Abstract
Background
Apolipoprotein is genetically associated with the risk of Alzheimer’s disease (AD). The APOA1, APOC3, and APOA4 genes are closely linked and located on human chromosome 11. Therefore, this gene cluster may be related to the risk of AD.
Patients and methods
A total of 147 AD patients and 160 healthy controls were randomly recruited from June 2013 to August 2014. APOA1, APOC3, and APOA4 levels were measured using real-time quantitative reverse-transcriptase polymerase chain reaction and enzyme-linked immunosorbent assay.
Results
APOA1, APOC3 and APOA4 levels were significantly lower in AD patients than controls (P<0.01). APOA1, APOC3, and APOA4 levels were negatively related with the severities of AD determined by Clinical Dementia Rating scores (P<0.01). APOA1, APOC3, and APOA4 levels showed a negative relation with Montgomery–Åsberg Depression Rating Scale scores and a positive relation with RAND 36-item health-survey scores (P<0.01). There was a decreased trend for levels of APOA1, APOC3, and APOA4 in AD patients.
Conclusion
Low levels of APOA1, APOC3, and APOA4 are associated with risk of AD. APOA1, APOC3, and APOA4 should be developed as combined drugs for the therapy of AD.
Keywords: Alzheimer’s disease, APOA1–APOC3–APOA4 gene cluster, enzyme-linked immunosorbent assay, Montgomery–Åsberg Depression Rating Scale, RAND 36-item health survey, real-time quantitative reverse-transcriptase PCR
Introduction
Alzheimer’s disease (AD) is a leading cause of dementia among the elderly and affects over 24 million people worldwide, and this number could increase twofold within two decades.1 The number of AD patients is increasing with the aging of the world population. AD is mainly characterized by amyloid plaques that are often surrounded by neurofibrillary tangles in the brain and poor memory.2,3 However, the etiology of AD is complex, and more molecular mechanisms still need to be explored.
To define ideal therapy strategies, early diagnosis and early intervention is crucial for timely therapy of AD. Although the neuropathology of AD can be found by autopsy, confirming prognostic characteristics of AD patients is still far from straightforward.4 Cerebrospinal fluid (CSF) biomarkers, such as total tau, phosphorylated tau, and Aβ42, have high accuracy during the early diagnosis of AD patients.5 Current AD guidelines give these criteria for the diagnosis of AD patients according to increased levels of phosphorylated tau, total tau, and Aβ42 in CSF.6 However, a spinal tap is used to obtain CSF.7 On the other hand, taking biopsies is often invasive and extremely difficult to perform. Not all AD patients want to accept the diagnosis; therefore, new methods are much needed.
Potential biomarkers for AD patients have been widely reported. Theoretically, DNA damage should be associated with the development of AD. Biomarkers for DNA damage have been reported in AD patients.8 Many serum biomarkers for AD patients have been also reported, such as IGF-1. IGF-1 enters mammalian brains and promotes amyloid peptide clearing, which prevents amyloid peptide accumulation in the brain. Decreased IGF-1 levels have been observed in AD patients.9 Also, ADNP levels are reduced in the development of AD.10 ADNP maintains cell survival by regulating the levels of p53, which protects cerebral cortical neurons by inhibiting the aggregation of Aβ42 in the brain, and thus ADNP plays an important role in inhibiting the development of AD.11 Recent advances in AD biomarkers have inspired novel research criteria that reconceptualize the diagnosis of AD involving two aspects: cognitive variation and structural evidence of AD pathogenesis.12
APOE is a kind of apolipoprotein in the chylomicron and an intermediate-density lipoprotein, which is necessary for the normal catabolism of lipoprotein with rich triglyceride (TG). In the central nervous system, APOE is produced via astrocytes, and can transport cholesterol to neurons.13 The APOE allele has been reported to be related to the risk of AD.14,15 APOA4, a component of lipoprotein particles similar to APOE, has been suggested to play an important role in brain metabolism. APOA4 deficiency can increase the levels of Aβ deposition in the brain and result in cognitive damage in an animal model of AD.16 APOA4 polymorphism has been reported as a risk factor for an unfavorable lipid serum profile.17 On the other hand, a growing body of evidence suggests that obesity, hypertriglyceridemia, and reduced high-density lipoprotein cholesterol (HDL-c) is important in the development of cognitive impairment and AD. APOA1 was also observed at lower levels in AD patients via proteomic two-dimensional gel electrophoresis in 2006.18 A clinical study has shown low HDL-c and APOA1 levels in patients with AD.19 APOC3 polymorphism has been reported to be associated with AD in a Chinese population.20 Furthermore, the APOA1, APOC3, and APOA4 genes are closely linked on the long arm of human chromosome 11.21 The APOA1 and APOA4 genes are located on the same strand, while the APOA1 and APOC3 genes are convergently transcribed. Increased APOC3 levels induce the progression of hypertriglyceridemia.22 On the other hand, the gene polymorphism has been widely reported to be associated with AD related to cerebral lipoprotein homoeostasis, including APOA1, APOC3, and APOA4.23 Therefore, all these genes may have synergistic functions, and their low-level expression may be associated with the risk of AD.
Patients and methods
Participants
All the protocols were approved by the institutional ethical committee of China Medical University and conducted in accordance with the Declaration of Helsinki.24 All subjects were Han Chinese from Shenyang. Informed consent was obtained from each subject directly or from his or her guardian. From June 2013 to August 2014, a total of 147 patients were recruited at the Fourth Affiliated Hospital of China Medical University (Shenyang). All AD patients were diagnosed with National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria according to a previous report.25 All familial cases of AD were excluded in this study. Patients were excluded if they had a history of suicidal behavior, severe substance abuse, difficulty with verbal conversation, and brain injuries or other brain disorders.
Study design
After all AD patients had been screened, 147 patients were eligible for the experiment. Meanwhile, 160 healthy subjects were selected as a control group. The two groups were similar in terms of sex ratio, age, body mass index (BMI), educational levels, alcohol consumption, and cigarette smoking.
Neuropsychological tests
All cases were diagnosed by licensed neurological physicians according to the Diagnostic and Statistical Manual of Mental Disorders (DSM): neurological examination, blood tests, and neuroimaging data (computed tomography or magnetic resonance imaging). The Clinical Dementia Rating (CDR) scale is frequently used to detect memory impairment and quantify the severity of dementia.26 The aim of the clinical diagnosis for some dementia (as a frontal-temporal dementia) is not memory impairment in the acute phase.27 The CDR is used to assess three domains of cognition (memory, orientation, problem solving) and three domains of function (community affairs, home and hobbies, personal care).28 The Mini Mental State Examination (MMSE) is used to diagnose dementia, assess its progression and severity, and examine memory problems.
All participants were evaluated with the Kimberley Indigenous Cognitive Assessment of Depression based on the World Health Organization International Classification of Diseases and the DSM-IV-TR of the American Psychiatric Association.29 There are eleven items in the Kimberley Indigenous Cognitive Assessment of Depression, each of which is assigned from “never” to “sometimes”, “a lot”, and “all the time” based on a frequency scale.
There are numerous factors causing AD. In order to reduce the interference of these factors, age, sex, years of education, and career were compared between a control group and an experimental group.30,31 Furthermore, to exclude the effects of compound factors, all subjects were selected from Shenyang and lived in families with similar educational and economic background. Depression is a frequent condition in AD patients, so depression was also considered here. The severity of depression was examined based on the Montgomery–Åsberg Depression Rating Scale (MADRS).32 There are ten items in the MADRS, scored from 0 to 60. Severity of depression is graded as severe, moderate, and mild based on cutoff scores.33 Psychopathological dysfunction in emotion is often described using anorexia, which is also related with depression.
Anxiety is often associated with AD and measured based on the standard of the Hospital Anxiety and Depression Scale.34–36 Hospital Anxiety and Depression Scale anxiety scores are presented from 0 to 21, and higher scores stand for severe anxiety. Alexithymia was investigated based on the Toronto Alexithymia Scale.37 General functioning was tested by the Global Assessment of Functioning scale.38 Life quality was evaluated using the RAND 36-item health-survey (RAND-36), which assesses well-being and functioning in eight dimensions.39
mRNA extraction
Venous blood (5 mL) was taken from every participant after obtaining signed consent forms. Venous blood was centrifuged at 3,000 g for 10 minutes and the serum collected. Total RNA was isolated from serum by an RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands).
Real-time qRT-PCR
Messenger RNA (mRNA; 1 μL) was reverse-transcribed to complementary DNA by reverse transcription using AMV Reverse Transcriptase (Promega Corporation, Fitchburg, WI, USA). The gene fragments were further amplified using quantitative real-time polymerase chain reaction (qRT-PCR) with primers for APOA1 (forward primer 5′-gtgctcaaagacagcggcag-3′, reverse primer 5′-ctcatctcctgcctcaggcc-3′, 200 bp), APOC3 (forward primer 5′-tcagaggccgaggatgcctc-3′, reverse primer 5′-cttgtccttaacggtgctcc-3′, 180 bp), APOA4 (forward primer 5′-ctcttccaggacaaacttgg-3′, reverse primer 5′-tccagctccttcccaatctc-3′, 140 bp) and GAPDH (forward primer 5′-gatccctccaaaatcaagtg-3′, reverse primer 5′-atacttctcatggttcacac-3′, 180 bp). PCR was conducted with a LightCycler FastStart DNA Master SYBR Green I (Hoffman-La Roche Ltd., Basel, Switzerland) on an ABI 7500 real-time PCR instrument (Thermo Fisher Scientific, Waltham, MA, USA). The PCR was performed as follows: 45 cycles (denaturation, 5 seconds at 95°C; annealing, 5 seconds at 60°C; extension, 20 seconds at 72°C).
ELISA analysis for protein levels of APOA1, APOC3, and APOA4
The protein levels of APOA1, APOC3, and APOA4 were measured in serum using a Human APOA1 ELISA (enzyme-linked immunosorbent assay) Kit (ab108804; Abcam, Cambridge, MA, USA), a Human APOC3 ELISA Kit (ab154131; Abcam), and a Human APOA4 ELISA Kit (P06727; Beacon Analytical Systems Inc, Saco, ME, USA). To determine serum levels of APOA1, APOC3, and APOA4, 5 mL blood was drawn into a vacuum tube. The blood was centrifuged at 3,000 g for 10 minutes, and the serum was stored at −80°C. Serum APOA1, APOC3, and APOA4 were measured using ELISA kits.
Biochemical enzyme analysis
APOA1 may reduce the oxidative stress in mammals, considering APOA1 can increase concentrations of HDL, which has potential antioxidative activities.40 SOD, AST, ALT and GSH have been regarded as biomarkers for oxidative stress.41–46 Therefore, the levels of all enzymes were examined using the blood samples of participants. AST and ALT levels were measured with a biochemical analysis instrument (Beckman Coulter Inc, Pasadena, CA, USA). SOD activity was analyzed using a previously reported method.47 GSH levels were determined using a fluorometric method.48
Analysis of lipid profiles
APOA1, APOC3, and APOA4 are key molecules for modulating lipid metabolism, which is associated with changes in TG, total cholesterol (TC), HDL-c, and low-density lipoprotein cholesterol (LDL-c) concentrations. Therefore, TG, TC, HDL-c and LDL-c levels were also measured in blood samples using a biochemical analyzer. Malondialdehyde, a biomarker of oxidative damage, was analyzed according to a previously reported method.49
Statistical analyses
All statistical analyses were performed with SPSS 20.0 (IBM Corporation, Armonk, NY, USA). AD patients and controls were compared using t-tests. One-way analysis of variance was performed to explore the association of APOA1, APOC3, and APOA4 levels and AD patients. There was a statistically significant difference if P<0.05. Spearman’s rank correlation coefficient was used to investigate the relationship between the degrees of depression (MADRS or RAND-36) and the protein levels of APOA1, APOC3, and APOA4. The sample size of the association was measured using Quanto 1.2. The level of significance was set at P=0.05, and the confidence interval (CI) set at 95%.
Results
Baseline characteristics of participants
The a priori sample size for the AD study was estimated with a study power of 90% to have a decrease in serum APOA1, APOC3, and APOA4 levels in AD patients compared with the controls, assuming an α-level of 0.05. The present study was initiated because of previous concerns over AD patients enrolled in our hospital. Finally, the number of AD patients was more than our expectations, and a priori size was determined and attained in the study.
Table 1 shows baseline characteristics of all participants. A total of 147 AD patients received medical and neurological test (male/female 63/84, mean age at study 68.9 years, range 57.1–80.7 years) (Table 1). All 160 controls were diagnosed as cognitively intact after receiving medical and neurological tests (male/female 66/94, mean age at study 69.6 years, range 57.1–82.1 years).
Table 1.
Baseline characteristics of AD patients and healthy controls
| AD patients | Control (n=160) | t-value/χ2 statistic | P-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 68.9 (11.8) | 69.6 (12.5) | 0.145 | 0.892a |
| Male, n (%) | 63 (42.9) | 66 (41.3) | 0.081 | 0.776b |
| Smoker/nonsmoker | 72/75 | 78/82 | 0.158 | 0.691b |
| Drinker/nondrinker | 73/74 | 79/81 | 0.003 | 0.960b |
| Education level (>9 years), n (%) | 32 (21.8) | 34 (21.3) | 0.012 | 0.912b |
| Spouse, n (%) | 132 (89.8) | 136 (85.0) | 1.589 | 0.207b |
| Career (working overtime), n (%) | 93 (63.3) | 98 (61.3) | 0.132 | 0.716b |
| Anxiety (cutoff score 8 in Hospital Anxiety and Depression Scale), n (%) | 44 (29.9) | 5 (3.1) | 41.044 | 0.000b |
| Current medication, n (%) | ||||
| Antidepressant medication | 51 (34.7) | 0 | 152.031 | 0.000b |
| Selective serotonin-reuptake inhibitors | 63 (42.9) | 0 | 86.276 | 0.322b |
| Serotonin–noradrenaline-reuptake inhibitors | 21 (14.3) | 0 | 24.535 | 0.000b |
| Psychiatric test scores, mean (SD) | ||||
| Montgomery–Åsberg Depression Rating Scale | 23.9 (7.8) | 14.2 (2.4) | 2.141 | 0.004a |
| Anxiety score (Hospital Anxiety and Depression Scale) | 12.6 (4.5) | 4.3 (1.1) | 2.649 | 0.002a |
| Global Assessment of Functioning score | 59.4 (6.8) | 78.1 (8.3) | 3.517 | 0.001a |
| Toronto Alexithymia Scale – 20 | 54.6 (13.8) | 23.2 (6.3) | 4.388 | 0.001a |
| RAND-36 score | 52.3 (16.1) | 83.6 (10.9) | 3.046 | 0.002a |
| MMSE scores | 19.8±4.9 | 31.8±4.3 | 3.061 | 0.002a |
| CDR scale | ||||
| CDR 0, n | 0 | 160 | 307.021 | 0.000b |
| CDR 0.5, n | 84 | 0 | 125.868 | 0.000b |
| CDR 1, n | 36 | 0 | 44.388 | 0.000b |
| CDR 2+, n | 27 | 0 | 32.221 | 0.000b |
Notes:
Student’s t-test;
standard χ2 statistic.
Abbreviations: AD, Alzheimer’s disease; SD, standard deviation; MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating.
Controls matched AD patients well with regard to sex and age (P>0.05). According to previous reports, BMI is related to the development of AD.50,51 To avoid the interference of BMI, the controls were carefully chosen to make sure that there was no significant statistically difference between AD patients and controls (P>0.05) (Table 1). Therefore, BMI was not a risk factor for AD patients. Diabetes affects many elderly people worldwide, with a deep decrease in life quality. Meanwhile, blood pressure and heart disorders are also risk factors of AD.52,53 On the other hand, smoking is related to a decrease in gray-matter density in brains, which contributes to the onset of AD.54 Drinking alcohol impairs brain function and causes dementia and geriatric cognitive disorders.55,56 Individuals with long-term education will have better cognitive function than those with short-term education.30,57 During the recruiting period, all the parameters were also carefully measured to avoid heterogeneous effects on final results (Table 1).
Neuropsychological tests showed that all AD subjects had MMSE scores less than 24.7, while all controls had MMSE scores more than 27. AD patients had an overall CDR score of 0.5 or more than 0.5, while overall CDR scores of all controls were 0 (Table 1). These results suggested that AD patients had clinical cognitive diseases, while controls were free of cognitive disorders.
mRNAlevels of serum APOA1, APOC3, and APOA4
The qRT-PCR showed that mRNA serum APOA1, APOC3, and APOA4 levels were lower in AD patients than controls. mRNA serum APOA1, APOC3 and APOA4 levels decreased when CDR scores increased from 0.5 to 2+ in AD patients. mRNA levels of APOA1, APOC3, and APOA4 were the lowest in AD patients with CDR 2+ (P<0.05) (Figure 1). Serum mRNA APOA1, APOC3, and APOA4 levels were negatively related with the development of AD (P<0.01).
Figure 1.
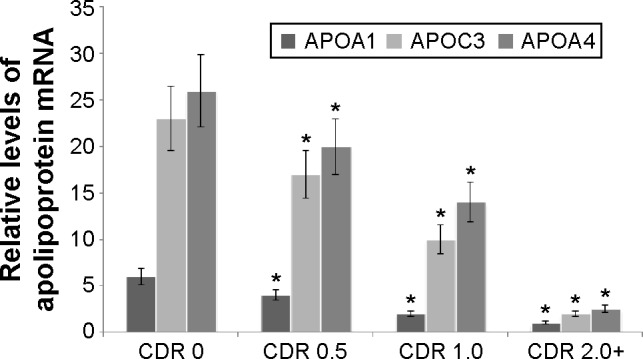
Quantitative RT-PCR analysis for APOA1, APOC3, and APOA4 messenger RNA (mRNA) levels in AD patients and controls.
Notes: Quantitative RT-PCR showed higher levels of APOA1, APOC3, and APOA4 mRNA from controls than those from AD patients. Each bar represents the mean ± SD of five independent cases. *P<0.05 via CDR 0.
Abbreviations: RT-PCR, reverse-transcriptase polymerase chain reaction; AD, Alzheimer’s disease; SD, standard deviation; CDR, Clinical Dementia Rating.
ELISA analysis
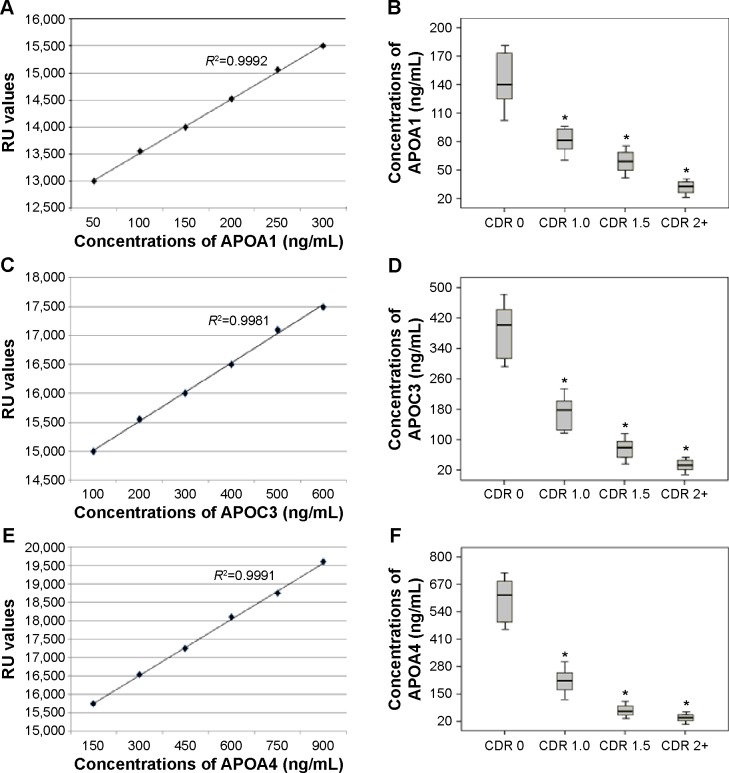
The concentrations of APOA1, APOC3, and APOA4 in serum were determined by responding standard curves (Figure 2A, C, and E). APOA1 concentrations were 140±40 ng/mL (95% CI 125–173 ng/mL) for healthy participants with CDR 0, 78±16 ng/mL (95% CI 70–90 ng/mL) for AD patients with CDR 0.5, 55±15 ng/mL (95% CI 50–65 ng/mL) for AD patients with CDR 1, and 35±15 ng/mL (95% CI 28–42 ng/mL) for AD patients with CDR 2+ (Figure 2B). APOC3 concentrations were 395±95 ng/mL (95% CI 320–440 ng/mL) for healthy participants with CDR 0, 180±60 ng/mL (95% CI 130–210 ng/mL) for AD patients with CDR 0.5, 80±40 ng/mL (95% CI 60–100 ng/mL) for AD patients with CDR 1, and 38±23 ng/mL (95% CI 30–50 ng/mL) for AD patients with CDR 2+ (Figure 2D). APOA4 concentrations were 595±145 ng/mL (95% CI 475–690 ng/mL) for healthy participants with CDR 0, 220±80 ng/mL (95% CI 170–240 ng/mL) for AD patients with CDR 0.5, 90±50 ng/mL (95% CI 50–100 ng/mL) for AD patients with CDR 1, and 45±25 ng/mL (95% CI 35–55 ng/mL) for AD patients with CDR 2+ (Figure 2F). Serum APOA1, APOC3, and APOA4 levels were also negatively associated with the progression of AD (P<0.01).
Figure 2.
Measurement of serum APOA1, APOC3, and APOA4 by ELISA.
Notes: (A) A standard curve was made by measuring the concentration of APOA1 and absorbing values at 450 nm. (B) Bar diagram indicating the differences in serum APOA1 among all participants with different CDR degrees. (C) A standard curve was made by measuring the concentration of APOC3 and absorbing values at 450 nm. (D) Bar diagram indicating the differences in serum APOC3 among all participants with different CDR degrees. (E) A standard curve was made by measuring the concentration of APOA1 and absorbing values at 450 nm. (F) Bar diagram indicating the differences in serum APOA1 among all participants with different CDR degrees. The CDR score of healthy controls was 0 (n=160 cases), and the CDR scores of AD patients were 0.5 (n=73 cases), 1.0 (n=44 cases), and 2.0+ (n=30 cases). *P<0.05 via CDR 0.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; AD, Alzheimer’s disease; CDR, Clinical Dementia Rating; RU, repopulating unit.
Oxidative stress in AD pathogenesis
Next, we explored whether oxidative stress plays an important role in AD pathogenesis. We measured SOD, AST, ALT, and GSH levels, which are biomarkers of oxidative stress.41–46 As Table 2 shows, SOD and GSH levels were lower in AD patients than those in controls. In contrast, AST and ALT levels were higher in AD patients than those from controls (Table 2). The results suggested that oxidative stress is involved in AD pathogenesis.
Table 2.
Biochemical parameters of enzyme activities
| Group | SOD (U/mL) | GSH (ng/L) | ALT (U/mL) | AST (U/mL) |
|---|---|---|---|---|
| AD | 11.252±4.361 | 12.223±1.973 | 89.791±6.485 | 212.247±19.698 |
| Controls | 27.144±3.082 | 26.258±2.567 | 47.228±10.83 | 112.554±24.077 |
| t-value | 5.482 | 6.948 | 7.125 | 5.916 |
| P-value | 0.002 | 0.001 | 0.001 | 0.002 |
Notes: All data presented as mean ± SD (n=147 for AD patients and n=160 for healthy controls). Student’s t-test used to evaluate statistically significant differences (P<0.05).
Abbreviations: GSH, glutathione; AD, Alzheimer’s disease; SD, standard deviation; SOD, superoxide dismutase; ALT, alamine aminotransferase; AST, asparate aminotransferase.
Serum lipid levels in AD patients
Biochemical analysis showed that serum TC, TG, LDL-c and malondialdehyde levels were higher in AD patients than controls (P<0.05) (Table 3). In contrast, serum HDL-c levels were lower in AD patients than controls (P<0.05) (Table 3). The results suggested that lipid per-oxidation product was increased and antioxidant product was decreased in AD patients, which contributes to the development of AD. Elevated TC, LDL, and TG and decreased HDL characterized the lipid profile in AD, which is different from a previous report.58 Further work is needed to validate the lipid profile as a biomarker for the development of AD.
Table 3.
Biochemical parameters of lipid components
| Group | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | MDA (nmol/L) |
|---|---|---|---|---|---|
| AD | 1.29±0.42 | 3.59±0.82 | 0.97±0.49 | 2.85±0.76 | 1.43±0.41 |
| Controls | 0.83±1.17 | 2.56±0.88 | 1.35±0.59 | 1.93±0.48 | 0.86±0.14 |
| t-value | 1.589 | 1.884 | 2.241 | 3.589 | 2.547 |
| P-value | 0.045 | 0.040 | 0.024 | 0.016 | 0.036 |
Notes: All data presented as mean ± SD (n=147 for AD patients and n=160 for healthy controls). Student’s t-test was used to evaluate statistically significant differences (P<0.05).
Abbreviations: TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDA, malondialdehyde; AD, Alzheimer’s disease; SD, standard deviation.
Association between APOA1, APOC3, and APOA4 levels and MADRS scores
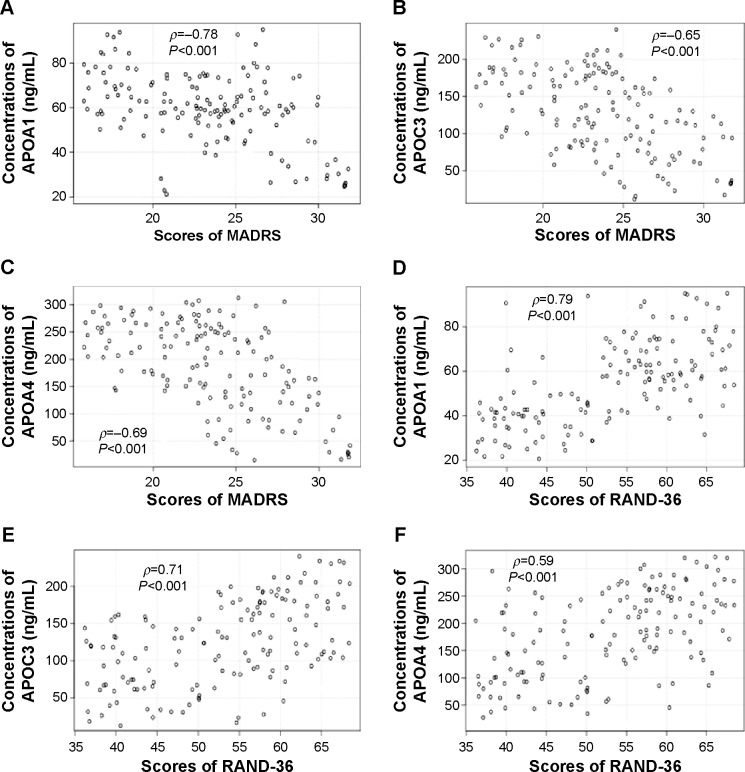
When severity of AD was stratified by MADRS, significant differences in APOA1, APOC3 and APOA4 levels were observed in different scores of MADRS. APOA1, APOC3, and APOA4 levels were reduced when MADRS scores increased in AD patients and controls (Figures 3A and C, 4A and C) (P<0.001). APOA1, APOC3, and APOA4 levels were negatively related with MADRS scores, suggested that APOA1, APOC3, and APOA4 inhibited the development of AD, since MADRS scores are often used to evaluate the severity of AD symptoms.59
Figure 3.
Relationship between MADRS or RAND-36 scores and APOA1/APOC3/APOA4 levels in AD patients.
Notes: (A) Relationship between MADRS scores and APOA1 levels. (B) Relationship between MADRS scores and APOC3 levels. (C) Relationship between MADRS scores and POA4 levels. (D) Relationship between RAND-36 scores and APOA1 levels. (E) Relationship between RAND-36 scores and APOC3 levels. (F) Relationship between RAND-36 scores and APOA4 levels. Statistical analysis performed using Spearman’s rank correlation test. There is a strong negative correlation if ρ-values are between −1 and −0.5, and there is a strong positive correlation if ρ-values are between 0.5 and 1.
Abbreviations: MADRS, Montgomery–Åsberg Depression Rating Scale; AD, Alzheimer’s disease.
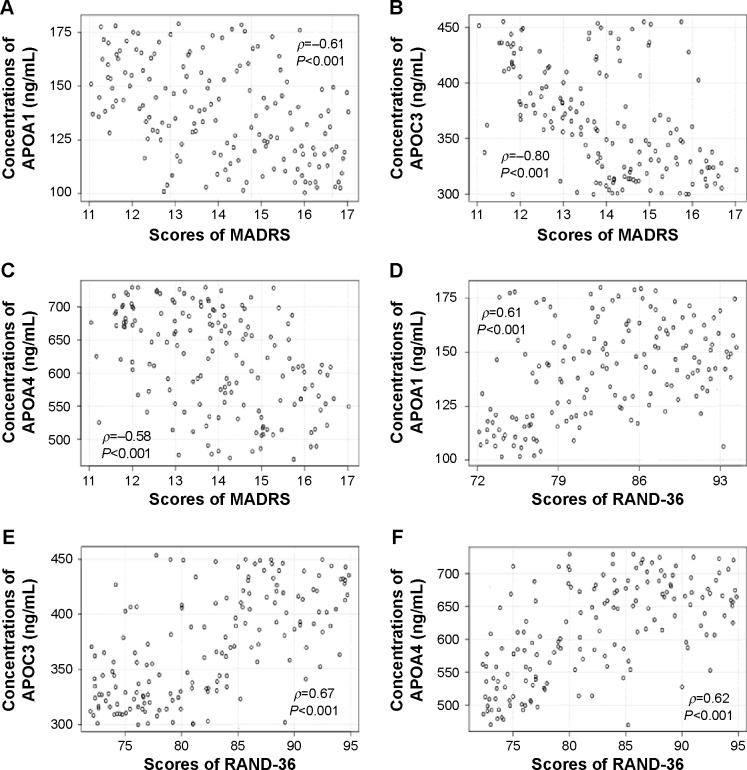
Figure 4.
Relationship between MADRS or RAND-36 scores and APOA1/APOC3/APOA4 levels in healthy subjects.
Notes: (A) Relationship between MADRS scores and APOA1 levels. (B) Relationship between MADRS scores and APOC3 levels. (C) Relationship between MADRS scores and APOA4 levels. (D) Relationship between RAND-36 scores and APOA1 levels. (E) Relationship between RAND-36 scores and APOC3 levels. (F) Relationship between RAND-36 scores and APOA4 levels. Statistical analysis was performed using Spearman’s rank correlation test. There is a strong negative correlation if ρ-values are between −1 and −0.5. There is a strong positive correlation if the ρ-values are between 0.5 and 1.
Abbreviation: MADRS, Montgomery–Åsberg Depression Rating Scale.
Association between APOA1, APOC3, and APOA4 levels and RAND-36 scores
RAND-36 is a reliable, valid, and sensitive method for measuring general health.60 When the severity of AD was stratified by RAND-36, different APOA1, APOC3, and APOA4 levels were observed with different RAND-36 scores. APOA1, APOC3, and APOA4 levels increased when RAND-36 scores increased (Figures 3D and F, 4D and F) (P<0.001). APOA1, APOC3, and APOA4 levels were positively related with MADRS scores, suggesting that APOA1, APOC3, and APOA4 inhibit the development of AD, since RAND-36 score is often used to evaluate general health.
Discussion
The high incidence and financial burden of AD greatly reduces the life quality of human beings. Although pharmaceutical therapy has been accepted as the first choice for AD patients, serious adverse effects and high-cost medical therapies have limited the utilization of the medicine. A new concept is also needed to understand the mechanisms by studying symptoms, behaviors, or biomarkers, which are different to traditional classification for mental disorders.61 Meanwhile, the study can also provide valuable information for the therapy of widespread depression.
Early detection of AD is also a great challenge for the therapy of dementia, and more molecular mechanisms urgently need to be explored. A combination of diet, lifestyle, and vascular and genetic elements that promote the onset and course of AD may be more likely the cause of AD. The possibility that AD risk can be reduced by diet is of great importance, and suggests a nonpharmaceutical therapy for AD. Because of the importance of lipid diets and metabolism in preventive treatment against AD, lipid metabolism may play an important role in AD risk.62 AD patients have significantly higher TC and LDL than patients with other dementia, which has been confirmed in a larger cohort. The result showed that increasing certainty of AD was closely associated with higher-level TC and LDL.63 In contrast, a conflict finding has been also reported: lipid profile is connected with the etiology and progress of AD. There was an association between low serum cholesterol and LDL-c levels and cognitive decline in patients with AD.58
Presently, the molecular mechanisms linking lipid and AD pathology remain unclear. No data are available on serum apolipoprotein in AD patients yet. Furthermore, the cause of AD is much more complex and not only a single genetic cause. Three genes – APOA1, APOC3, and APOA4 in a gene cluster – may have synergetic enhancement effects on a healthy lipid profile and are often closer to one another. Therefore, we examined the association between the expression levels of the three genes and AD risk. The results showed that low serum levels of three apolipoproteins were correlated with development of AD based on CDR scores. Apolipoprotein concentrations declined significantly in AD patients, but not in controls. Therefore, decreased expression of serum APOA1, APOC3, and APOA4 may be related with risk of AD.
Nevertheless, one important question should be clarified. The AD pathological process occurs in brain. How can APOA1–APOC3–APOA4 levels in serum reflect AD pathogenesis? Serum lipid may be independent of the brain, but an unfavorable lipid profile may cause hyperlipidemia and subtle changes in brain lipid metabolism.64 Also, Yu et al found that a long-term high-lard diet improved serum TC and LDL-c levels, which also could affect the brain’s lipid composition, memory, and learning activities.65 Here, we considered more that serum apolipoproteins can be measured directly and will be useful as indicators to evaluate AD risk.
Compared with other serum biomarkers of AD patients,9,10 serum apolipoproteins APOA1, APOC3, and APOA4 seem to be more functional proteins: 1) three proteins can be used to predict the severity of AD more accurately than a single protein, 2) all the proteins affect the progression of AD,66,67 and 3) the polymorphisms of these apolipoprotein genes may result in the pathogenesis and development of AD.20,68 With more certain results, we hope that apolipoprotein-gene cluster of APOA1, APOC3, and APOA4 can be widely applied in the diagnosis of AD.
Certainly, there are some limitations in the present work. Firstly, the present trial had its limitations in a statistical sense and was not conducted in a larger population. More work is needed to be performed in a larger population to validate the present results. Secondly, a gene cluster still cannot present a complete study of AD. The molecular mechanism for the effects of APOA1, APOC3, and APOA4 on the progression of AD remains unknown. A series of silencing and overexpression studies are needed to make sure of their functional role in the risk of AD. Thirdly, the polymorphism of these apolipoprotein genes will result in the pathogenesis and development of AD, which was not considered here. Nonetheless, the present work will be beneficial for understanding molecular mechanisms for the risks of AD.
In sum, the results of this work suggest that decreased expression of the APOA1–APOC3–APOA4 gene cluster may be associated with AD risk. APOA1, APOC3, and APOA4 can reduce the severity of AD symptoms by increasing RAND-36 scores and reducing MADRS scores. Therefore, APOA1, APOC3, and APOA4 should be developed as new combined biomarkers for the early diagnosis of AD patients.
Acknowledgments
We are very grateful to the three anonymous reviewers who provided us with instructive comments, which have significantly improved our manuscript. The authors alone are responsible for the contents of the paper.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(8):a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzmán-Vélez E, Feinstein JS, Tranel D. Feelings without memory in Alzheimer disease. Cogn Behav Neurol. 2014;27(3):117–129. doi: 10.1097/WNN.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano-Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, Hyman BT. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol. 2013;72(12):1182–1192. doi: 10.1097/NEN.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2(10):605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 7.Zwahlen A, Nydegger UE, Vaudaux P, Lambert PH, Waldvogel FA. Complement-mediated opsonic activity in normal and infected human cerebrospinal fluid: early response during bacterial meningitis. J Infect Dis. 1982;145(5):635–646. doi: 10.1093/infdis/145.2.635. [DOI] [PubMed] [Google Scholar]
- 8.Watabe-Rudolph M, Song Z, Lausser L, et al. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology. 2012;78(8):569–577. doi: 10.1212/WNL.0b013e318247caa1. [DOI] [PubMed] [Google Scholar]
- 9.Trueba-Sáiz A, Cavada C, Fernandez AM, et al. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry. 2013;3:e330. doi: 10.1038/tp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang MH, Yang YH, Lu CY, et al. Activity-dependent neuroprotector homeobox protein: a candidate protein identified in serum as diagnostic biomarker for Alzheimer’s disease. J Proteomics. 2012;75(12):3617–3629. doi: 10.1016/j.jprot.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Ranford JC, Coates AR, Henderson B. Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev Mol Med. 2000;2(8):1–17. doi: 10.1017/S1462399400002015. [DOI] [PubMed] [Google Scholar]
- 12.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 13.Singh PP, Singh M, Mastana SS. Genetic variation of apolipoproteins in North Indians. Hum Biol. 2002;74(5):673–682. doi: 10.1353/hub.2002.0057. [DOI] [PubMed] [Google Scholar]
- 14.Malkki H. Alzheimer disease: effects of the APOE ε4 allele on brain development. Nat Rev Neurol. 2014;10(1):4. doi: 10.1038/nrneurol.2013.258. [DOI] [PubMed] [Google Scholar]
- 15.Rassas AA, Khiari HM, Fredj SH, et al. High APOE epsilon 4 allele frequencies associated with Alzheimer disease in a Tunisian population. Neurol Sci. 2012;33(1):33–37. doi: 10.1007/s10072-011-0663-8. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Huang M, He Y, Zhang S, Luo Y. Genetic ablation of apolipoprotein A-IV accelerates Alzheimer’s disease pathogenesis in a mouse model. Am J Pathol. 2011;178(3):1298–1308. doi: 10.1016/j.ajpath.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ota VK, Chen ES, Ejchel TF, et al. APOA4 polymorphism as a risk factor for unfavorable lipid serum profile and depression: a cross-sectional study. J Investig Med. 2011;59(6):966–970. doi: 10.2310/JIM.0b013e31822467cd. [DOI] [PubMed] [Google Scholar]
- 18.Liu HC, Hu CJ, Chang JG, et al. Proteomic identification of lower apolipoprotein A-I in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(3):155–161. doi: 10.1159/000090676. [DOI] [PubMed] [Google Scholar]
- 19.Lewis TL, Cao D, Lu H, et al. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J Biol Chem. 2010;285(47):36958–36968. doi: 10.1074/jbc.M110.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Shi JJ, Zhang SH, et al. The APOC3 SstI polymorphism is weakly associated with sporadic Alzheimer’s disease in a Chinese population. Neurosci Lett. 2005;380(3):219–222. doi: 10.1016/j.neulet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Antonarakis SE, Oettgen P, Chakravarti A, et al. DNA polymorphism haplotypes of the human apolipoprotein APOA1-APOC3-APOA4 gene cluster. Hum Genet. 1988;80(3):265–273. doi: 10.1007/BF01790095. [DOI] [PubMed] [Google Scholar]
- 22.Garenc C, Aubert S, Laroche J, et al. Population prevalence of APOE, APOC3 and PPAR-α mutations associated to hypertriglyceridemia in French Canadians. J Hum Genet. 2004;49(12):691–700. doi: 10.1007/s10038-004-0208-6. [DOI] [PubMed] [Google Scholar]
- 23.Carter CJ. Convergence of genes implicated in Alzheimer’s disease on the cerebral cholesterol shuttle: APP, cholesterol, lipoproteins, and atherosclerosis. Neurochem Int. 2007;50(1):12–38. doi: 10.1016/j.neuint.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Palacios R. Post-trial access and the new version of the Declaration of Helsinki. Colomb Med (Cali) 2013;44(4):206–207. [PMC free article] [PubMed] [Google Scholar]
- 25.Tamaoka A. Alzheimer’s disease: definition and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) Nihon Rinsho. 2011;69 Suppl 10(Pt 2):240–245. Japanese. [PubMed] [Google Scholar]
- 26.Inoue K, Meguro K, Akanuma K, Meguro M, Yamaguchi S, Fukuda H. Impaired memory and executive function associated with decreased medial temporal and prefrontal blood flow in Clinical Dementia Rating 0.5 status: the Osaki-Tajiri project. Psychogeriatrics. 2012;12(1):27–33. doi: 10.1111/j.1479-8301.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- 27.Pirani A, Brodaty H, Martini E, Zaccherini D, Neviani F, Neri M. The validation of the Italian version of the GPCOG (GPCOG-It): a contribution to cross-national implementation of a screening test for dementia in general practice. Int Psychogeriatr. 2010;22(1):82–90. doi: 10.1017/S104161020999113X. [DOI] [PubMed] [Google Scholar]
- 28.Cedarbaum JM, Jaros M, Hernandez C, et al. Rationale for use of the Clinical Dementia Rating Sum of Boxes as a primary outcome measure for Alzheimer’s disease clinical trials. Alzheimers Dement. 2013;9(1 Suppl):S45–S55. doi: 10.1016/j.jalz.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Salvatore P, Baldessarini RJ, Khalsa H, et al. Predicting diagnostic change among patients diagnosed with first-episode DSM-IV-TR major depressive disorder with psychotic features. J Clin Psychiatry. 2013;74(7):723–731. doi: 10.4088/JCP.12m08328. [DOI] [PubMed] [Google Scholar]
- 30.Pradier C, Sakarovitch C, Le Duff F, et al. The Mini Mental State Examination at the time of Alzheimer’s disease and related disorders diagnosis, according to age, education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. PLoS One. 2014;9(8):e103630. doi: 10.1371/journal.pone.0103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortel KF, Meyer JS, Herod B, Thornby J. Education and occupation as risk factors for dementias of the Alzheimer and ischemic vascular types. Dementia. 1995;6(1):55–62. doi: 10.1159/000106922. [DOI] [PubMed] [Google Scholar]
- 32.Alonzo A, Chan G, Martin D, Mitchell PB, Loo C. Transcranial direct current stimulation (tDCS) for depression: analysis of response using a three-factor structure of the Montgomery-Åsberg depression rating scale. J Affect Disord. 2013;150(1):91–95. doi: 10.1016/j.jad.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Birnbaum H, Kessler R, Joish V, et al. Healthcare resource utilization and costs of mild, moderate, and severe depression in the workforce in the United States. Eur Psychiatry. 2009;24(Suppl 1):S614. [Google Scholar]
- 34.Mormont E, Jamart J, Jacques D. Symptoms of depression and anxiety after the disclosure of the diagnosis of Alzheimer disease. J Geriatr Psychiatry Neurol. 2014;27(4):231–236. doi: 10.1177/0891988714532021. [DOI] [PubMed] [Google Scholar]
- 35.Ramakers IH, Verhey FR, Scheltens P, et al. Anxiety is related to Alzheimer cerebrospinal fluid markers in subjects with mild cognitive impairment. Psychol Med. 2013;43(5):911–920. doi: 10.1017/S0033291712001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz A, Finck C, Gómez Y, Daig I, Glaesmer H, Singer S. Anxiety and depression in the general population in Colombia: reference values of the Hospital Anxiety and Depression Scale (HADS) Soc Psychiatry Psychiatr Epidemiol. 2014;49(1):41–49. doi: 10.1007/s00127-013-0714-y. [DOI] [PubMed] [Google Scholar]
- 37.Melin EO, Thulesius HO, Persson BA. Affect School for chronic benign pain patients showed improved alexithymia assessments with TAS-20. Biopsychosoc Med. 2010;4(1):5. doi: 10.1186/1751-0759-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mello AF, Blay SL, Kohn R. Global Assessment of Relational Functioning Scale (GARF): a validity study in patients with recurrent major depression in Brazil. Transcult Psychiatry. 2007;44(1):55–64. doi: 10.1177/1363461507074969. [DOI] [PubMed] [Google Scholar]
- 39.Mattila K, Lahtela M, Hynynen M. Health-related quality of life following ambulatory surgery procedures: assessment by RAND-36. BMC Anesthesiol. 2012;12:30. doi: 10.1186/1471-2253-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Linthout S, Spillmann F, Riad A, et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation. 2008;117(12):1563–1573. doi: 10.1161/CIRCULATIONAHA.107.710830. [DOI] [PubMed] [Google Scholar]
- 41.Dong X, Li D, Liu H, Zhao Y. SOD3 and eNOS genotypes are associated with SOD activity and NO. Exp Ther Med. 2014;8(1):328–334. doi: 10.3892/etm.2014.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su S, Li Q, Liu Y, et al. Sesamin ameliorates doxorubicin-induced cardiotoxicity: involvement of Sirt1 and Mn-SOD pathway. Toxicol Lett. 2014;224(2):257–263. doi: 10.1016/j.toxlet.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 43.Kumar SM, Swaminathan K, Clemens DL, Dey A. GSH protects against oxidative stress and toxicity in VL-17A cells exposed to high glucose. Eur J Nutr. 2015;54(2):223–234. doi: 10.1007/s00394-014-0703-2. [DOI] [PubMed] [Google Scholar]
- 44.Jung S, Kim OY, Kim M, Song J, Lee SH, Lee JH. Age-related increase in alanine aminotransferase correlates with elevated levels of plasma amino acids, decanoylcarnitine, Lp-PLA2 activity, oxidative stress, and arterial stiffness. J Proteome Res. 2014;13(7):3467–3475. doi: 10.1021/pr500422z. [DOI] [PubMed] [Google Scholar]
- 45.Yamada J, Tomiyama H, Yambe M, Yamashina A. Elevated serum alanine aminotransferase is a marker of inflammation and oxidative stress associated with the metabolic syndrome. Atherosclerosis. 2006;189(1):198–205. doi: 10.1016/j.atherosclerosis.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 46.Mansour HH, Ismael Nel S, Hafez HF. Ameliorative effect of septilin, an ayurvedic preparation against γ-irradiation-induced oxidative stress and tissue injury in rats. Indian J Biochem Biophys. 2014;51(2):135–141. [PubMed] [Google Scholar]
- 47.Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- 48.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 49.Gheita TA, Kenawy SA. Measurement of malondialdehyde, glutathione, and glutathione peroxidase in SLE patients. Methods Mol Biol. 2014;1134:193–199. doi: 10.1007/978-1-4939-0326-9_14. [DOI] [PubMed] [Google Scholar]
- 50.Besser LM, Gill DP, Monsell SE, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28(1):36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidoni ED, Townley RA, Honea RA, Burns JM. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77(21):1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E ε4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 53.Arlt S, Kontush A, Müller-Thomsen T, Beisiegel U. Lipid peroxidation as a common pathomechanism in coronary heart disease and Alzheimer disease. Z Gerontol Geriatr. 2001;34(6):461–465. doi: 10.1007/s003910170019. German. [DOI] [PubMed] [Google Scholar]
- 54.Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(1):92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- 55.Wiscott R, Kopera-Frye K, Seifert L. Possible consequences of social drinking in the early stages of Alzheimer disease. Geriatr Nurs. 2001;22(2):100–104. doi: 10.1067/mgn.2001.115201. quiz 105. [DOI] [PubMed] [Google Scholar]
- 56.Marinho V, Laks J, Engelhardt E, Conn D. Alcohol abuse in an elderly woman taking donepezil for Alzheimer disease. J Clin Psychopharmacol. 2006;26(6):683–685. doi: 10.1097/01.jcp.0000246213.41647.9f. [DOI] [PubMed] [Google Scholar]
- 57.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh compound B uptake. Arch Neurol. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Presećki P, Mück-Seler D, Mimica N, et al. Serum lipid levels in patients with Alzheimer’s disease. Coll Antropol. 2011;35(Suppl 1):115–120. [PubMed] [Google Scholar]
- 59.Lebedev AV, Beyer MK, Fritze F, Westman E, Ballard C, Aarsland D. Cortical changes associated with depression and antidepressant use in Alzheimer and Lewy body dementia: an MRI surface-based morphometric study. Am J Geriatr Psychiatry. 2014;22(1):4–13.e1. doi: 10.1016/j.jagp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Laakkonen ML, Hölttä EH, Savikko N, Strandberg TE, Suominen M, Pitkälä KH. Psychosocial group intervention to enhance self-management skills of people with dementia and their caregivers: study protocol for a randomized controlled trial. Trials. 2012;13:133. doi: 10.1186/1745-6215-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badcock JC, Hugdahl K. A synthesis of evidence on inhibitory control and auditory hallucinations based on the Research Domain Criteria (RDoC) framework. Front Hum Neurosci. 2014;8:180. doi: 10.3389/fnhum.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008;585(1):176–196. doi: 10.1016/j.ejphar.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 63.Lesser GT. Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama study. Neurology. 2012;78(16):1280. doi: 10.1212/WNL.0b013e318254f6ad. [DOI] [PubMed] [Google Scholar]
- 64.Bruder ED, Lee PC, Raff H. Dexamethasone treatment in the newborn rat: fatty acid profiling of lung, brain, and serum lipids. J Appl Physiol (1985) 2005;98(3):981–990. doi: 10.1152/japplphysiol.01029.2004. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Bi Y, Ma W, et al. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int J Dev Neurosci. 2010;28(3):271–276. doi: 10.1016/j.ijdevneu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Furuya TK, Chen ES, Ota VK, et al. Association of APOA1 and APOA5 polymorphisms and haplotypes with lipid parameters in a Brazilian elderly cohort. Genet Mol Res. 2013;12(3):3495–3499. doi: 10.4238/2013.February.28.7. [DOI] [PubMed] [Google Scholar]
- 67.Macesic M, Lalic NM, Kostic VS, et al. Alzheimer’s disease in normoglycaemic patients: an association with decreased insulin sensitivity and atherogenic profile of lipid abnormalities; Poster presented at: 46th EASD Meeting; September 20–24, 2010; Stockholm, Sweden. [Google Scholar]
- 68.Shibata N, Nagata T, Shinagawa S, et al. Genetic association between APOA1 and APOD polymorphisms and Alzheimer’s disease in a Japanese population. J Neural Transm. 2013;120(11):1599–1603. doi: 10.1007/s00702-013-1036-7. [DOI] [PubMed] [Google Scholar]