3.28, p = .004.
Figure 5.
Average priming effects for the control participants (top) and participants with aphasia (bottom) when including only monomorphemic nouns. All participants in both groups were included in this analysis. Error bars reflect 95% confidence intervals. * reflects priming effects significant at p<.05, and ** reflects priming effects significant at p<.01.
Effects of age
Preliminary analysis of the data collected in this study suggested significant age effects in the control group, with individuals over the age of 70 showing, as a group, no significant priming (though some individuals did prime in some conditions). Therefore, given that there was a significant age range represented in both participant groups for this study, we analyzed the relationship between age and the strength of priming effects, to determine whether age might influence the priming effects that were observed, or might need to be considered in final interpretation of results. An average priming effect (across all ISI conditions) was calculated for each participant as a general way to capture that person’s susceptibility to masked primes. These averages were then entered into linear regression against participant age. Control participants showed a significant inverse relationship between age and degree of priming, with r2 = .275 (p = .002). Participants with aphasia did not show a consistent relationship between age and degree of priming, with r2 = .002 (p = .85).
Effects of aphasia severity
Linear regression analysis of the relationship between aphasia severity and the degree of priming seen over all ISI conditions was conducted, yielding an r2 = .001, p = .89. Analysis of each ISI condition revealed only one condition (70 ms ISI) in which the linear regression approached significance (r2 = .166, p = .067), indicating a trend toward a direct relationship between WAB AQ and priming effects but no significant relationships. Specifically, participants with more severe aphasia trended toward interference effects, while those less severe had overall priming effects close to zero.
Discussion
This study was investigated the responses of individuals with aphasia, and anomia in particular, to masked repetition priming. This study was undertaken to explore both the use of masked priming methodology with this population and the status of automatic spreading activation, the mechanism often invoked to explain how the cognitive-linguistic system processes information automatically and without conscious awareness. This investigation demonstrated that people with aphasia and anomia continue to show masked priming effects, indicating that the processes of automatic spreading activation are present, but that these effects occur in a different time-course relative to typical adults. Interestingly, the differences in automatic processing found in this study were essentially the same for individuals with both fluent and non-fluent aphasia, with both delay and reduced maintenance of spreading activation. This finding is in contrast to prior work that has found differences in priming patterns between fluent and non-fluent aphasia (e.g., Blumstein, et al., 1982; Milberg & Blumstein, 1981; Prather, 1994; Prather, et al., 1997), but is consistent with Hagoort’s (1993) observation of similar priming patterns for both groups, although he attributed them to different mechanisms for fluent vs. non-fluent aphasia. As with other researchers, Hagoort suggested that the pattern of priming noted for non-fluent aphasics may have been due to an altered time course of automatic spreading activation, with rapid decay of activation (as noted in this study, as well). In contrast, based on performance on an explicit semantic judgment task, the same pattern of priming obtained for fluent aphasics was interpreted as being due to an impairment of post-lexical integration of the prime and target. Given that the present study did not involve conscious access to the prime words, it is unclear how a controlled post-lexical integration explanation would apply to the current results, and we suggest that, indeed, there may be differences in the automatic spread of activation in fluent, as well as non-fluen+t, aphasia.
Given the similarities between data from the fluent and non-fluent groups, we combined these data for comparison with typical control participants. This grouping of aphasia subtypes is compatible with the notion that the fluent/non-fluent dichotomy is not always clear, consistently applied, or productive for either research or clinical purposes (Gordon, 1998; Marshall, 1986; McNeil & Copland, 2011; Trupe, 1984), and with models of aphasia that suggest that it is a unitary disorder (e.g., Schuell, Jenkins, & Jiménez-Pabón, 1964). Given that all of the participants in this study shared a common symptom, anomia, it is possible that the measure of lexical activation used here captured a common source of impairment for word retrieval, regardless of fluency classification. Overall, these combined data support the first and second research hypotheses discussed earlier (delayed activation and impaired maintenance), but not the third (impaired suppression).
Considering the first research hypothesis, concerning delayed activation, the 50 ms difference in the strongest activation between participant groups may be interpreted as reflecting an overall slowing in the automatic spread of activation among the individuals with aphasia. This conclusion is consistent with previous findings (Prather, et al., 1997; Prather, et al., 1992) of alterations in the time course of priming effects in individuals with non-fluent aphasia, and the suggestion (Schwartz, Saffran, Bloch, & Dell, 1994) that aphasia may, at times, involve a deficit in activation transmission.
In relation to the range of intervals showing priming (i.e., the impaired maintenance of activation hypothesis), the control group exhibited priming effects across a wide range of ISIs, indicating that the spreading activation induced by presentation of the masked primes was maintained over an extended period of time. In contrast, individuals with aphasia exhibited priming over a much more restricted range of ISIs. Consistent with the second research hypothesis, we suggest that these results demonstrate that individuals with aphasia have an impaired ability to sustain automatic spreading activation. While this explanation is inconsistent with Prather et al.’s (1997) suggestion that fluent aphasia may involve difficulty suppressing activation, it is consistent with suggested mechanisms from previous findings of abbreviated time-courses of priming effects for non-fluent aphasia (e.g., Ferrill, et al., 2011; Hagoort, 1993) and with the suggestion (Schwartz, et al., 1994) that aphasia may involve a deficit in representation integrity, wherein the system is unable to maintain activation of the desired information long enough for it to be selected for further processing.
It might be expected that priming effects for the individuals with aphasia would be influenced by the severity of the aphasic deficit, as a more severe language deficit might correlate with reduced sensitivity to priming due to greater impairments in spreading activation. This was not observed, however, in the current data set; aphasia severity does not appear to be an indicator of a person’s sensitivity to masked primes. It is possible, however, that a stronger relationship would become evident with a larger sample including greater representation of participants on the more severe end of the aphasia continuum. Further investigation of the relationship between aphasia severity and responses to masked priming would be appropriate to better understand the role that automatic spreading activation may play in determining aphasia severity.
Age effects were also investigated to determine if there was a need to account for age in interpreting the priming data. While control participants showed a significant inverse linear relationship between age and the degree of priming elicited by masked primes, this relationship was not seen for individuals with aphasia. While this investigation yields no clear explanation for this difference, three possible simple explanations are that 1) the participants with aphasia were not adequately distributed along the age continuum (especially on the young end) to capture age effects; 2) there was so much variability between the participants with aphasia, and within participants at the various ISIs, that clear patterns could not emerge given the sample size; and/or 3) responses from the participants with aphasia were so slow overall that the measure was simply not as sensitive to age differences for this group as it was for the control participants. The current data set does not allow determination of which of these explanations, if any, is most likely; indeed, it is possible that all of three of these factors play a role in the age effect differences that emerged between groups.
A more complex possible explanation for the lack of age effects in the participants with aphasia might be found in considering the potential effects of age-related changes in attention on the masked priming task. While masked priming effects are obtained with no conscious awareness of the presence of the prime items, as discussed earlier, it has also been demonstrated that implicit cognitive effects can be modulated by attention or task demands (e.g., Dagenbach, et al., 1989; Fabre, Lemaire, & Grainger, 2007; Forster & Davis, 1984). At the same time, investigators have suggested that controlled processes (i.e., those requiring attention) may decline with age, while automatic processes do not (Balota, Black, & Cheney, 1992; Balota & Duchek, 1988; Fabre & Lemaire, 2005). It may be, therefore, that the control participants’ responses to the masked primes in this experiment were modulated by their understanding of the task, some aspect of stimulus presentation, or other strategic, attention-related effects, and that these effects were more pronounced for younger control participants and less so for older control participants. At the same time, however, attention deficits in aphasia are well documented (e.g., Glosser & Goodglass, 1990; Laures, 2005; Murray, 2000; Tseng, McNeil, & Milenkovic, 1993). If the participants with aphasia manifested attention deficits of some type, it is possible that they did not engage strategic/attention resources in the same way that the control participants did to perform the lexical decision task and, therefore, did not show differences across the age span. This is not to say that the priming effects observed in this investigation reflect controlled or attention-related priming; instead, we suggest that any effects of these attentional processes on implicit priming may have been mitigated with age for the control participants, while participants of any age with aphasia may not have had been influenced by attentional processes.
In spite of the remaining questions regarding severity and age, these results are both theoretically and practically meaningful. Theoretically, they confirm that priming through implicit processes occurs for people with aphasia, which is consistent with prior research (e.g., Blumstein, et al., 1982; Hagoort, 1993; Milberg & Blumstein, 1981), although it occurs with different parameters than for typical adults. This is a novel contribution to the literature in that prior research suggesting differences in the time course of automatic priming responses have been conducted using primes that were available for conscious processing. As a result, prior results could not be confidently interpreted in terms of the implicit, unconscious process of automatic spreading activation. This study, however, involved presenting primes in a manner that precluded their being consciously processed. The use of masked primes provides confidence that the differences seen between groups are attributable to unconscious, automatic processing, presumably in the form of automatic spreading activation.
In the same vein, this study is a first step toward using masked priming to further elucidate the loci of deficits in aphasia. If implicit priming were not apparent in individuals with aphasia, the locus of impairment would more likely be early orthographic processing or representation integrity, rather than automatic spreading activation for lexical access. Given that priming effects were obtained, however, it appears from these data that one important locus of impairment in aphasia is likely to be automatic spreading activation within the language processing system. This is consistent with theories of aphasia that invoke deficits in linguistic access, rather than representation.
Practically, these results provide an initial guide for the parameters under which future investigations using masked priming in aphasia can be conducted. In addition, they suggest that it may be appropriate to consider whether treatments that focus on explicit levels of processing, which are typical of aphasia rehabilitation, are the most efficient or effective methods to rehabilitate language impairments in aphasia. If one of the fundamental impairments underlying lexical retrieval is in automatic, implicit processing, then perhaps effective rehabilitative approaches that directly target the implicit processes supporting language function could be developed.
Methodological differences between this study and previous work may explain the differences between present and previous findings (as suggested by Prather, 1994). First, this study used repetition primes, rather than semantic or phonological primes as were used in much of the previous work, so different processing mechanisms may be engaged than in those previous studies. It may also be that masked priming effects are differentially sensitive to some aspects of lexical processing so these results reflect mechanisms not captured by other priming paradigms. Finally, it is possible that the present results may reflect a single source of impairment, regardless of fluency classification, that underlies lexical access for all individuals with word retrieval impairments.
Conclusion and future directions
The participants with aphasia and anomia in this study demonstrated evidence of automatic spreading activation, although in a different time course than seen in typical adults. Specifically, there appears to be both a delay in spreading activation and impaired maintenance of activated information. These differences were the same for participants with both fluent and non-fluent aphasia. This suggests that there may be a common mechanism underlying lexical retrieval impairments regardless of fluency classification, and that other differences between fluent and non-fluent aphasia likely emerge later in the time course of language processing. These findings have implications for understanding the source of word retrieval deficits in aphasia, informing exploration of the most efficient, effective ways to remediate these deficits, and guiding the use of masked priming as a tool for further investigation of aphasia.
Supplementary Material
Acknowledgments
This work was supported by NIH 5T32DC000033-14, NIH 1F31DC008736-02, and NIH UW Research Core Grant, University of Washington P30 DC04661. Portions of these data were presented in posters at the 2009 Clinical Aphasiology Conference and the 2011 Annual Meeting of the International Neuropsychological Society. A methodological analysis of the visibility task described here, based on the data collected for this project, has been previously published. The authors thank Kristie Spencer, Susan Joslyn, Cathy Off, Holly Kavalier, Rebecca Hunting-Pompon, Lina Huang, Laine Anderson, Coralee Choules, and the UW Aphasia Research Lab for assistance with data collection and processing and intellectual support. Most of all, we thank the research participants, especially those with aphasia, who graciously provided the data for this work.
Contributor Information
JoAnn P. Silkes, Department of Speech and Hearing Sciences, University of Washington
Margaret A. Rogers, Department of Speech and Hearing Sciences, University of Washington
References
- Avila C, Lambon Ralph MA, Parcet M, Geffner D, Gonzalez-Darder J. Implicit word cues facilitate impaired naming performance: Evidence from a case of anomia. Brain and Language. 2001;79:185–200. doi: 10.1006/brln.2001.2472. doi: 10.1006/brln.2001.2472. [DOI] [PubMed] [Google Scholar]
- Balota DA, Black SR, Cheney M. Automatic and attentional priming in young and older adults: reevaluation of the two-process model. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:485–502. doi: 10.1037//0096-1523.18.2.485. doi: 10.1037/0096-1523.18.2.485. [DOI] [PubMed] [Google Scholar]
- Balota DA, Duchek JM. Age-related differences in lexical access, spreading activation, and simple pronunciation. Psychology and Aging. 1988;3:84–93. doi: 10.1037//0882-7974.3.1.84. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Milberg W, Shrier R. Semantic processing in aphasia: evidence from an auditory lexical decision task. Brain and Language. 1982;17:301–315. doi: 10.1016/0093-934x(82)90023-2. doi: 10.1016/0093-934X(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Dabul B. Apraxia Battery for Adults. C. C. Publications; Trigard: 1979. [Google Scholar]
- Dagenbach D, Carr TH, Wilhelmsen A. Task-induced strategies and near-threshold priming: Conscious influences on unconscious perception. Journal of Memory and Language. 1989;28:412–443. doi: 10.1016/0749-596X(89)90020-X. [Google Scholar]
- Dell GS. A spreading-activation theory of retrieval in sentence production. Psychological Review. 1986;93:283–321. doi: 10.1037/0033-295X.93.3.283. [PubMed] [Google Scholar]
- Draine SC, Greenwald AG. Replicable unconscious semantic priming. Journal of Experimental Psychology: General. 1998;127:286–303. doi: 10.1037/0096-3445.127.3.286. [PubMed] [Google Scholar]
- Fabre L, Lemaire P. Age-related differences in automatic stimulus-response associations: insights from young and older adults’ parity judgments. Psychonomic Bulletin and Review. 2005;12:1100–1105. doi: 10.3758/bf03206450. doi: 10.3758/BF03206450. [DOI] [PubMed] [Google Scholar]
- Fabre L, Lemaire P, Grainger J. Attentional modulation of masked repetition and categorical priming in young and older adults. Cognition. 2007;105:513–532. doi: 10.1016/j.cognition.2006.10.011. doi: 10.1016/j.cognition.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ferrand L. The masked repetition priming effect dissipates when increasing the inter-stimulus interval: Evidence from word naming. Acta Psychologica. 1996;91:15–25. doi: 10.1016/0001-6918(95)00010-0. [Google Scholar]
- Ferrand L, Grainger J. Effects of orthography are independent of phonology in masked form priming. Quarterly Journal of Experimental Psychology A. 1994;47:365–382. doi: 10.1080/14640749408401116. doi: 10.1080/14640749408401116. [DOI] [PubMed] [Google Scholar]
- Ferrill M, Love-Geffen T, Shapiro L. The time-course of lexical activation during sentence comprehension in aphasia. Paper presented at the Clinical Aphasiology Conference; Ft. Lauderdale, FL. 2011. [Google Scholar]
- Forster KI. Category size effects revisited: frequency and masked priming effects in semantic categorization. Brain and Language. 2004;90:276–286. doi: 10.1016/S0093-934X(03)00440-1. doi: 10.1016/S0093-934X(03)00440-1. [DOI] [PubMed] [Google Scholar]
- Forster KI, Davis C. Repetition priming and frequency attenuation in lexical access. Journal of Experimental Psychology: Learning, Memory and Cognition. 1984;10:680–698. doi: 10.1037/0278-7393.10.4.680. [Google Scholar]
- Forster KI, Mohan K, Hector J. The mechanics of masked priming. In: Kinoshita S, Lupker SJ, editors. Masked Priming: The State of the Art. Psychology Press; New York: 2003. pp. 3–37. [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Houghton Mifflin Company; Boston: 1982. [Google Scholar]
- Glosser G, Goodglass H. Disorders in executive control functions among aphasic and other brain-damaged patients. Journal of Clinical and Experimental Neuropsychology. 1990;12:485–501. doi: 10.1080/01688639008400995. doi: 10.1080/01688639008400995. [DOI] [PubMed] [Google Scholar]
- Gordon JK. The fluency dimension in aphasia. Aphasiology. 1998;12:673–688. [Google Scholar]
- Greenwald AG, Draine SC, Abrams RL. Three cognitive markers of unconscious semantic activation. Science. 1996;273:1699–1702. doi: 10.1126/science.273.5282.1699. doi: 10.1126/science.273.5282.1699. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Impairments of lexical-semantic processing in aphasia: evidence from the processing of lexical ambiguities. Brain and Language. 1993;45:189–232. doi: 10.1006/brln.1993.1043. doi: 10.1006/brln.1993.1043. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febinger; Philadelphia: 2001. [Google Scholar]
- Kertesz A. The Western Aphasia Battery. Grune and Stratton; New York: 1982. [Google Scholar]
- LaPointe LL, Horner J. Reading Comprehension Battery for Aphasia. G.C. Publications, Inc; Tegoid, OR: 1979. [Google Scholar]
- Laures JS. Reaction time and accuracy in individuals with aphasia during auditory vigilance tasks. Brain and Language. 2005;95:353–357. doi: 10.1016/j.bandl.2005.01.011. doi: 10.1016/j.bandl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Producing spoken language: A blueprint of the speaker. In: Brown CM, Hagoort P, editors. The Neurocognition of Language. Oxford University Press; Oxford: 1999. [Google Scholar]
- Love T, Swinney D, Walenski M, Zurif E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain and Language. 2008;107:203–219. doi: 10.1016/j.bandl.2007.11.004. doi: 10.1016/j.bandl.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel AJ. Conscious and unconscious perception: Experiments on visual masking and word recognition. Cognitive Psychology. 1983;15:197–237. doi: 10.1016/0010-0285(83)90009-9. doi: 10.1016/0010-0285(83)90009-9. [DOI] [PubMed] [Google Scholar]
- Marshall JC. The description and interpretatino of aphasic language disorder. Neuropsychologia. 1986;34:5–24. doi: 10.1016/0028-3932(86)90040-0. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Copland D. Aphasia theory, models, and classification. In: LaPointe LL, editor. Aphasia and Related Neurogenic Language Disorders. Thieme; New York: 2011. pp. 27–47. [Google Scholar]
- Milberg W, Blumstein S, Dworetzky B. Phonological processing and lexical access in aphasia. Brain and Language. 1988;34:279–293. doi: 10.1016/0093-934x(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein S, Giovanello KS, Misiurski C. Summation priming in aphasia: Evidence for alterations in semantic integration and activation. Brain and Cognition. 2003;51:31–47. doi: 10.1016/s0278-2626(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein SE. Lexical decision and aphasia: evidence for semantic processing. Brain and Language. 1981;14:371–385. doi: 10.1016/0093-934x(81)90086-9. doi: 10.1016/0093-934X(81)90086-9. [DOI] [PubMed] [Google Scholar]
- Milberg W, McGlinchey-Berroth R, Duncan KM, Higgins JA. Alterations in the dynamics of semantic activation in Alzheimer’s disease: evidence for the Gain/Decay hypothesis of a disorder of semantic memory. Journal of the International Neuropsychological Society. 1999;5(7):641–658. doi: 10.1017/s1355617799577072. [DOI] [PubMed] [Google Scholar]
- Morris J, Grainger J, Holcomb PJ. An electrophysiological investigation of early effects of masked morphological priming. Language and Cognitive Processes. 2008;23:1021–1056. doi: 10.1080/01690960802299386. doi: 10.1080/01690960802299386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LL. The effects of varying attentional demands on the word retrieval skills of adults with aphasia, right hemisphere brain damage, or no brain damage. Brain and Language. 2000;72:40–72. doi: 10.1006/brln.1999.2281. doi: 10.1006/brln.1999.2281. [DOI] [PubMed] [Google Scholar]
- Perea M, Gotor A. Associative and semantic priming effects occur at very short stimulus-onset asynchronies in lexical decision and naming. Cognition. 1997;62:223–240. doi: 10.1016/s0010-0277(96)00782-2. doi: 10.1016/S0010-0277(96)00782-2. [DOI] [PubMed] [Google Scholar]
- Prather P. The time course of lexical activation in fluent and non-fluent aphasia. Linguistics and Cognitive Neuroscience. 1994;6:128–144. [Google Scholar]
- Prather P, Shapiro L, Zurif E, Swinney D. Real-time examinations of lexical processing in aphasics. Journal of Psycholinguistic Research. 1991;20:271–281. doi: 10.1007/BF01067219. [DOI] [PubMed] [Google Scholar]
- Prather P, Zurif E, Love T, Brownell H. Speed of lexical activation in nonfluent Broca’s aphasia and fluent Wernicke’s aphasia. Brain and Language. 1997;59:391–411. doi: 10.1006/brln.1997.1751. doi: 10.1006/brln.1997.1751. [DOI] [PubMed] [Google Scholar]
- Prather P, Zurif E, Stern C, Rosen TJ. Slowed lexical access in nonfluent aphasia: A case study. Brain and Language. 1992;43:336–348. doi: 10.1016/0093-934x(92)90134-z. doi: 10.1016/0093-934X(92)90134-Z. [DOI] [PubMed] [Google Scholar]
- Raven JC. Coloured Progressive Matrices: Set A, Ab, B. Oxford Psychologists Press; Oxford, UK: 1976. [Google Scholar]
- Schuell H, Jenkins JJ, Jiménez-Pabón E. Aphasia in adults: diagnosis, prognosis and treatment. Hoeber Medical Division: Harper & Row Publishers; New York: 1964. [Google Scholar]
- Schwartz MF, Saffran EM, Bloch DE, Dell GS. Disordered speech production in aphasic and normal speakers. Brain and Language. 1994;47:52–88. doi: 10.1006/brln.1994.1042. doi: 10.1006/brln.1994.1042. [DOI] [PubMed] [Google Scholar]
- Silkes JP, Rogers MA. Perception of visually masked stimuli by individuals with aphasia: A methodological assessment and preliminary theoretical implications. Aphasiology. 2010;24:763–774. doi: 10.1080/02687030903509340. doi: 10.1080/02687030903509340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney D, Zurif E, Nicol J. The effects of focal brain damage on sentence processing: An examination of the neurological organization of a mental module. Journal of Cognitive Neuroscience. 1989;1:25–37. doi: 10.1162/jocn.1989.1.1.25. [DOI] [PubMed] [Google Scholar]
- Trupe EH. Reliability of rating spontaneous speech in the Western Aphasia Battery: Implications for classification. In: Brookshire R, editor. Clinical Aphasiology: Proceedings of the Conference; Minneapolis. BRK Publishers; 1984. pp. 55–69. [Google Scholar]
- Tseng CH, McNeil MR, Milenkovic P. An investigation of attention allocation deficits in aphasia. Brain and Language. 1993;45:276–296. doi: 10.1006/brln.1993.1046. doi: 10.1006/brln.1993.1046. [DOI] [PubMed] [Google Scholar]
- Turner-Stokes L, Kalmus M, Hirani D, Clegg F. The Depression Intensity Scale Circles (DISCs): A first evaluation of a simple assessment tool for depression in the context of brain injury. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:1273–1278. doi: 10.1136/jnnp.2004.050096. doi: 10.1136/jnnp.2004.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.