Abstract
In this paper, we present the results of a reanalysis of the data of two large randomized, double-blind, parallel group studies with a similar design, comparing the efficacy of an angiotensin-receptor blocker (olmesartan medoxomil) with that of an angiotensin-converting enzyme inhibitor (ramipril), by applying two different blood pressure targets recently recommended by hypertension guidelines for all patients, irrespective of the presence of diabetes (<140/90 mmHg), and for elderly hypertensive patients (<150/90 mmHg). The efficacy of olmesartan was not negatively affected by age, sex, hypertension type, diabetes status or other concomitant clinical conditions, or cardiovascular risk factors. In most cases, olmesartan provided better blood pressure control than ramipril. Olmesartan was significantly more effective than ramipril in male patients, in younger patients (aged 65–69 years), in those with metabolic syndrome, obesity, dyslipidemia, preserved renal function, diastolic ± systolic hypertension, and, in general, in patients with a high or very high cardiovascular risk. Interestingly, patients previously untreated or treated with two or more antihypertensive drugs showed a significantly larger response with olmesartan than with ramipril. Thus, our results confirm the good efficacy of olmesartan in elderly hypertensives even when new blood pressure targets for antihypertensive treatment are considered. Such results may be relevant for the clinical practice, providing some hint on the possible different response of elderly hypertensive patients to two different drugs acting on the renin–angiotensin system, when patients are targeted according to the blood pressure levels recommended by recent hypertension guidelines.
Keywords: arterial hypertension, elderly, guidelines, olmesartan medoxomil, ramipril
Introduction
Until recently, major guidelines recommended two distinct blood pressure targets for treated hypertensives, namely <140/90 mmHg in low-moderate risk individuals and <130/80 mmHg in high-risk ones.1,2 According to these guidelines, the blood pressure goal in treated older patients had to be the same as in younger patients, namely <140/90 mmHg or below, if tolerated.1,2 However, such recommendations were not supported by incontrovertible trial evidence. As a matter of fact, in all the large randomized trials of antihypertensive treatment in the elderly, showing a reduction in cardiovascular events through lowering blood pressure, the average systolic and diastolic blood pressure levels attained with treatment were never <140/90 mmHg.3,4 Other trials of more vs less intensive blood pressure lowering were unable to demonstrate benefits, in either aged individuals or high-risk hypertensive patients, by lowering systolic blood pressure <140 mmHg.5–11 Additionally, the results of extensive reviews of randomized controlled trials showed that recommendation to lower blood pressure <130/80 mmHg in patients with diabetes or a history of cardiovascular or renal disease was not supported by any evidence.3,12–14
Taken together, results of all these studies suggested that evidence-based recommendations could be a most appropriate and modern approach to hypertension treatment management. Accordingly, most recent guidelines now recommend that patients with arterial hypertension associated with diabetes or chronic kidney disease must be treated to attain the goal of systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg. They also suggest that in older persons it may be sufficient to treat high blood pressure to a target of 150/90 mmHg or lower.1,2,14–17
Given these premises, the question arises as whether the current available antihypertensive armamentarium, and particularly monotherapies, may be suitable to achieve modern blood pressure targets in older individuals, regardless of the presence of associated clinical conditions or additional cardiovascular risk factors. The availability of a large database of elderly hypertensive patients enrolled in two randomized, double-blind, parallel group studies with a similar design, comparing the efficacy of an angiotensin-receptor blocker (ARB), olmesartan medoxomil, with that of the angiotensin-converting enzyme (ACE)-inhibitor, ramipril, gave us the possibility to explore such a scenario.18,19 The original studies were devised at the time when old recommendations were still valid, and thus blood pressure targets differed between nondiabetics (<140/90 mmHg) and diabetics (<130/80 mmHg). Therefore, in the present paper we reanalyzed the data and compared the results by applying two different blood pressure targets indicated by the new hypertension guidelines: <140/90 mmHg, irrespective of the presence of diabetes, and <150/90 mmHg, as recommended for older hypertensives.
Methodology
The details on the study design and population can be found in previous publications.18–20 Briefly, the two original studies18,19 had a multicenter, randomized, double-blind, parallel group design, consisting of a 2-week washout with placebo, followed by 12 weeks of treatment with olmesartan medoxomil or ramipril at initial doses of 10 or 2.5 mg once daily, respectively. The initial drug dose could be doubled after the 2nd or 6th week of treatment in case of lack of normalization (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg for nondiabetic, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg for diabetic patients). Elderly patients aged between 65 and 89 years, of either sex, with grade 1 or 2 essential hypertension (systolic blood pressure between 140 and 179 mmHg and diastolic blood pressure between 90 and 109 mmHg) were studied.
All patients gave their written informed consent before being enrolled in the study. The study was approved by the Ethics Committees of each study center. As in the original studies, analysis was performed on patients who were eligible for intention-to-treat, defined as all randomized patients receiving at least one dose of active treatment drug and having at least one office blood pressure measurement after randomization, using the last-observation-carried-forward method for patients prematurely leaving the study.
Response to antihypertensive treatment was evaluated by using a blood pressure target of <140/90 mmHg in all patients, irrespective of the presence of diabetes, and <150/90 mmHg, as recommended for older hypertensives. Analysis of variance was used to assess the differences between groups for continuous variables, whereas comparison of normalized patients was performed by the chi-squared test. Subgroup analyses for sex, age groups, level of cardiovascular risk, type of hypertension, metabolic status, renal functions status, number and type of previous antihypertensive drugs, and according to drug tolerability were also made. The level of statistical significance was kept at 0.05 throughout the whole study. Data are shown as means ± standard deviation (SD) or as numbers and percentages.
Results
Treatment efficacy according to new blood pressure targets in the whole study group
The pooled dataset from the two studies consisted of 1,426 patients (intention-to-treat population) of which 712 were treated with olmesartan at an average dose of 27.2±12.6 mg (47.1% of patients taking the full drug dosage) and 714 treated with ramipril at an average dose of 7.3±3.1 mg (55.3% of patients taking the full drug dosage, P=0.008 vs olmesartan).
As shown in Table 1, no statistically significant differences existed between the two treatment groups for the main demographic and clinical characteristics at baseline.
Table 1.
Demographic and clinical characteristics of the 1,426 patients of the intention-to-treat population of the two studies pooled together
| Olmesartan 10–40 mg (n=712) | Ramipril 2.5–10 mg (n=714) | P-value | |
|---|---|---|---|
| Age (years) | 72.0±5.2 | 72.1±5.0 | 0.689 |
| 65–69 | 298 (41.9) | 299 (41.9) | 0.954 |
| 70–79 | 351 (49.3) | 355 (49.7) | |
| >80 | 63 (8.8) | 60 (8.4) | |
| Sex | |||
| Male | 355 (49.9) | 362 (50.7) | 0.751 |
| Female | 357 (50.1) | 352 (49.3) | |
| Height (cm) | 165.8±8.7 | 165.5±8.7 | 0.623 |
| Weight (kg) | 73.8±11.8 | 74.1±11.5 | 0.613 |
| BMI (kg/m2) | 26.8±3.5 | 27.0±3.2 | 0.339 |
| Waist circumference (cm)a | 96.1±11.6 | 96.3±11.5 | 0.790 |
| Significant medical history | 586 (82.3) | 588 (82.4) | 0.980 |
| Concomitant treatments | 480 (67.4) | 492 (68.9) | 0.545 |
| Hypertension medication in the previous 3 months | 538 (75.6) | 537 (75.2) | 0.877 |
| Number of previous antihypertensive drugs | |||
| None | 174 (24.4) | 179 (25.1) | 0.682 |
| 1 | 348 (48.9) | 333 (46.6) | |
| 2 or more | 190 (26.7) | 202 (28.3) | |
| Type of previous antihypertensive drugsb | |||
| ACE inhibitors | 208 (38.7) | 214 (39.9) | 0.672 |
| Angiotensin II receptor blockers | 215 (40.0) | 210 (39.2) | 0.793 |
| Calcium channel blockers | 170 (31.6) | 127 (23.7) | 0.004 |
| Diuretics | 54 (10.0) | 73 (13.6) | 0.069 |
| Beta-blockers | 85 (15.8) | 100 (18.7) | 0.215 |
| Alpha-blockers | 36 (6.7) | 43 (8.0) | 0.403 |
| Others | 10 (1.9) | 12 (2.2) | 0.660 |
| Metabolic syndrome | 372 (52.2) | 363 (50.8) | 0.595 |
| Central or peripheral obesity | 557 (78.2) | 573 (80.3) | 0.347 |
| Dyslipidemia | 601 (84.4) | 610 (85.4) | 0.589 |
| Diabetes | 138 (19.4) | 153 (21.4) | 0.338 |
| CKD stages | |||
| Normal or increased eGFR (≥90 mL/min/1.73 m2) | 89 (12.5) | 92 (12.9) | 0.973 |
| Slightly reduced eGFR (60–90 mL/min/1.73 m2) | 421 (59.1) | 419 (58.7) | |
| Moderately or severely reduced eGFR (<60 mL/min/1.73 m2) | 202 (28.4) | 203 (28.4) | |
| Cardiovascular risk level | |||
| Low-moderate (≤5%) | 80 (11.2) | 79 (11.1) | 0.918 |
| High-very high (>5%) | 632 (88.8) | 635 (88.9) | |
| Office SBP (mmHg) | 157.1±10.0 | 156.6±10.0 | 0.407 |
| Office DBP (mmHg) | 91.8±6.7 | 91.3±6.7 | 0.103 |
| Type of hypertension | |||
| Diastolic ± systolic | 550 (77.2) | 527 (73.8) | 0.131 |
| Isolated systolic | 162 (22.8) | 187 (26.2) | |
Notes: Data are shown as means (± SD) or as absolute (n) and relative (%) frequencies. P-values for between-treatment difference are also reported.
Available for 699 patients randomized to olmesartan and for 703 patients randomized to ramipril.
Percentages refer to treated patients (n=1,074).
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; ACE, angiotensin-converting enzyme.
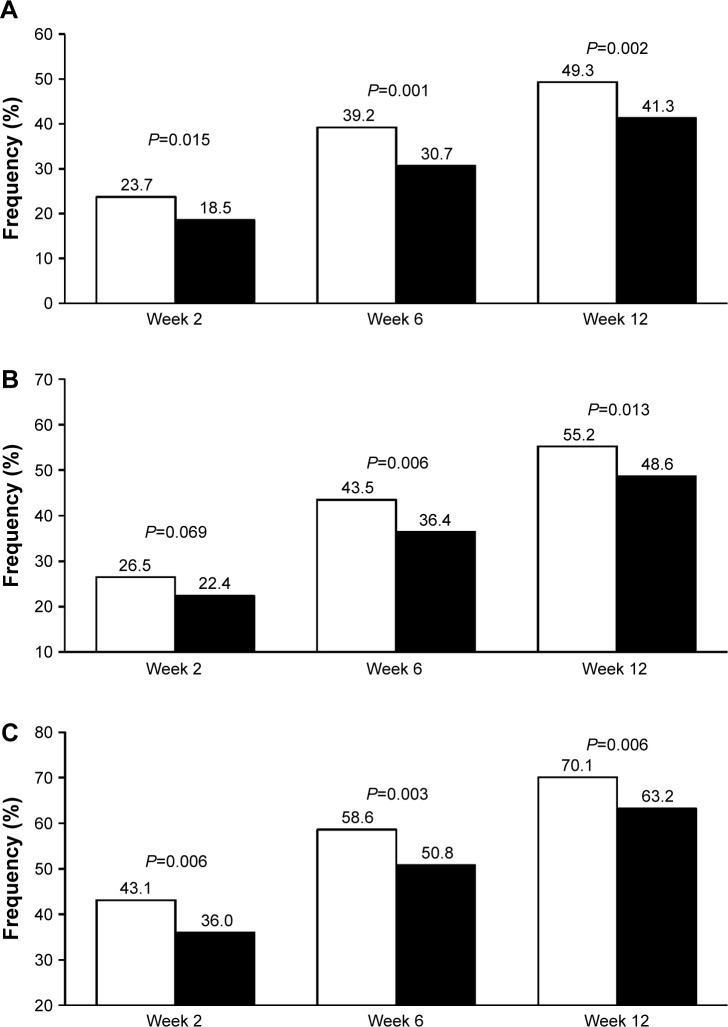
As expected, using the new cutoffs, the rate of normalization at 12 weeks (study end) increased as compared to the original reports, being still significantly larger under olmesartan than under ramipril (Figure 1). When the blood pressure target was set at <140/90 mmHg for all patients, 55.2% of olmesartan-treated patients attained blood pressure normalization vs 48.6% of ramipril-treated patients (P=0.013). The use of a less rigid target (<150/90 mmHg), as indicated for the elderly, resulted in 70.1% rate of normalization under olmesartan and 63.2% under ramipril (P=0.006) at the end of the study. The superiority of olmesartan was observed at each study time point and the difference vs ramipril was statistically significant, except in one case.
Figure 1.
Percentage of normalized patients according to different thresholds.
Notes: (A) Original study thresholds, <140/90 mmHg in nondiabetics and <130/80 mmHg in diabetics. (B) <140/90 mmHg for all patients. (C) <150/90 mmHg for all patients after 2, 6, and 12 weeks of treatment with olmesartan 10–40 mg (white bars) or ramipril 2.5–10 mg (black bars). P-values for between-treatment difference are also reported.
A summary of other studies assessing the efficacy of olmesartan monotherapy in elderly hypertensive patients is reported in Table 2. Although the studies performed so far are not entirely homogenous for design, inclusion criteria, study duration, and endpoints, some similarities with our results may be observed, at least for some studies. For instance, the application of new blood pressure targets to our data gave results superimposable to those observed in a similar study by Kereiakes et al which made use of olmesartan at dosages of 20 or 40 mg with blood pressure targets evaluated at <140/90 mmHg (Table 2).21 Our responder rate was also not dissimilar from that observed in a study by Saito et al employing olmesartan monotherapy at dosages of 5–40 mg, with the addition of other antihypertensive drugs.22 In the other three studies, the percentages were higher than in our studies, but one study enrolled patients with entry blood pressure levels higher than those of our population and evaluated only diastolic blood pressure response.23 Another study was characterized by a very long follow-up.24 A last study included only patients with systolic hypertension and the proportion of responders, larger than in our study, was estimated taking into account the systolic blood pressure only.25
Table 2.
Blood pressure response to olmesartan monotherapy in elderly patients with systolic and/or diastolic hypertension or isolated systolic hypertension in different open-label or double-blind randomized studies
| Author, year (ref) | Country | Number of patients | Study design | Age (years) | BP at entry | Washout (weeks) | Treatment duration | Type of treatment | Blood pressure normalization |
|---|---|---|---|---|---|---|---|---|---|
| Kereiakes et al 200921 | USA | 176 | Open-label, randomized, blinded endpoint | ≥65 | DBP ≥90 mmHg and/or SBP ≥140 mmHg | 2 (placebo) | 12 weeks | Olmesartan 20 or 40 mg | BP <140/90 mmHg: 52.3% |
| Saito et al 200822 | Japan | 481 | Open-label, randomized, prospective | ≥65 | DBP ≥90 mmHg and/or SBP ≥140 mmHg | None | 24 weeks | Olmesartan 5, 10, 20 or 40 mg (+ other antihypertensive drugs) | BP <140/90 mmHg: 50.0% |
| Heagerty and Mallion 200923 | Europe | 251 | Double-blind, randomized | ≥65 | DBP 100–114 mmHg SBP 151–200 mmHg |
2 (placebo) | 52 weeks | Olmesartan 20 or 40 mg | DBP ≤90 mmHg: 74.5% |
| Ogawa et al 201224 | Japan | 578 | Open-label, randomized, prospective, blinded endpoint | 65–84 | DBP ≥90 mmHg and/or SBP ≥140 mmHg | 2–4 (olmesartan 20 mg) | 3 years | Olmesartan 40 mg | BP <140/90 mmHg: 62.1% |
| Mallion et al 200725 | Europe | 256 | Double-blind, randomized | ≥65 | DBP <90 mmHg SBP 161–200 mmHg |
2 (placebo) | 24 weeks | Olmesartan 20 or 40 mg | SBP ≤135 mmHg: 67.6% |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; BP, blood pressure.
Interestingly, in the present study, the proportion of patients achieving a blood pressure <140/90 mmHg was only marginally lower than that observed in a large cohort of 5,141 individuals aged 65–85 years receiving olmesartan 5–40 mg combined with a calcium channel blocker (amlodipine 2.5 or 5 mg, azelnidipine 8 or 16 mg) or a low-dose diuretic.26 As a matter of fact, at the end of the 3.3 years of median follow-up, 68.0% of patients achieved the target blood pressure levels of <140/90 mmHg.
Blood pressure response according to sex, age, and absolute level of cardiovascular risk
In the original study report, blood pressure response was significantly better with olmesartan than with ramipril, in both men and women, as well as in younger individuals (65–69 years).20 When the new therapeutic targets were applied, in the present analysis, the difference observed in favor of olmesartan was no more statistically significant for women (Table 3). Such differences as respect to the main study may be explained by the retrospective nature of the analysis, but we cannot exclude sex differences in response to olmesartan or ramipril, as postulated by recent studies in humans and animals.27,28 Concerning age, olmesartan was still significantly more effective than ramipril in patients younger than 70 years, whereas no superiority was observed in the other age categories, as in the original study (Table 3).
Table 3.
Percentage of normalized and normalized or responder patients after 12 weeks of treatment with olmesartan medoxomil 10–40 mg (n=712) or ramipril 2.5–10 mg (n=714), according to sex, age and 10-year cardiovascular risk category (low-moderate: <5% and high-very high: ≥5%)
| Normalized patients (<140/90 mmHg)
|
Normalized patients (<150/90 mmHg)
|
|||||
|---|---|---|---|---|---|---|
| Olmesartan 10–40 mg | Ramipril 2.5–10 mg | P-value | Olmesartan 10–40 mg | Ramipril 2.5–10 mg | P-value | |
| Sex | ||||||
| Male (n=717) | 199 (56.1) | 174 (48.1) | 0.032 | 253 (71.3) | 222 (61.3) | 0.005 |
| Female (n=709) | 194 (54.3) | 173 (49.1) | 0.166 | 246 (68.9) | 229 (65.1) | 0.276 |
| Age | ||||||
| 65–69 years (n=597) | 183 (61.4) | 147 (49.2) | 0.003 | 221 (74.2) | 184 (61.5) | 0.001 |
| 70–79 years (n=706) | 174 (49.6) | 172 (48.5) | 0.766 | 232 (66.1) | 226 (63.7) | 0.498 |
| >80 years (n=123) | 36 (57.1) | 28 (46.7) | 0.245 | 46 (73.0) | 41 (68.3) | 0.568 |
| 10-year Cardiovascular risk | ||||||
| Low-moderate (n=159) | 54 (67.5) | 46 (60.8) | 0.376 | 64 (80.0) | 57 (72.2) | 0.246 |
| High-very high (n=1,267) | 339 (53.6) | 299 (47.1) | 0.020 | 435 (68.8) | 394 (62.0) | 0.011 |
When patients were classified according to the 10-year absolute risk of fatal cardiovascular disease, according to the SCORE (Systematic COronary Risk Evaluation) algorithm,29 a larger proportion of patients in the low-moderate (≤5%) risk category responded to either treatment, with a statistically significant superiority of olmesartan over ramipril in the high- or very high-risk category (>5%) (Table 3). Although application of the SCORE algorithm to elderly population may be not completely appropriate, given the fact that this population is at high risk for itself, such results may support the use of ARBs as an alternative to ACE inhibitors for the achievement of adequate blood pressure control with less intensive treatment in older hypertensive patients at higher risk of cardiovascular events. This is particularly relevant because there is consistent evidence that olmesartan may reduce cardiovascular risk by simultaneously normalizing blood pressure and reversing the proatherogenic effects of angiotensin II, an effect which is particularly desirable in the elderly.30,31
Blood pressure response according to type of hypertension
Olmesartan medoxomil also proved to be effective in controlling blood pressure, regardless of the type of hypertension. Most of the studied patients (75.5%) were affected by diastolic ± systolic hypertension. In these patients, the chance of attaining blood pressure normalization was significantly larger under olmesartan than under ramipril for both the thresholds considered (<140/90 mmHg: 54.4% vs 46.7%, P=0.012; <150/90 mmHg: 68.4% vs 60.3%, P=0.006), confirming the results of the original analysis. In three previous studies, which enrolled a total of 1,235 hypertensive patients aged ≥65 years, the proportion of patients with a blood pressure <140/90 mmHg at the end of the study who were given olmesartan at doses 5–40 mg was 56.0%, thus very close to our finding.21,22,24
In our population, isolated systolic hypertension was less common than diastolic ± systolic hypertension (24.5%), but the chance of achieving blood pressure normalization with treatment was similar for the two hypertension subtypes. As in the original publication, no statistically significant differences were observed between the treatment groups in terms of blood pressure normalization either considering the 140/90 mmHg (olmesartan 58.0% vs ramipril 54.0%, P=0.451) or the 150/90 mmHg cutoff (75.9% vs 71.1%, P=0.311). Such results indicate that also in a relatively small subgroup of high-risk patients such as those with isolated systolic hypertension, olmesartan is capable of adequately controlling blood pressure.
Blood pressure response in patients with metabolic disorders
The metabolic syndrome is characterized by the association of different cardiovascular risk factors such as abdominal obesity, atherogenic dyslipidemia, insulin resistance or glucose intolerance, and blood pressure elevation.32 Patients suffering from this condition have a higher risk of cardiovascular fatal and nonfatal events than healthy people, particularly in the presence of diabetes mellitus.33,34 Treatment with a drug acting on the renin–angiotensin system (RAS) has been shown to be particularly effective for controlling blood pressure and reducing major cardiovascular events, in the presence of metabolic abnormalities, such as metabolic syndrome or diabetes.35–37
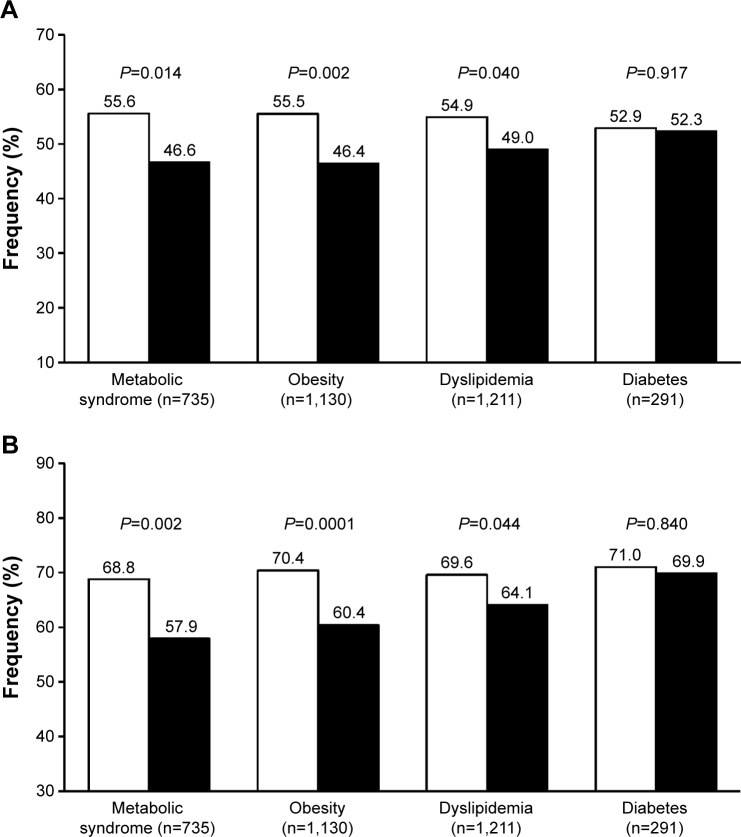
In the pooled analysis of our studies, we analyzed the antihypertensive effect of olmesartan and ramipril in patients with metabolic syndromes, defined according to the International Diabetes Federation criteria and observed a significantly higher proportion of normalized patients under olmesartan, irrespective of the blood pressure target considered (Figure 2).38,39 In addition to this finding, we observed a superior efficacy of olmesartan vs ramipril in patients with central or peripheral obesity (waist circumference >102 cm in men and >88 cm in women, or body mass index ≥30 kg/m2), as well as in those with dyslipidemia (total cholesterol >190 mg/dL, or low-density lipoprotein cholesterol >115 mg/dL, or high-density lipoprotein cholesterol <40 mg/dL in men and <46 mg/dL in women, or triglycerides >150 mg/dL, or under specific treatment with a lipid lowering drug).1 The superiority of olmesartan for controlling blood pressure in patients with metabolic disorders may be explained by an overexpression of vascular angiotensin II type 1 (AT1)-receptors and overactivation of the RAS, which are the targets for the drug, and by a more specific inhibiting action of the ARB on the systemic and adipose tissue RAS.40,41
Figure 2.
Percentage of normalized patients.
Notes: (A) <140/90 mmHg. (B) <150/90 mmHg after 12 weeks of treatment with olmesartan 10–40 mg (white bars) or ramipril 2.5–10 mg (black bars) according to the presence of specific metabolic abnormalities. P-values for between-treatment difference are also reported.
Despite a high rate of blood pressure normalization in the subgroup of diabetics, no statistically significant differences were observed between the two treatment groups (Figure 2). The fact that olmesartan is as effective as ramipril in diabetic patients is in line with the results of a recent meta-analysis of 23 randomized controlled studies comparing ARBs and ACE inhibitors: no significant difference was found in the proportion of patients who achieved successful blood pressure control on a single antihypertensive agent of the ARB or ACE-inhibitor class.36,42
Blood pressure goal attainment according to renal function status
An impaired renal function is a frequent finding in hypertensive patients and constitutes a very potent predictor of future cardiovascular events.43 Current evidence supports the use of ARBs or ACE inhibitors as the therapy of choice for hypertension in patients with chronic kidney disease, due to specific renoprotective effects of these drugs, which are beyond their antihypertensive effect.44–46
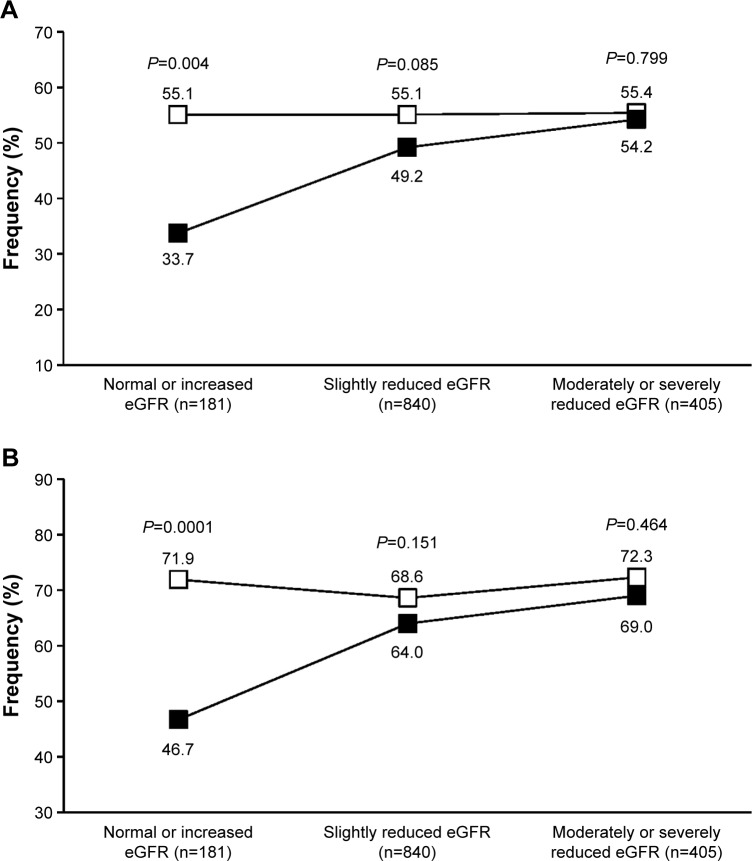
A post hoc analysis of the two pooled studies has previously shown that olmesartan medoxomil is efficacious in controlling blood pressure in the elderly patients of the study, independently of their renal function status, assessed by estimated glomerular filtration rate (eGFR), using the Cockroft–Gault equation.47 The efficacy of olmesartan proved to be generally superior to that of ramipril, in terms of blood pressure normalization, particularly in patients with normal or increased eGFR (≥90 mL/min/1.73 m2) and in those with slightly reduced eGFR (60–90 mL/min/1.73 m2). Data reanalysis based on the currently recommended blood pressure targets (<140/90 or <150/90 mmHg) confirmed a statistically significant superiority of olmesartan vs ramipril in the normal or increased eGFR subgroup, with a comparable efficacy of the two drugs in the other two categories (Figure 3).
Figure 3.
Percentage of normalized patients.
Notes: (A) <140/90 mmHg. (B) <150/90 mmHg after 12 weeks of treatment with olmesartan 10–40 mg (open square) or ramipril 2.5–10 mg (full square) according to estimated glomerular filtration rate (eGFR). P-values for between-treatment difference are also reported.
Thus, it seems that RAS inhibition is effective in controlling blood pressure in older hypertensives, regardless of the renal status of the patients, even when less tight blood pressure control is required. Also in this case, as in the case of a tighter blood pressure control, olmesartan may help achieving a better blood pressure control in the subgroup of patients with a preserved renal function or at an early stage of the kidney disease.
Patients at target according to number and types of previous antihypertensive treatment
Patients with hypertension at highest risk of cardiovascular complications, such as older persons, have a greater chance of being resistant to particular classes of drugs or may not adequately respond to monotherapy.1 In order to gain further insight into the mechanisms behind the efficacy of olmesartan and ramipril in our pooled datasets of aged hypertensives, we evaluated response rate in subgroups of patients according to the number of drugs and type or RAS antagonist (ARB or ACE inhibitor) used at the time of entering the studies.
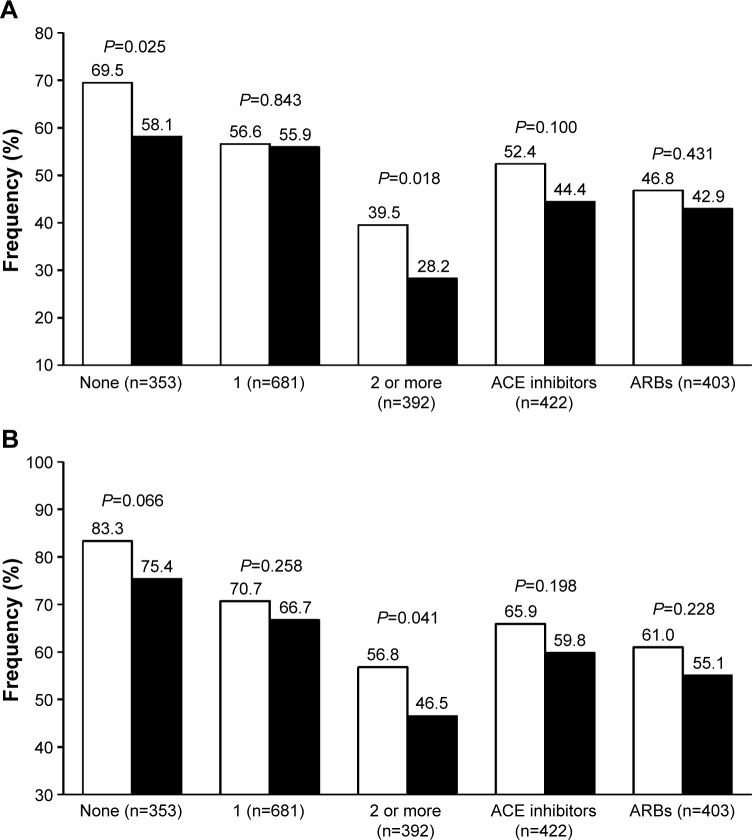
In never-treated patients (24.8% of the whole study population), olmesartan ability to achieve the blood pressure target, whether it was based on the 140/90 or the 150/90 mmHg threshold, was better than that of ramipril (with statistically significant differences for the <140/90 mmHg target), suggesting that the subgroup of patients of our population previously receiving no antihypertensive drug was more prone to respond to the ARB than to the ACE inhibitor (Figure 4). Additionally, olmesartan appeared to be significantly superior to ramipril in the subgroup of patients that were previously treated with a combination therapy, suggesting that an olmesartan-based monotherapy may be a possible choice for patients that are less susceptible to an adequate blood pressure response to previous multiple antihypertensive treatment.
Figure 4.
Percentage of normalized patients.
Notes: (A) <140/90 mmHg. (B) <150/90 mmHg after 12 weeks of treatment with olmesartan 10–40 mg (white bars) or ramipril 2.5–10 mg (black bars) according to the number and type of previous antihypertensive drugs. P-values for between-treatment difference are also reported.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker.
Blood pressure response to a RAS inhibitor may be reduced or event blunted in patients previously not responding to a drug from the same class. In our population, the rate of blood pressure control was similar with olmesartan and ramipril, independently of the kind of previous antihypertensive treatment, whether it was based on an ACE inhibitor or an ARB (Figure 4).
Drug safety according to blood pressure targets
As previously shown, 44 patients (3.1%) reported 67 adverse events attributed to study treatment: 21 patients received olmesartan (33 adverse events) and 23 ramipril (34 adverse events) (P=0.767 between treatments).20 The rate of patients with drug-related adverse events was the same in the group normalized at a target of <140/90 mmHg (23 of 740 patients, 3.1%) and of <150/90 mmHg (20 of 950 patients, 3.1%, P=0.948). In these two target groups, the rate of patients reporting adverse events attributed to study drug never differed between treatments (<140/90 mmHg: olmesartan 2.0% vs ramipril 4.3%, P=0.074; <150/90 mmHg: olmesartan 2.2% vs ramipril 4.0%, P=0.110). Thus, in our study, the risk of reporting an adverse drug reaction was not related to the blood pressure level achieved during treatment.
Discussion and overall conclusion
All current hypertension guidelines have raised the target blood pressure goals in older hypertensive patients, while eliminating the tighter control recommendations in patients with diabetes and renal disease.1,2,15–17 Notwithstanding such recommendations, strong debate exists among scientists on whether, in older or high-risk populations, blood pressure cutoffs should remain more conservative, namely kept higher, or rather a more aggressive approach should be followed, as it was in the past.7,48–50 The disagreement between studies and the difficulty in weighting the available evidence in the absence of definite data are reflected in the guidelines: some of them recommend a blood pressure target of <150/90 mmHg for persons older than 60 years,2 whereas others recommend a goal of <140/90 mmHg, in persons aged 80 years or younger and <150/90 mmHg only in frail persons aged 80 years or more.1,15–17
We attempted to provide a better insight into this controversy, by reanalyzing the results of two large randomized studies at the light of the new blood pressure targets recommended by present hypertension guidelines. As in the original study, the efficacy of olmesartan was not negatively affected by age, sex, hypertension type, diabetes status, or other concomitant clinical conditions or cardiovascular risk factors. In most cases, olmesartan provided better blood pressure control than ramipril. Olmesartan was significantly more effective than ramipril in male patients, in younger patients (aged 65–59 years), in patients with a normal eGFR, and in those with diastolic ± systolic hypertension. Olmesartan showed better results than ramipril also in specific categories of high-risk patients, such as those with metabolic syndrome, obesity, dyslipidemia, and in general, in patients with a high or very high cardiovascular risk. Interestingly, patients previously untreated or treated with two or more antihypertensive drugs showed a significantly larger response with olmesartan than with ramipril.
Having said so, we must acknowledge some limitations of our post hoc analysis. First, although we pooled together data from two adequately powered, randomized, double-blind, parallel group studies with an identical design, the fact of raising the target of adequate blood pressure control and applying less stringent criteria increased per se the rate of responders in both study treatments. This is because in the original study drug, uptitration and treatment tailoring were based on targets lower than those used in this reanalysis (<140/90 mmHg in nondiabetic patients and <130/80 mmHg in diabetic patients). Second, we should acknowledge as a potential source of difference among treatment groups the fact that the antihypertensive effect of the maximum dose of ramipril employed in our study (10 mg) might not correspond in terms of efficacy to that of olmesartan (40 mg). The use of higher doses of ramipril could have allowed achieving better responses, but in the original study, comparisons were limited to the maximum doses currently recommended for the two drugs. Third, one meta-analysis documented that while the blood pressure dependent effects of ACE inhibitors and ARBs on the risk of stroke, coronary heart disease, and heart failure are similar, ACE inhibitors but not ARBs have blood pressure independent effect on the risk of major coronary disease events.51 Very recently, a systematic review showed that ACE inhibitors are more effective in preventing coronary heart disease and less in preventing stroke, whereas ARBs are inferior in preventing coronary heart disease.52 However, the blood pressure lowering effect of the two classes of drugs in hypertensive patients seems to be quite superimposable.36,42 Thus, in spite of some superiority in terms of antihypertensive effect of a given ARB over a given ACE inhibitor, as in our study, we must admit that there is no evidence in medical literature to recommend ARB over ACE inhibitor therapy. Differences found among active principles may instead suggest specific choices in specific conditions, or preferable combinations of drugs and doses. Fourth, in our study we showed a better blood pressure response with olmesartan, but we could not demonstrate any superiority in terms of prevention of cardiovascular outcomes because these endpoints were not assessed in the study. Thus, we cannot conclude that olmesartan is superior to ramipril in terms of cardiovascular protection in the elderly hypertensive patient.
Notwithstanding these limitations, our results may be relevant for the clinical practice, providing some indication on the possible different response of elderly hypertensive patients to two different RAS inhibitors, when patients are targeted according to the blood pressure levels recommended by recent hypertension guidelines.
Acknowledgments
This work was financially supported by Menarini International Operations Luxembourg through an unconditional and unrestricted grant. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
SO, EM, JMM, and MV have occasionally received grants for lectures by the manufacturers of olmesartan or ramipril. MV has been consultant in scientific advisory board of Daiichi Sankyo, manufacturer of olmesartan.
References
- 1.ESH/ESC Task Force for the Management of Arterial Hypertension 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Zanchetti A, Grassi G, Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens. 2009;27(5):923–934. doi: 10.1097/HJH.0b013e32832aa6b5. [DOI] [PubMed] [Google Scholar]
- 4.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 5.JATOS Study Group Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 6.Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang X, Liu L, Zanchetti A. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J. 2011;32(12):1500–1508. doi: 10.1093/eurheartj/ehr039. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B, Byington RP, Furberg CD, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102(13):1503–1510. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 10.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364(9437):849–857. doi: 10.1016/S0140-6736(04)16980-8. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277. doi: 10.1002/14651858.CD008277.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database Syst Rev. 2009;3:CD004349. doi: 10.1002/14651858.CD004349.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154(8):541–548. doi: 10.7326/0003-4819-154-8-201104190-00335. [DOI] [PubMed] [Google Scholar]
- 15.National Clinical Guideline Centre (UK) Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London: Royal College of Physicians (UK); 2011. [Accessed July 31, 2015]. Internet. Available from: http://www.ncbi.nlm.nih.gov/books/NBK83274/ [PubMed] [Google Scholar]
- 16.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens. 2011;5(4):259–352. doi: 10.1016/j.jash.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014;16(1):14–26. doi: 10.1111/jch.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A, ESPORT Study Group Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28(11):2342–2350. doi: 10.1097/HJH.0b013e32833e116b. [DOI] [PubMed] [Google Scholar]
- 19.Mallion JM, Omboni S, Barton J, et al. Antihypertensive efficacy and safety of olmesartan and ramipril in elderly patients with mild to moderate systolic and diastolic essential hypertension. Blood Press Suppl. 2011;1:3–11. doi: 10.3109/08037051.2010.532332. [DOI] [PubMed] [Google Scholar]
- 20.Omboni S, Malacco E, Mallion JM, Fabrizzi P, Volpe M. Olmesartan vs ramipril in elderly hypertensive patients: review of data from two published randomized, double-blind studies. High Blood Press Cardiovasc Prev. 2014;21(1):1–19. doi: 10.1007/s40292-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 21.Kereiakes DJ, Neutel J, Stoakes KA, et al. The effects of an olmesartan medoxomil-based treatment algorithm on 24-hour blood pressure levels in elderly patients aged 65 and older. J Clin Hypertens (Greenwich) 2009;11(8):411–421. doi: 10.1111/j.1751-7176.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito I, Kushiro T, Hirata K, et al. The use of olmesartan medoxomil as monotherapy or in combination with other antihypertensive agents in elderly hypertensive patients in Japan. J Clin Hypertens (Greenwich) 2008;10(4):272–279. doi: 10.1111/j.1751-7176.2008.07460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heagerty AM, Mallion JM. Olmesartan medoxomil in elderly patients with essential or isolated systolic hypertension: efficacy and safety data from clinical trials. Drugs Aging. 2009;26(1):61–76. doi: 10.2165/0002512-200926010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa H, Kim-Mitsuyama S, Matsui K, et al. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125(10):981–990. doi: 10.1016/j.amjmed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Mallion JM, Heagerty A, Laeis P. Systolic blood pressure reduction with olmesartan medoxomil versus nitrendipine in elderly patients with isolated systolic hypertension. J Hypertens. 2007;25(10):2168–2177. doi: 10.1097/HJH.0b013e328287ad0d. [DOI] [PubMed] [Google Scholar]
- 26.Ogihara T, Saruta T, Rakugi H, et al. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. J Hypertens. 2014;32(10):2054–2063. doi: 10.1097/HJH.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui K, Kim-Mitsuyama S, Ogawa H, et al. Sex differences in response to angiotensin II receptor blocker-based therapy in elderly, high-risk, hypertensive Japanese patients: a subanalysis of the OSCAR study. Hypertens Res. 2014;37(6):526–532. doi: 10.1038/hr.2014.23. [DOI] [PubMed] [Google Scholar]
- 28.Seeland U, Regitz-Zagrosek V. Sex and gender differences in cardiovascular drug therapy. Handb Exp Pharmacol. 2012;(214):211–236. doi: 10.1007/978-3-642-30726-3_11. [DOI] [PubMed] [Google Scholar]
- 29.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 30.Rosei EA. Reduction of cardiovascular risk through angiotensin II type 1 receptor antagonism: focus on olmesartan medoxomil. High Blood Press Cardiovasc Prev. 2008;15(4):231–243. doi: 10.2165/0151642-200815040-00003. [DOI] [PubMed] [Google Scholar]
- 31.Volpe M, Tocci G. Olmesartan in the treatment of hypertension in elderly patients: a review of the primary evidence. Drugs Aging. 2013;30(12):987–998. doi: 10.1007/s40266-013-0130-8. [DOI] [PubMed] [Google Scholar]
- 32.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 34.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25(6):375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 35.Nakao YM, Teramukai S, Tanaka S, et al. Effects of renin-angiotensin system blockades on cardiovascular outcomes in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;96(1):68–75. doi: 10.1016/j.diabres.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Powers B, Greene L, Balfe LM. Updates on the treatment of essential hypertension: a summary of AHRQ’s comparative effectiveness review of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and direct renin inhibitors. J Manag Care Pharm. 2011;17(8 Suppl):S1–S14. doi: 10.18553/jmcp.2011.17.s8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 39.Omboni S, Malacco E, Mallion JM, Volpe M. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly mild to moderate essential hypertensive patients with or without metabolic syndrome: a pooled post hoc analysis of two comparative trials. Drugs Aging. 2012;29(12):981–992. doi: 10.1007/s40266-012-0030-3. [DOI] [PubMed] [Google Scholar]
- 40.Sharma AM, Engeli S. The role of renin-angiotensin system blockade in the management of hypertension associated with the cardiometabolic syndrome. J Cardiometab Syndr. 2006;1(1):29–35. doi: 10.1111/j.0197-3118.2006.05422.x. [DOI] [PubMed] [Google Scholar]
- 41.Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89(2A):3A–10A. doi: 10.1016/s0002-9149(01)02321-9. [DOI] [PubMed] [Google Scholar]
- 42.Powers BJ, Coeytaux RR, Dolor RJ, et al. Updated report on comparative effectiveness of ACE inhibitors, ARBs, and direct renin inhibitors for patients with essential hypertension: much more data, little new information. J Gen Intern Med. 2012;27(6):716–729. doi: 10.1007/s11606-011-1938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380(9854):1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366(9502):2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 45.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 46.Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia. 2012;55(3):566–578. doi: 10.1007/s00125-011-2398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malacco E, Omboni S, Mallion JM, Volpe M. Antihypertensive efficacy of olmesartan medoxomil and ramipril in elderly patients with mild to moderate hypertension grouped according to renal function status: a retrospective analysis. High Blood Press Cardiovasc Prev. 2012;19(4):213–222. doi: 10.1007/BF03297633. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23(12):2157–2172. doi: 10.1097/01.hjh.0000194120.42722.ac. [DOI] [PubMed] [Google Scholar]
- 49.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mmHg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160(7):499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Goldberg SI, Shubina M, Turchin A. Optimal systolic blood pressure target, time to intensification, and time to follow-up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. doi: 10.1136/bmj.h158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blood Pressure Lowering Treatment Trialists’ Collaboration. Turnbull F, Neal B, et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 52.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs – overview and meta-analyses. J Hypertens. 2015;33:1321–1341. doi: 10.1097/HJH.0000000000000614. [DOI] [PubMed] [Google Scholar]