Abstract
Dental caries is the most common chronic disease of childhood. Cariogram is a well-recognized algorithm-based software program based on different caries-related risk factors and intended to aid clinicians in performing more objective and consistent dental caries risk assessments. This type of approach precedes the diagnosis of caries and allows the dentist to identify at-risk patients and then take appropriate preventive measures before caries develop further. One of the etiological factors favoring the development of dental caries is the mutans streptococci. These acidogenic dental plaque inhabitants can be effectively antagonized by the activity of bacteriocins released by the probiotic Streptococcus salivarius M18 (salivarius M18). Moreover, salivarius M18 after colonizing the human oral mucosa produces the enzymes dextranase and urease that are able to counteract plaque formation and saliva acidity, respectively. Seventy-six subjects at high risk of dental caries were randomized and then either treated or not treated for 90 days with an oral formulation containing the oral probiotic salivarius M18 (Carioblis®). The results indicate that the use of salivarius M18 increases the chances of avoiding new dental caries development in children, and its application could be proposed as a new tool in the dentist’s armory to be adopted in subjects considered at high risk on the basis of their Cariogram outcome.
Keywords: BLIS M18, caries prediction, dextranase, urease, Streptococcus mutans, Streptococcus sobrinus, plaque, salivary pH, bacteriocins
Introduction
Dental caries is the most common chronic disease of childhood and its prevalence continues to increase in many populations worldwide.1,2 It is a multifactorial disease mainly caused by interactions between mutans streptococci, especially Streptococcus mutans and Streptococcus sobrinus, and individual caries risk factors, such as saliva composition, fluoride exposure, and dietary habits.3 Despite dental caries being preventable and the many major technological advances in dentistry in recent years,4 dental caries remains a very diffuse and unsolved medical problem. Being a pathology sustained by microbial pathogens, treatments using conventional antistreptococcal antibiotics can be effective in the short term to reduce dental plaque levels and to decrease counts of the mutans streptococci. However, as most antibiotics have relatively broad-spectrum antimicrobial activity, they indiscriminately destroy both commensal and potentially harmful bacteria and thereby create population imbalances within the microflora.5 This outcome could be a consequence of using well-known natural or synthetic antibiotics and also of using new herbal medicines endowed with antibiotic activity.6,7 It is now becoming clear that the severity of some oral pathologies, including dental caries, otitis media, halitosis, and streptococcal pharyngotonsillitis, can be related to the development of oral microbiota disequilibria. The application of oral probiotics to help restore a balanced microbiota and thereby improve oral health is a relatively new concept.8 Some putative commensal bacteria have been assessed for their ability to help prevent dental caries. Some initial studies based on the use of intestinal probiotics have reported a reduction in levels of S. mutans and apparently fewer dental caries.9,10 However, because these strains have limitations in terms of their colonization of oral tissues, a new generation of probiotic strains sourced from the human oral cavity and belonging to commensal species known to have extremely low pathogenic potential has more recently been developed. In this regard, a key species is Streptococcus salivarius and the oral probiotic identified as strain K12 has been the most thoroughly studied in terms of its bacteriocin production, oral colonization, and oral persistence and also its efficacy in counteracting halitosis, oral candidosis, pharyngotonsillitis, and acute otitis media.11–22 Streptococcus salivarius M18 (salivarius M18) (IDA classification: DSM 14865),23 a strain originally isolated from a healthy female adult subject during a specific search for an oral commensal strain capable of inhibiting mutans streptococci, has subsequently been shown to have relatively broad spectrum bacteriocin-like inhibitory substance (BLIS) activity against S. mutans and S. sobrinus and to produce both dextranase and urease enzymes, the activities of which could potentially help limit the progression of dental caries by reducing plaque accumulation and plaque acidification, respectively.24–26 The whole genome of strain salivarius M18 has been published recently, and its bacteriocin repertoire includes the megaplasmid-encoded salivaricin A2, salivaricin MPS, and salivaricin 9, and the chromosomally encoded salivaricin M.23,24,27 Recent trials have revealed, along with its safety and tolerability profiles, the capability of salivarius M18 to colonize and persist in the human oral cavity,28 to reduce plaque formation and to lower S. mutans counts in colonized primary-school-aged children,29 and to reduce both moderate and severe gingivitis and periodontitis in adults.30 On the basis of these biochemical, microbiological, and clinical findings, we determine whether the oral and daily use of the strain salivarius M18 affects or modifies the Cariogram outcome after 90 days of treatment in children at high risk of developing new dental caries. Cariogram is an algorithm-based software program developed in Sweden in 1997 by the University of Malmö, based on nine different caries-related risk factors, along with physician judgment, intended to aid clinicians in performing more objective and consistent dental caries risk assessments.31 The performance of the program has been validated in preschool children, schoolchildren, young adults, and the elderly.32–38
Materials and methods
Subjects and criteria
Seventy-six children (aged 6–17 years) classified as high risk on the basis of the Cariogram results performed at day 0 (chance to avoid new cavities <25) were included in this randomized, controlled study after informed consent was obtained from their parents. Exclusion criteria were diagnosis of heart, respiratory, renal, liver, or intestinal disease, or undergoing current therapy with antibiotics and/or corticosteroids for the prevention/treatment of recurrent bacterial pathologies, such as cystitis, pharyngotonsillitis, and acute otitis media, or to counteract allergic reactions and/or asthma. According to the protocol, occasional use of acetaminophen or ibuprofen for fever and/or pain control and of physician-prescribed antibiotics was allowed. During the use of antibiotics, treated children were asked to stop using the salivarius M18-based product. As there were no dropouts, all the 76 children (38 children in the treated group and 38 in the control, untreated group) attended the follow-up examination performed after 90 days and were included in the statistical analysis.
Study scheme
This randomized, controlled study was conducted in the field of routine clinical practice in the area of Milan (Italy) between March and September 2014, in agreement with the criteria set by the Declaration of Helsinki and the Milan Ethical Board gave the approval for this study. The parents of all the participants in the study were informed of the trial methods and signed the consent and privacy-policy documents giving the authorization to publish the results. As shown in Figure 1, 76 of the 100 children analyzed were considered eligible for enrollment and were randomly assigned to be supplemented once a day for 3 months with the test product (treated group; n=38) or not to receive any treatment (control group; n=38). Randomization was carried out using the sealed envelope system. After 90 days, 76 children attended the follow-up examination and were subjected to their second Cariogram test. Every 15 days, during the study, all of the enrolled subjects were in contact with the dentists responsible for the study to report their medical condition and specific study parameters such as probiotic tolerability and dosing compliance, as well as to enable documentation of the occurrence of any side effects possibly linked to the treatment. The subjects were also provided with the possibility of daily access to the physicians responsible for the study.
Figure 1.
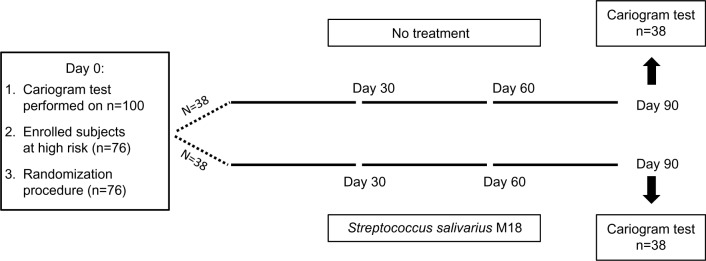
Scheme of the study.
Tested product
Salivarius M18 (IDA classification: DSM 14865), also named by the manufacturer as BLIS M18 (BLIS Technologies, Dunedin, New Zealand), was formulated as slowly dissolving oral tablets by SIIT (Trezzano S/N, Italy) and notified as nutritional supplement to the Italian Ministry of Health as Carioblis® by Omeopiacenza (Pontenure, Italy), according to the provisions of law 169 of 2004, on July 19, 2013 (notification number 69163). The preparation of Carioblis® used in our research contained no less than 1 billion colony-forming units (CFU)/tablet of strain salivarius M18.
Treatment protocol
Starting from day 0 to 90, one tablet of Carioblis® was administered to each subject every night, just before sleep. The tablet was allowed to slowly dissolve in the oral cavity, without biting or swallowing. Saliva production is typically reduced in the evening hours and this improves the effectiveness of oral colonization. Only for the very first treatment, the administration of the tablet was preceded, approximately 30 minutes before, by the use of a chlorhexidine-based (0.2%) mouthwash. This procedure improves the efficacy of oral colonization by BLIS M18 by creating bacteria-depleted niches in the oral tissues. In order to evaluate the level of subject adherence to the established protocol, the subjects were asked to return any unused product boxes and tablets. Acceptable adherence was considered to be the administration of not less than 95% of the allocated tablets.
Mutans, saliva, and plaque
To evaluate the presence of S. mutans, the GC Saliva-Check Mutans test (monoclonal antibody-based) was used. As regards saliva, to evaluate pH and quantity, the GC Saliva-Check Buffer test was used. In order to obtain the samples of saliva for the analysis of mutans streptococci, the saliva secretion rate (mL/min), and buffer capability, paraffin-stimulated whole saliva was collected from all children. The presence of dental plaque was assessed by using the GC Plaque Indicator test. All kits are supplied by GC Europe, Leuven, Belgium.
Study objectives
The principal objectives for the study were 1) to establish the safety and tolerability profiles of the salivarius M18-based product in children at high risk of developing new dental caries and 2) to evaluate in the same children whether any Cariogram modifications occurred after 90 days of treatment with the salivarius M18-based product.
Statistical analysis
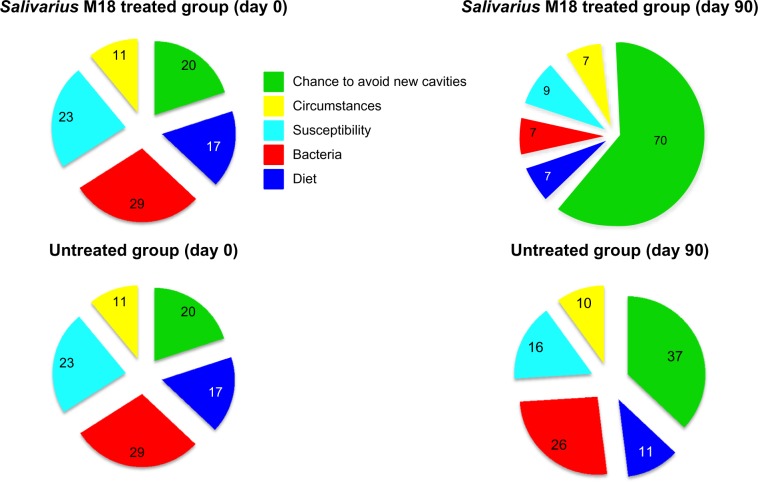
To study the null hypothesis of no effect of treatment on Carioblis® for each clinical variable, and for a global summation, we applied the two-tailed Wilcoxon test for matched pairs with signed ranks. To study the effect of Carioblis® therapy on Cariogram scores, using averaged clinical variables, we used the two-tailed Fisher’s exact test. Statistical software JMP® 10 for Mac OS X (SAS Institute Inc., Cary, NC, USA) was used, and the threshold for statistical significance was 95%. To calculate the caries risk of a group of subjects (results are shown in Table 1 and Figure 2), we used the average value of any single items of the Cariogram and considered equal to 0 if the decimal values stands between 0.1 and 0.4 and equal to 1 if the decimal values were between 0.5 and 0.9. The Cariogram software used the Java Internet 2004 version.
Table 1.
Cariogram values (at day 0 and day 90) calculated using the average value of the items listed
| Treatment, time period | Actual chance to avoid new cavities (%) | Diet (%) | Bacteria (%) | Susceptibility (%) | Circumstances (%) |
|---|---|---|---|---|---|
| Salivarius M18, day 0 | 20 | 17 | 29 | 23 | 11 |
| Salivarius M18*, day 90 | 70 | 7 | 7 | 9 | 7 |
| Untreated, day 0 | 20 | 17 | 29 | 23 | 11 |
| Untreated, day 90 | 37 | 11 | 26 | 16 | 10 |
Note:
All values of M18, day 90 are significant (P<0.01) vs treatment M18, day 0.
Abbreviation: Salivarius M18, Streptococcus salivarius M18.
Figure 2.
Graphical representation of Cariogram values (%) calculated using the average value of the items listed.
Abbreviation: Salivarius M18, Streptococcus salivarius M18.
Results
This randomized and controlled study has been carried out on 76 children at high risk of new dental caries development. Thirty-eight of these subjects were treated for 90 days with Carioblis® (a S. salivarius M18-based product) and the others served as controls (untreated group). There was no dropout, therefore all of the children were considered eligible for the statistical analysis. As shown in Table 2, no statistical differences existed between the two groups in terms of sex and age. Ninety days of treatment with strain M18 produced in the treated group a statistically significant reduction, by more than 30%, in the global Cariogram outcome. No statistical difference was observed in the control group (Table 3). By analyzing every individual parameter of the Cariogram results in the salivarius M18 treated group (Table 4) one observes that, other than for “caries experience”, “related diseases”, and “clinical judgment”, all of the parameters are improved. Some improvements are probably due to a better control of the aspects of diet (diet content and frequency) or changes in oral hygiene and/or in prophylaxis (fluoride program) and cannot be linked to the treatment, with clear evidence. Others, such as “plaque amount”, “mutans streptococci”, and “buffer capacity” could be a direct consequence of the treatment because salivarius M18 releases bacteriocins able to kill mutans streptococci, and dextranase and urease enzymes, which are capable of counteracting plaque formation and increasing saliva pH, respectively. Noteworthy, “plaque control” and “mutans streptococci” were reduced by approximately 50% and 75%, respectively. By contrast, as shown in Table 5, the untreated group did not show the same type of improvement and the only statistically significant changes are due to a better control of diet, oral hygiene, and prophylactic approach. The Cariogram software was uniquely implemented to calculate the caries risk of individual subjects. Nevertheless, we have exploited the algorithm of the Cariogram software and used the average value of any single items of the Cariogram to calculate the caries risk of a group of subjects. This nonvalidated procedure allows construction of an image representative of the likely impact that a treatment can have on a group of patients. As shown in Table 1 and Figure 2, treatment with the strain M18-based product significantly improves the “chances of avoiding new dental caries”, from 20 to 70, reduces the parameter “bacteria”, from 29 to 7, and reduces the “susceptibility”, from 23 to 9. No relevant variations were evident in the untreated group. Finally, in Table 6, the M18-based treatment demonstrated a very good safety profile with no treatment-related side effects and no subject dropout. Tolerability was assessed as “good” and “very good” in 35 of the 38 subjects and overlapping results were obtained with regard to compliance.
Table 2.
Characteristics* of the enrolled children
| Salivarius M18-treated group (n=38) | Untreated group (n=38) | |
|---|---|---|
| Males, n | 25 | 21 |
| Age° of males | 11.2±3.2 | 12.1±2.9 |
| Females, n | 13 | 17 |
| Age° of females | 11.5±3.6 | 11.8±3.8 |
Notes:
Nonsignificant differences between groups; °age expressed in years ± standard deviation.
Abbreviation: Salivarius M18, Streptococcus salivarius M18.
Table 3.
Global Cariogram outcome at day 0 and day 90
| Salivarius M18-treated group (n=38) | Untreated group (n=38) | |
|---|---|---|
| Day 0** | 15.9±2.6 (16) | 16.3±2.9 (16) |
| Day 90** | 11.1±2.0 (11)* | 14.4±3.2 (14) |
| Δ % vs day 0 | 30.2 | 11.7 |
Notes:
P<0.01 vs day 0;
data expressed as mean ± standard deviation (median).
Abbreviation: Salivarius M18, Streptococcus salivarius M18.
Table 4.
Cariogram: outcome of individual parameters in the salivarius M18-treated group (n=38)
| Day 0* | Day 90* | P | |
|---|---|---|---|
| Caries experience | 2.7±0.5 (3) | 2.7±0.5 (3) | ns |
| Related diseases | 0.0±0.2 (0) | 0.1±0.2 (0) | ns |
| Diet, content | 1.9±1.0 (2) | 1.4±0.7 (1) | <0.05 |
| Diet, frequency | 1.4±0.8 (1) | 1.2±0.5 (1) | <0.05 |
| Plaque amount | 2.0±0.8 (2) | 1.0±0.6 (1) | <0.01 |
| Mutans streptococci | 2.7±0.5 (3) | 0.7±0.8 (0) | <0.01 |
| Fluoride program | 2.4±0.7 (2) | 1.8±0.5 (2) | <0.01 |
| Saliva secretion | 1.7±0.9 (2) | 1.2±1.1 (1) | <0.05 |
| Buffer capacity | 0.0±0.2 (0) | 0.0±0.0 (0) | <0.05 |
| Clinical judgment | 1.1±0.4 (1) | 1.0±0.2 (1) | ns |
Note:
Data expressed as mean ± standard deviation (median).
Abbreviations: ns, not significant; salivarius M18, Streptococcus salivarius M18.
Table 5.
Cariogram: outcome of individual parameters in the untreated group (n=38)
| Day 0* | Day 90* | P | |
|---|---|---|---|
| Caries experience | 2.8±0.5 (3) | 2.8±0.6 (3) | ns |
| Related diseases | 0.1±0.2 (0) | 0.2±0.2 (0) | ns |
| Diet, content | 2.0±1.0 (2) | 1.3±0.4 (1) | <0.01 |
| Diet, frequency | 1.3±0.8 (1) | 1.1±0.7 (1) | <0.05 |
| Plaque amount | 2.0±0.9 (2) | 2.1±0.7 (2) | ns |
| Mutans streptococci | 2.6±0.5 (3) | 2.5±0.6 (3) | ns |
| Fluoride program | 2.3±0.7 (2) | 1.6±0.7 (1) | <0.01 |
| Saliva secretion | 1.8±0.9 (2) | 1.4±1.2 (1) | <0.05 |
| Buffer capacity | 0.2±0.2 (0) | 0.2±0.2 (0) | ns |
| Clinical judgment | 1.2±0.4 (1) | 1.2±0.1 (1) | ns |
Note:
Data expressed as mean ± standard deviation (median).
Abbreviation: ns, not significant.
Table 6.
Tolerability, compliance, and side effects in children (n=38) treated for 90 days by oral route with Streptococcus salivarius M18 as reported by themselves and/or parents and established by dentists responsible for the study
| Tolerability | Compliance | Side effects | |
|---|---|---|---|
| Very good | n=30 | n=32 | None |
| Good | n=5 | n=6 | None |
| Acceptable | n=3 | n=0 | None |
| Unacceptable | n=0 | n=0 | None |
Discussion
Caries risk assessment is an important tool assisting the dentist in obtaining a better understanding of the dental profile of a patient. The Cariogram software has been clinically proven to be effective in evaluating such a risk.39 Cariogram is based on a set of nine pathological and protective factors, in addition to the professional judgment of the expert dentist. Among these factors, the likely most relevant variable in caries risk prediction is “caries experience” and, as a matter of fact, a strong relationship has been shown between caries experience and caries risk profile.40,41 Apparently, microbial tests, aimed at evaluating the presence of mutans streptococci, do not seem to be equally relevant. This could be because, in the presence of fluoride, along with an appropriate diet in terms of quality and quantity, a high number of mutans streptococci may be tolerated without causing significant harm to the teeth.42 Fluoride is not the only potentially protective factor in the presence of an abundance of deleterious streptococci. Within the oral microbiota, populations of mutans streptococci can indeed be balanced by the presence of antagonizing bacteria. Among these, a particularly important role is thought to be played by S. salivarius, one of the most prevalent of the commensal oral bacteria. Different strains of S. salivarius have been shown capable of counteracting the growth of mutans streptococci8 and, of these, the strongest clinical potential has been shown by strain M18.29,30 On this basis, we decided to test the capability of salivarius M18 to modify the Cariogram outcome. According to our results, 90 days treatment with this oral probiotic has increased the chances of avoiding new cavities in children. This outcome is considered attributable to the specific anticariogenic characteristics of strain M18 that, after colonizing the oral mucosa, is able to release bacteriocins, limiting the growth of S. mutans and S. sobrinus, and the enzymes dextranase and urease, catalyzing the breakdown of dextran (aiding solubilization of plaque) and the hydrolysis of urea (increasing saliva pH). The present study does contain some bias: 1) it is not a blinded study; 2) there is no placebo group; 3) the control group comprises untreated subjects; and 4) the number of enrolled subjects is rather small. Nevertheless, this study represents one of the pioneer attempts to analyze the significance of salivarius M18 in dental practice. If these preliminary results can be confirmed with a larger number of subjects and in double-blind clinical conditions, the practical application of strain M18 could be proposed in the future as a new tool in the dentist’s armory, along with the already available strategies (eg, anticaries diets, fluoride, and oral hygiene) to be adopted in subjects considered at high risk on the basis of their Cariogram outcome. On the basis of the calculated risk to develop new dental caries, subjects are divided into three groups: low, medium, and high. Depending on these groups, the fundamental aspects of primary prevention are applied to different extents for protocol and rigor: light in those at low risk, moderate in those at intermediate risk, and close and manifold in high-risk individuals. Certainly, in patients defined at high risk, but possibly also in those of intermediate risk, the addition of a protocol incorporating the administration of the salivarius M18 could be crucial to addressing and further reducing the risk of tooth-decay receptivity. The caries risk is configured as the predisposition of an individual to be affected by the carious pathology, regardless of the fact of presenting caries at the time of the dental examination. This type of diagnosis precedes then the diagnosis of caries, allowing dentist to intercept the at-risk patient and take the appropriate preventive measures to intercept the development of tooth decay.
Acknowledgments
The authors thank Dr J Tagg for suggestions and review of the paper.
Footnotes
Disclosure
F Di Pierro is the Scientific Director of Velleja Research, the company that developed the finished product tested in this study. The other authors report no other conflicts of interest in this work.
References
- 1.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent. 2009;22:3–8. [PubMed] [Google Scholar]
- 2.Vachirarojpisan T, Shinada K, Kawaguchi Y, Laungwechakan P, Somkote T, Detsomboonrat P. Early childhood caries in children aged 6–19 months. Community Dent Oral Epidemiol. 2004;32(2):133–142. doi: 10.1111/j.0301-5661.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 3.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 4.Shah N, Bansal N, Logani A. Recent advances in imaging technologies in dentistry. World J Radiol. 2014;6(10):794–807. doi: 10.4329/wjr.v6.i10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ten Cate JM, Zaura E. The numerous microbial species in oral biofilms: how could antibacterial therapy be effective? Adv Dent Res. 2012;24(2):108–111. doi: 10.1177/0022034512450028. [DOI] [PubMed] [Google Scholar]
- 6.Silva JP, Castilho AL, Saraceni CH, Díaz IE, Paciencia ML, Suffredini IB. Anti-Streptococcal activity of Brazilian Amazon Rain Forest plant extracts presents potential for preventive strategies against dental caries. J Appl Oral Sci. 2014;22(2):91–97. doi: 10.1590/1678-775720130366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brighenti FL, Salvador MJ, Delbem AC, et al. Systematic screening of plant extracts from the Brazilian Pantanal with antimicrobial activity against bacteria with cariogenic relevance. Caries Res. 2014;48(5):353–360. doi: 10.1159/000357225. [DOI] [PubMed] [Google Scholar]
- 8.Wescombe PA, Hale JD, Heng NC, Tagg JR. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. 2012;7(12):1355–1371. doi: 10.2217/fmb.12.113. [DOI] [PubMed] [Google Scholar]
- 9.Caglar E, Sandalli N, Twetman S, Kavaloglu S, Ergeneli S, Selvi S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand. 2005;63:317–320. doi: 10.1080/00016350510020070. [DOI] [PubMed] [Google Scholar]
- 10.Nase L, Hatakka K, Savilahti E, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 11.Tagg JR. Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. Indian J Med. 2004;119:13–16. [PubMed] [Google Scholar]
- 12.Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. 2007;73(4):1107–1113. doi: 10.1128/AEM.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Verma KK. Skin and soft tissue infection. Indian J Pediatr. 2001;68(Suppl 3):S46–S50. [PubMed] [Google Scholar]
- 14.Wescombe PA, Burton JP, Cadieux PA, et al. Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek. 2006;90(3):269–280. doi: 10.1007/s10482-006-9081-y. [DOI] [PubMed] [Google Scholar]
- 15.van Zon A, van der Heijden GJ, van Dongen TM, Burton MJ, Schilder AG. Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev. 2012;9:CD009163. doi: 10.1002/14651858.CD009163.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Power DA, Burton JP, Chilcott CN, Dawes PJ, Tagg JR. Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur J Clin Microbiol Infect Dis. 2008;27(12):1261–1263. doi: 10.1007/s10096-008-0569-4. [DOI] [PubMed] [Google Scholar]
- 17.Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. 2007;22(2):126–130. doi: 10.1111/j.1399-302X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 18.Burton JP, Wescombe PA, Moore CJ, Chilcott CN, Tagg JR. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl Environ Microbiol. 2006;72(4):3050–3053. doi: 10.1128/AEM.72.4.3050-3053.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2011;49(9):2356–2364. doi: 10.1016/j.fct.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Di Pierro F, Adami T, Rapacioli G, Giardini N, Streitberger C. Clinical evaluation of the oral probiotic Streptococcus salivarius K12 in the prevention of recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes in adults. Expert Opin Biol Ther. 2013;13(3):339–343. doi: 10.1517/14712598.2013.758711. [DOI] [PubMed] [Google Scholar]
- 21.Di Pierro F, Donato G, Fomia F, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med. 2012;5:991–997. doi: 10.2147/IJGM.S38859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. 2014;6:15–20. doi: 10.2147/DHPS.S59665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chilcott CN, Tagg JR. Antimicrobial composition. 7226590. United States patent US. 2007
- 24.Heng NC, Haji-Ishak NS, Kalyan A, et al. Genome sequence of the bacteriocin producing oral probiotic Streptococcus salivarius strain M18. J Bacteriol. 2011;193:6402–6403. doi: 10.1128/JB.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YY, Clancy KA, Burne RA. Streptococcus salivarius urease: genetic and biochemical characterization and expression in a dental plaque streptococcus. Infect Immun. 1996;64:585–592. doi: 10.1128/iai.64.2.585-592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi Y, Kubo S, Ono Y, et al. Cloning and sequencing of the gene coding for dextranase from Streptococcus salivarius. Gene. 1995;156:93–96. doi: 10.1016/0378-1119(95)00071-d. [DOI] [PubMed] [Google Scholar]
- 27.Wescombe PA, Upton M, Renault P, et al. Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology. 2011;157:1290–1299. doi: 10.1099/mic.0.044719-0. [DOI] [PubMed] [Google Scholar]
- 28.Burton JP, Wescombe PA, Macklaim JM, et al. Persistence of the oral probiotic Streptococcus salivarius M18 is dose dependent and megaplasmid transfer can augment their bacteriocin production and adhesion characteristics. PLoS One. 2013;8(6):e65991. doi: 10.1371/journal.pone.0065991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton JP, Drummond BK, Chilcott CN, et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol. 2013;62(Pt 6):875–884. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 30.Litty S, Nagarathna D, Merline V. Probiotics in periodontal therapy. Int J Pharm Bio Sci. 2015;6(1):242–250. [Google Scholar]
- 31.Bratthall D, Hänsel PG. Cariogram: a multifactorial risk assessment model for a multifactorial disease. Community Dent Oral Epidemiol. 2005;33:256–264. doi: 10.1111/j.1600-0528.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 32.Holgerson PL, Twetman S, Stecksèn-Blicks C. Validation of an age-modified caries risk assessment program (Cariogram) in preschool children. Acta Odontol Scand. 2009;67:106–112. doi: 10.1080/00016350802714734. [DOI] [PubMed] [Google Scholar]
- 33.Hänsel Petersson G, Twetman S, Bratthall D. Evaluation of a computer program for caries risk assessment in schoolchildren. Caries Res. 2002;36:327–340. doi: 10.1159/000065963. [DOI] [PubMed] [Google Scholar]
- 34.Campus G, Cagetti MG, Sale S, Carta G, Lingström P. Cariogram validity in schoolchildren: a two-year follow-up study. Caries Res. 2012;46:16–22. doi: 10.1159/000334932. [DOI] [PubMed] [Google Scholar]
- 35.Petersson GH, Isberg PE, Twetman S. Caries risk assessment in school children using a reduced Cariogram model without saliva tests. BMC Oral Health. 2010;19(10):5. doi: 10.1186/1472-6831-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zukanović A. Caries risk assessment models in caries prediction. Acta Med Acad. 2013;42:198–208. doi: 10.5644/ama2006-124.87. [DOI] [PubMed] [Google Scholar]
- 37.Alian AY, McNally ME, Fure S, Birkhed D. Assessment of caries risk in elderly patients using the Cariogram model. J Can Dent Assoc. 2006;72:459–463. [PubMed] [Google Scholar]
- 38.Celik EU, Gokay N, Ates M. Efficiency of caries risk assessment in young adults using Cariogram. Eur J Dent. 2012;6:270–279. [PMC free article] [PubMed] [Google Scholar]
- 39.Tellez M, Gomez J, Ellwood R, Ismail AI. Evidence on existing caries risk assessment systems: are they predictive of future caries? Community Dent Oral Epidemiol. 2013;41(1):67–78. doi: 10.1111/cdoe.12003. [DOI] [PubMed] [Google Scholar]
- 40.Campus G, Cagetti MG, Sacco G, Benedetti G, Strohmenger L, Lingström P. Caries risk profiles in Sardinian schoolchildren using Cariogram. Acta Odontol Scand. 2009;67(3):146–152. doi: 10.1080/00016350902740498. [DOI] [PubMed] [Google Scholar]
- 41.Hänsel Petersson G, Twetman S, Bratthall D. Evaluation of a computer program for caries risk assessment in schoolchildren. Caries Res. 2002;36(5):327–340. doi: 10.1159/000065963. [DOI] [PubMed] [Google Scholar]
- 42.Baehni PC, Guggenheim B. Potential of diagnostic microbiology for treatment and prognosis of dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7(3):259–277. doi: 10.1177/10454411960070030401. [DOI] [PubMed] [Google Scholar]