Abstract
Background
While a number of pro-hemostatic agents that are applied intraoperatively have been introduced to minimize bleeding, little is known about the patterns of use and the factors that influence use. We examined the use of hemostatic agents in patients undergoing major surgery.
Methods
All patients who underwent major general, gynecologic, urologic, cardiothoracic, or orthopedic surgery from 2000–2010 who were recorded in the Perspective database were analyzed.
Results
Among 3,633,799 patients, hemostatic agents were used in 30.3% (n=1,102,267). The use of hemostatic agents increased from 28.5% in 2000 to 35.2% in 2010. Over the same period, the rates of transfusion declined for pancreatectomy (−14.4%), liver resection (−15.0%), gastrectomy (−11.7%), prostatectomy (−6.6%), nephrectomy (−4.6%), hip arthroplasty (−10.4%), and knee arthroplasty (−6.6%). Over the same time period the transfusion rate increased for colectomy (6.0%), hysterectomy (3.7%), CABG (8.4%), valvuloplasty (4.2%), lung resection (1.9%), and spine surgery (1.6%). Transfusion remained relatively stable for thyroidectomy (0.2%).
Conclusion
The use of hemostatic agents has increased rapidly even for surgeries associated with a small risk of transfusion and bleeding complications.
Summary
The use of hemostatic agents has increased rapidly even for surgeries associated with a small risk of transfusion and bleeding complications. In addition to patient characteristics, surgeon and hospital factors exerted substantial influence on the allocation of hemostatic agents.
Keywords: Hemostatic agents, hemostasis, bleeding, hemorrhage, blood
Introduction
Bleeding is one of the most feared complications of surgery and a frequent cause of significant perioperative morbidity.1,2 Current estimates suggest that 60–70% of all transfused red blood cells are used in the surgical setting.3,4 In addition to infectious complications, transfusion is associated with a number of non-infectious side effects and is accompanied by substantial costs to the healthcare system.2,5–7 Surgical site bleeding leads not only to transfusion, but can require reoperation and is associated with other complications including coagulopathy and hematoma formation.
Reducing the risk of bleeding complications requires meticulous intraoperative hemostasis. While hemostasis is typically achieved through suturing, electrocautery, or surgical clips, a number of adjuvant pro-hemostatic agents have been developed for use over the last two decades.3,8–11 These agents have been broadly classified into three groups: topical hemostats that cause blood to clot at a bleeding surface, sealants which prevent leakage from tissues including vessels, and adhesives which bond tissues.8,9 There is overlap among these categories and many compounds can be classified into multiple groups. Topical hemostats are the most commonly used, and typically consist of a mechanical surface to promote clot formation often with either thrombin or fibrinogen or a combination thereof.
In 1998 the Food and Drug Administration (FDA) approved the first fibrin sealant in the United States.9 Since then, a wide range of hemostatic agents have received approval, often for a narrow spectrum of surgical procedures after the demonstration of safety.8,9 A Cochrane review noted that fibrin sealants were associated with reductions in postoperative blood loss and reduced requirements for allogenic red blood cell transfusion. However, the review also noted that the beneficial effects of these agents were most pronounced for orthopedic procedures and that the efficacy of these agents for other operations was not clinically significant. Notably, the majority of trials included in the review were small with over three quarters of the studies included consisting of fewer than 50 patients.3
Despite the availability of many hemostatic agents, a number of questions remain unanswered. The majority of trials of hemostatic agents have been small, often non-randomized, and limited by relatively uncommon endpoints and with a lack of standardized protocols for transfusion.3 In addition, little is known about how these agents are being used by surgeons in the community. Given the limited data describing the use of surgical hemostatic agents, we conducted a population-based analysis to determine the utilization of hemostatic agents and examined the trends in perioperative transfusion over the last decade.
Methods
Data Source
We utilized the Perspective database (Premier, Charlotte, North Carolina), a voluntary, fee-supported database developed to measure resource utilization and quality. Perspective collects data on inpatient admissions from more than 500 acute-care hospitals located across the United States.12 In addition to demographics, disease characteristics, and procedures, Perspective collects information on all billed services. The database has been validated and utilized in a number of outcomes studies.13,14 Perspective collected approximately 5.5 million hospital discharges in 2006, which represents approximately 15% of nationwide hospitalizations.12,14 In addition to clinical and demographic data, Perspective collects data on all drugs and therapeutic agent utilized during a patient's hospitalization. Perspective has been utilized in a number of prior studies to explore the use of drugs and therapeutic agents using billing data.15,16
Cohort Selection and Surgical Procedures
Our analysis included patients who underwent major surgery between 2000 and 2010 (Table 1). The procedures were classified into the following groups: general surgical procedures (colectomy, thyroidectomy, pancreatectomy, liver resection, and gastrectomy), gynecologic and urologic procedures (prostatectomy, hysterectomy, nephrectomy), cardiothoracic procedures (coronary artery bypass graft, valvuloplasty, lung resection), and orthopedic procedures (hip arthroplasty, knee arthroplasty, spine surgery). Patients who underwent a minimally invasive procedure were not included.
Table 1.
Procedures selected for the analysis.
| Procedure | ICD-9 procedure codes | ICD-9 site specific cancer codes |
|---|---|---|
| Colectomy | 45.7, 45.71, 45.72, 45.73, 45.74, 45.75, 45.76, 45.79, 45.8, 45.82, 45.83 | 153–153.9 |
| Thyroidectomy | 06.2–06.6 | 193–193.99 |
| Pancreatectomy | 52.5, 52.51, 52.52, 52.53, 52.59, 52.6, 52.7 | 157–157.9 |
| Liver resection | 50.22, 50.3, 50.4 | 140–239 |
| Gastrectomy | 43.5, 43.6, 43.7, 43.81, 43.89, 43.9, 43.91,43.99 | 151–151.9 |
| Prostatectomy | 60.2, 60.21, 60.29, 60.3, 60.4, 60.5, 60.62 | 185 |
| Hysterectomy | 68.4, 68.9 | 182.0–182.8, 183.0–183.9 |
| Nephrectomy | 55.4, 55.5, 55.51, 55.52, 55.53, 55.54 | 189–189.9 |
| Coronary artery bypass graft | 36.1, 36.10, 36.11, 36.12, 36.13, 36.14, 36.15, 36.16, 36.17, 36.19 | 164–164.9 |
| Valvuloplasty | 35.1, 35.10, 35.11, 35.12, 35.13, 35.14, 35.2 ,35.20, 35.21, 35.22, 35.23, 35.24, 35.25, 35.26, 35.27, 35.28 | 164–164.9 |
| Lung resection | 32.3, 32.39, 32.3, 32.39, 32.4, 32.49, 32.4, 32.49, 32.5, 32.59 | 162–163.9 |
| Knee replacement | 81.54 | 140–209.69 |
| Hip replacement | 81.51 | 140–209.69 |
| Spine surgery | 80.50, 80.51, 81.0, 81.00, 81.01, 81.02, 81.03, 81.04, 81.05, 81.06, 81.07, 81.08, | 140–209.69 |
Hemostatic Agent Use and Transfusion Requirements
Hemostatic agent use was determined for each patient by review of billing records. We recorded use of any topical fibrin or thrombin-based product as well as commercially available topical hemostatic agents classified as a topical hemostat, sealant, or adhesive, as previously described.8,9 Given the overlap in the composition of many of these products, the analysis was not separated into individual products. Each patient was classified as having received or not received a hemostatic agent. Transfusion was defined as either an ICD-9 code for transfusion of autologous or donor blood products (ICD-9 99.0x) or through the identification of a billing code in Perspective for use of blood component therapy. A priori we chose not to attempt to analyze the direct effect of hemostatic agents on transfusion rates given the likely substantial influence of unmeasured confounders. Rather, we present an ecologic analysis of hemostatic agent use in combination with transfusion rates.
Clinical and Demographic Characteristics
Demographic data analyzed included age (<60 and ≥60 years of age), gender (male, female), race (white, black, other, unknown), marital status (married, single, unknown), year of surgery (2000 through 2010), and insurance status (Medicare, Medicaid, commercial, self-pay, and unknown). The presence of cancer at the site of surgery was analyzed for each patient (Table 1). Risk adjustment for comorbid conditions was performed using the Charlson comorbidity index.17 The ICD-9 coding to define the Charlson index as reported by Deyo and colleagues was utilized.18
We characterized the hospitals in which patients were treated based on location (metropolitan, non-metropolitan), region of the country (northeast, midwest, west, south), size (<400 beds, 400–600 beds, and >600 beds) and teaching status (teaching, non-teaching). Both hospital and surgeon volume were analyzed. For each surgeon and hospital, we determined the total number of each procedure that was performed during the study period. Given that not all physicians and hospitals contributed data for the entire study period, annualized procedure volumes were calculated. Annualized procedure volumes were estimated by dividing the total number of subjects who underwent a procedure by the number of years a given surgeon or hospital contributed at least one procedure. The volumes were then divided to create three approximately equal tertiles of surgeon and hospital volume: low, intermediate, and high.19,20 Separate volume estimates were determined for each procedure.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. The use of hemostatic agents as well as trends in transfusion rates are presented graphically by year of diagnosis. Multivariable hierarchical mixed-effects logistic regression models were used to analyze the influence of patient, physician, and hospital characteristics on use of hemostatic agents. The models included all of the patient, physician and hospital facts as well as a hospital-specific random effect. Separate models were developed for general surgical, gynecologic/urologic, cardiothoracic, and orthopedic procedures. Results are reported as odds ratios and 95% confidence intervals. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided.
Results
We identified a total of 3,633,799 patients who underwent the procedures of interest, including 1,102,267 (30.3%) who received a hemostatic agent and 2,531,532 (69.7%) who did not. The use of hemostatic agents was highest for spine surgery (82.2%) and liver resection (51.6%) and lowest for prostatectomy (6.6%), hip arthroplasty (7.3%), knee arthroplasty (8.3%), and colectomy (8.5%). The use of hemostatic agents increased over time from 28.5% in 2000 to 35.2% in 2010 (P<0.0001) (Table 2).
Table 2.
Characteristics of the cohort stratified by hemostatic agent use.
| No hemostatic agent | Hemostatic agent | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | (%) | N | (%) | P-value | ||
| 2,531,532 | (69.7) | 1,102,267 | (30.3) | |||
| Age | <0.0001 | |||||
| <60 | 1,047,589 | (63.8) | 594,507 | (36.2) | ||
| ≥60 | 1,483,943 | (74.5) | 507,760 | (25.5) | ||
| Gender | <0.0001 | |||||
| Male | 1,118,719 | (66.8) | 555,519 | (33.2) | ||
| Female | 1,412,813 | (72.1) | 546,748 | (27.9) | ||
| Race | <0.0001 | |||||
| White | 1,780,327 | (68.4) | 822,295 | (31.6) | ||
| Black | 246,256 | (72.2) | 94,850 | (27.8) | ||
| Other | 504,902 | (73.2) | 185,112 | (26.8) | ||
| Unknown | 47 | (0) | 10 | (0) | ||
| Year of diagnosis | <0.0001 | |||||
| 2000 | 162,341 | (71.5) | 64,842 | (28.5) | ||
| 2001 | 233,121 | (72.3) | 89,454 | (27.7) | ||
| 2002 | 239,039 | (72.4) | 91,130 | (27.6) | ||
| 2003 | 251,973 | (71.9) | 98,657 | (28.1) | ||
| 2004 | 248,809 | (71.3) | 100,343 | (28.7) | ||
| 2005 | 246,935 | (70.7) | 102,432 | (29.3) | ||
| 2006 | 280,001 | (69.6) | 122,330 | (30.4) | ||
| 2007 | 269,225 | (68.6) | 123,375 | (31.4) | ||
| 2008 | 265,617 | (66.8) | 132,057 | (33.2) | ||
| 2009 | 269,677 | (65.5) | 142,385 | (34.6) | ||
| 2010 | 64,794 | (64.8) | 35,262 | (35.2) | ||
| Insurance | <0.0001 | |||||
| Commercial | 1,131,764 | (68.1) | 529,605 | (31.9) | ||
| Medicare | 1,166,987 | (74.1) | 409,016 | (26.0) | ||
| Medicaid | 100,897 | (66.5) | 50,784 | (33.5) | ||
| Uninsured | 50,298 | (67.5) | 24,262 | (32.5) | ||
| Unknown | 81,586 | (47.9) | 88,600 | (52.1) | ||
| Marital status | <0.0001 | |||||
| Married | 1,443,383 | (68.9) | 652,496 | (31.1) | ||
| Single | 312,615 | (66.9) | 154,619 | (33.1) | ||
| Unknown | 775,534 | (72.4) | 295,152 | (27.6) | ||
| Area of residence | <0.0001 | |||||
| Metropolitan | 2,258,005 | (69.1) | 1,007,905 | (30.9) | ||
| Non-metropolitan | 273,527 | (74.4) | 94,362 | (25.7) | ||
| Region | <0.0001 | |||||
| Eastern | 365,797 | (75.7) | 117,246 | (24.3) | ||
| Midwest | 556,597 | (70.5) | 233,221 | (29.5) | ||
| South | 1,195,830 | (67.1) | 586,953 | (32.9) | ||
| West | 413,308 | (71.5) | 164,847 | (28.5) | ||
| Cancer | <0.0001 | |||||
| No | 1,399,264 | (60.4) | 918,150 | (39.6) | ||
| Yes | 65,759 | (71.2) | 26,581 | (28.8) | ||
| Comorbidity | <0.0001 | |||||
| 0 | 1,126,403 | (68.4) | 520,210 | (31.6) | ||
| 1 | 609,469 | (71.1) | 247,935 | (28.9) | ||
| ≥2 | 795,660 | (70.4) | 334,122 | (29.6) | ||
| Hospital type | <0.0001 | |||||
| Non-teaching | 1,407,799 | (70.6) | 585,488 | (29.4) | ||
| Teaching | 1,123,733 | (68.5) | 516,779 | (31.5) | ||
| Hospital size | <0.0001 | |||||
| <400 beds | 1,188,154 | (72.2) | 458,633 | (27.9) | ||
| 400–600 beds | 737,025 | (68.9) | 332,284 | (31.1) | ||
| >600 beds | 606,353 | (66.1) | 311.350 | (33.9) | ||
| Hospital volume | <0.0001 | |||||
| Low | 838,647 | (69.2) | 372,916 | (30.8) | ||
| Intermediate | 851,190 | (70.9) | 349,615 | (29.1) | ||
| High | 841,695 | (68.9) | 379,736 | (31.1) | ||
| Surgeon volume | <0.0001 | |||||
| Low | 785,465 | (69.2) | 349,540 | (30.8) | ||
| Intermediate | 787,459 | (69.3) | 349,596 | (30.8) | ||
| High | 796,221 | (69.9) | 342,734 | (30.1) | ||
| Unknown | 162,387 | (72.9) | 60,397 | (27.1) | ||
| Procedure | ||||||
| Colectomy | 321,587 | (91.5) | 29,984 | (8.5) | ||
| Thyroidectomy | 60,301 | (74.9) | 20,237 | (25.1) | ||
| Pancreatectomy | 9066 | (63.7) | 5175 | (36.3) | ||
| Liver resection | 4840 | (48.4) | 5168 | (51.6) | ||
| Gastrectomy | 26,869 | (80.1) | 6665 | (19.9) | ||
| Prostatectomy | 261,219 | (93.4) | 18,483 | (6.6) | ||
| Hysterectomy | 394,102 | (85.9) | 64,979 | (14.2) | ||
| Nephrectomy | 48,826 | (64.3) | 27,082 | (35.7) | ||
| Coronary artery bypass graft | 243,112 | (57.0) | 183,521 | (43.0) | ||
| Valvuloplasty | 71,523 | (50.8) | 69,324 | (49.2) | ||
| Lung resection | 43,429 | (75.5) | 14,125 | (24.5) | ||
| Knee arthroplasty | 611,968 | (91.7) | 55,655 | (8.3) | ||
| Hip arthroplasty | 309,851 | (92.7) | 24,486 | (7.3) | ||
| Spine surgery | 124,839 | (17.8) | 577,383 | (82.2) | ||
In the multivariable models, more recent year of treatment was the strongest predictor of receipt of a hemostatic agent across all four classes of surgical procedures (Table 3). For patients undergoing general surgical procedures, use was higher in black patients, those with Medicare or Medicaid, residents of the Midwest and south, patients with comorbidities and those treated at intermediate and high volume hospitals; hemostatic agents were less frequently used in women, patients with cancer, and for patients treated by intermediate and high-volume surgeons (P<0.05 for all). These trends were similar for the other procedure types except that patients with cancer undergoing gynecologic/urologic, cardiovascular or orthopedic surgery were more likely to receive a hemostatic agent (P<0.05 for all). Additionally, those undergoing gynecologic or urologic surgery with commercial insurance, cancer, and those treated by treated by intermediate and high-volume surgeons were more likely to receive a hemostatic agent (P<0.05 for all).
Table 3.
Multivariable model of predictors of use of hemostatic agents.
| General surgery | Gynecologic and urologic | Cardiovascular | Orthopedic | ||
|---|---|---|---|---|---|
| Age | |||||
| <60 | Referent | Referent | Referent | Referent | |
| ≥60 | 1.01 (0.99–1.03) | 0.91 (0.89–0.93)* | 1.01 (1.00–1.02) | 1.01 (1.00–1.02)* | |
| Gender | |||||
| Male | Referent | Referent | Referent | Referent | |
| Female | 0.94 (0.93–0.96)* | 0.97 (0.95–1.00)* | 0.99 (0.98–1.00) | 1.00 (0.99–1.00) | |
| Race | |||||
| White | Referent | Referent | Referent | Referent | |
| Black | 1.03 (1.01–1.06)* | 1.04 (1.02–1.06)* | 1.01 (1.00–1.03) | 1.00 (0.99–1.01) | |
| Other | 1.01 (0.99–1.04) | 1.01 (0.9901.03) | 0.97 (0.96–0.99)* | 1.01 (1.01–1.02)* | |
| Year of diagnosis | |||||
| 2000 | Referent | Referent | Referent | Referent | |
| 2001 | 1.08 (1.01–1.16)* | 1.03 (0.98–1.09) | 0.96 (0.94–0.99)* | 1.03 (1.01–1.05)* | |
| 2002 | 1.17 (1.09–1.25)* | 1.13 (1.07–1.19)* | 0.98 (0.95–1.01) | 1.05 (1.03–1.08)* | |
| 2003 | 1.21 (1.13–1.30)* | 1.29 (1.22–1.36)* | 1.02 (0.99–1.05) | 1.07 (1.05–1.09)* | |
| 2004 | 1.28 (1.20–1.37)* | 1.43 (1.35–1.50)* | 1.14 (1.10–1.17)* | 1.11 (1.08–1.13)* | |
| 2005 | 1.37 (1.28–1.47)* | 1.57 (1.49–1.65)* | 1.17 (1.13–1.20)* | 1.14 (1.11–1.16)* | |
| 2006 | 1.46 (1.37–1.56)* | 1.79 (1.70–1.89)* | 1.26 (1.22–1.30)* | 1.18 (1.11–1.16)* | |
| 2007 | 1.60 (1.50–1.72)* | 2.04 (1.94–2.15)* | 1.34 (1.30–1.38)* | 1.22 (1.20–1.24)* | |
| 2008 | 1.73 (1.62–1.85)* | 2.29 (2.17–2.41)* | 1.39 (1.35–1.43)* | 1.28 (1.25–1.30)* | |
| 2009 | 1.82 (1.70–1.94)* | 2.53 (2.40–2.66)* | 1.38 (1.34–1.42)* | 1.30 (1.27–1.33)* | |
| 2010 | 1.82 (1.68–1.96)* | 2.73 (2.57–2.89)* | 1.37 (1.32–1.42)* | 1.34 (1.31–1.37)* | |
| Insurance | |||||
| Commercial | Referent | Referent | Referent | Referent | |
| Medicare | 1.03 (1.01–1.05)* | 0.81 (0.79–0.82)* | 1.03 (1.03–1.04)* | 1.00 (0.99–1.00) | |
| Medicaid | 1.08 (1.04–1.12)* | 0.95 (0.93–0.98)* | 1.02 (1.00–1.05)* | 0.99 (0.98–1.00) | |
| Uninsured | 1.02 (0.98–1.07) | 0.91 (0.88–0.95)* | 1.00 (0.98–1.03) | 0.98 (0.96–1.00) | |
| Unknown | 1.04 (0.99–1.09) | 0.97 (0.94–1.01) | 1.03 (1.00–1.05)* | 1.01 (1.00–1.02)* | |
| Marital status | |||||
| Married | Referent | Referent | Referent | Referent | |
| Single | 1.00 (0.97–1.02) | 0.93 (0.92–0.95)* | 1.01 (0.99–1.02) | 1.00 (0.99–1.00) | |
| Unknown | 1.01 (0.99–1.03) | 0.98 (0.97–1.00)* | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | |
| Area of residence | |||||
| Metropolitan | Referent | Referent | Referent | Referent | |
| Non-metropolitan | 0.85 (0.69–1.03) | 0.89 (0.71–1.10) | 0.84 (0.63–1.12) | 0.63 (0.50–0.80)* | |
| Region | |||||
| Eastern | Referent | Referent | Referent | Referent | |
| Midwest | 1.79 (1.39–2.31)* | 1.35 (1.02–1.78)* | 1.36 (0.99–1.87) | 1.61 (1.20–2.16)* | |
| South | 2.09 (1.65–2.66)* | 1.51 (1.16–1.97)* | 1.59 (1.18–2.14)* | 2.28 (1.73–3.00)* | |
| West | 1.18 (0.88–1.58) | 0.88 (0.64–1.21) | 1.21 (0.84–1.73) | 1.60 (1.15–2.23)* | |
| Cancer | |||||
| No | Referent | Referent | Referent | Referent | |
| Yes | 0.87 (0.85–0.88)* | 1.42 (1.40–1.45)* | 1.11 (1.07–1.16)* | 1.02 (1.00–1.05)* | |
| Comorbidity | |||||
| 0 | Referent | Referent | Referent | Referent | |
| 1 | 1.17 (1.14–1.20)* | 1.30 (1.28–1.32)* | 1.04 (1.03–1.05)* | 1.00 (1.00–1.01) | |
| ≥2 | 1.43 (1.40–1.46)* | 1.24 (1.21–1.26)* | 1.08 (1.07–1.10)* | 1.01 (1.00–1.01)* | |
| Hospital type | |||||
| Non-teaching | Referent | Referent | Referent | Referent | |
| Teaching | 0.98 (0.81–1.19) | 1.15 (0.93–1.43) | 1.00 (0.80–1.26) | 0.93 (0.75–1.16) | |
| Hospital size | |||||
| <400 beds | Referent | Referent | Referent | Referent | |
| 400–600 beds | 1.21 (0.99–1.49) | 1.10 (0.88–1.39) | 1.11 (0.87–1.41) | 1.33 (1.04–1.68)* | |
| >600 beds | 1.16 (0.87–1.54) | 1.03 (0.74–1.42) | 1.17 (0.84–1.63) | 1.12 (0.80–1.57) | |
| Hospital volume | |||||
| Low | Referent | Referent | Referent | Referent | |
| Intermediate | 1.14 (1.11–1.18)* | 0.98 (0.95–1.01) | 1.03 (1.01–1.05)* | 1.12 (1.10–1.14)* | |
| High | 1.29 (1.24–1.34)* | 1.30 (1.26–1.35)* | 0.99 (0.97–1.02) | 1.05 (1.02–1.07)* | |
| Surgeon volume | |||||
| Low | Referent | Referent | Referent | Referent | |
| Intermediate | 0.86 (0.85–0.88)* | 1.09 (1.07–1.11)* | 0.95 (0.94–0.96)* | 1.03 (1.02–1.04)* | |
| High | 0.83 (0.82–0.85)* | 1.23 (1.21–1.26)* | 0.93 (0.92–0.94)* | 1.01 (1.00–1.01) | |
| Unknown | 0.92 (0.86–0.99)* | 1.08 (1.03–1.15)* | 0.91 (0.88–0.94)* | 1.05 (1.03–1.08)* | |
| Procedure | |||||
| Colectomy | Referent | Referent | Referent | - | |
| Thyroidectomy | 3.21(3.14–3.27)* | - | - | - | |
| Pancreatectomy | 3.84 (3.72–3.97)* | - | - | - | |
| Liver resection | 5.72 (5.53–5.92)* | - | - | - | |
| Gastrectomy | 2.11 (2.05–2.16)* | - | - | - | |
| Prostatectomy | - | Referent | - | - | |
| Hysterectomy | - | 2.67 (2.58–2.75)* | - | - | |
| Nephrectomy | - | 4.83 (4.72–4.94)* | - | - | |
| Coronary artery bypass graft | - | - | Referent | - | |
| Valvuloplasty | - | - | 1.13 (1.12–1.14)* | - | |
| Lung resection | - | - | 0.50 (0.48–0.52)* | - | |
| Hip arthroplasty | - | - | - | Referent | |
| Knee arthroplasty | - | - | - | 1.08 (1.06–1.10)* | |
| Spine surgery | - | - | - | 11.71 (11.55–11.87)* | |
P<0.05
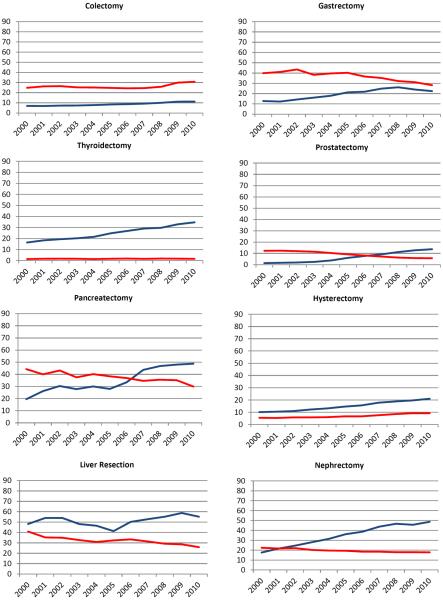
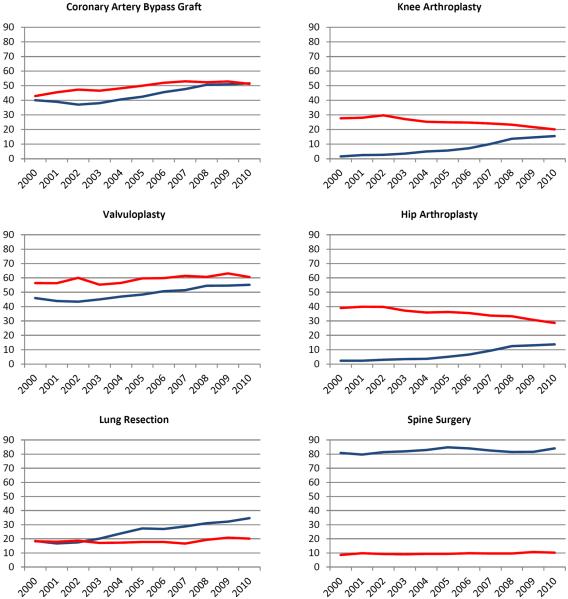
The use of hemostatic agents increased over time for all procedures (Figure 1). The greatest increase in use of hemostatic agents was noted for nephrectomy (31.1%) and pancreatectomy (29.3%). There was a moderate (10–25%) increase in use for thyroidectomy, prostatectomy, hysterectomy, CABG, lung resection, hip arthroplasty, and knee arthroplasty. Use increased by <10% for colectomy, liver resection, gastrectomy, valvuloplasty, and spine surgery. The trends in use from 2000 to 2010 for each procedure were: colectomy (7.0% to 11.3%), thyroidectomy (16.4% to 34.7%), pancreatectomy (19.5% to 48.8%), liver resection (48.2% to 55.2%), and gastrectomy (12.8% to 22.4%), prostatectomy (1.4% to 13.7%), hysterectomy (10.1% to 21.0%), nephrectomy (17.6% to 48.7%), CABG (40.1% to 51.6%), valvuloplasty (45.9% to 55.2%), lung resection (18.4% to 34.6%), hip arthroplasty (2.3% to 13.7%), knee arthroplasty (1.6% to 15.5%), and spine surgery (80.8% to 84.0%).
Figure 1.
Use of hemostatic agents (blue) and transfusion requirements (red) stratified by year of diagnosis for each procedure.
The trends in use of blood transfusion were highly variable. From 2000 to 2010, the rates of transfusion use declined for pancreatectomy (−14.4%), liver resection (−15.0%), gastrectomy (−11.7%), prostatectomy (−6.6%), nephrectomy (−4.6%), hip arthroplasty (−10.4%), and knee arthroplasty (−6.6%). Over the same time period, the transfusion rate increased for colectomy (6.0%), hysterectomy (3.7%), CABG (8.4%), valvuloplasty (4.2%), lung resection (1.9%), and spine surgery (1.6%). Transfusion remained relatively stable for thyroidectomy (0.2%).
Discussion
We noted a rapid increase in the use of hemostatic agents over time with more than a third of patients receiving one of these compounds by 2010. The increased use of hemostatic agents was noted for all of the procedures examined, including operations associated with a relatively small risk of transfusion and other bleeding related complications. In addition to patient characteristics, surgeon and hospital factors exerted substantial influence on the utilization of hemostatic agents.
Conducting high-quality studies to examine the efficacy of drugs and devices that minimize transfusion requirements has proven difficult. Prior data has suggested that cell salvage and anti-fibrinolytic agents provide worthwhile reductions in blood loss but other drugs, such as desmopression, and interventions, such as normovolemic hemodilution, are of limited utility.21–24 Data regarding hemostatic agents derives mainly from small trials.25–29 A 2009 meta-analysis reported that fibrin sealants reduced blood loss by an average of 161 mL per patient and were associated with a 32% reduction in the rate of allogeneic blood transfusion. These findings were most pronounced for orthopedic procedures in which blood loss is often substantial but were not clinically significant for other operations. Based on the quality of studies included the authors suggested that more methodologically rigorous randomized trials were needed.3
Although the number of commercially available hemostatic agents is increasing rapidly, relatively little is known about how these agents are currently being in used in clinical practice.30–35 A hospital-level survey conducted by the International Study of Peri-operative Transfusion (ISPOT) collaborative and reported in 2001 found that techniques such as cell salvage (82%) and preoperative autologous blood donation (83%) were used more frequently by hospitals than pharmacologic interventions such as aprotinin (61%), desmopression (52%), and epsilon-aminocaproic acid (50%).34 Similar trends were noted in a study of Canadian hospitals in 2000.32 These studies have consistently shown that interventions to reduce blood loss are most frequently used in cardiac procedures.32,33 Data specifically describing use of topical hemostats, sealants, and adhesives is largely lacking. It is likely that practice patterns have changed substantially over the last decade as a number of new hemostatic agents have become available and as safety concerns have arisen for other commonly used procoagulant drugs such as aprotinin.36,37
The difficulty of how to incorporate procoagulant and hemostatic drugs into surgical practice is well demonstrated by the case of recombinant factor VIIa (rFVIIa). Recombinant factor VIIa was approved in 1999 for the treatment of patients with hemophilia but is now commonly used for a variety of off-label indications. A study of rFVIIa noted that use of the agent increased 143-fold from 2000 to 2008 with an astonishing 96% of cases representing off-label use.35 Once approved, drugs are often used for other indications or in different dosing regimens and schedules.38–41 Off-label use is of concern not only because a patient may not derive benefit, but also because many drugs and products are associated with substantial toxicity and cost.38,41,42 Use of hemostatic agents is somewhat different in that many of these agents have been approved with a general indication for surgical hemostasis. Notably however, most clinical trials of hemostatic agents have focused on procedures associated with significant bleeding risks and it is doubtful that widespread use of these agents for low-risk procedures is cost-effective.
Regardless of the efficacy of hemostatic agents, their rapid uptake raises potential concerns regarding side effects and cost. While strong selection bias and unmeasured confounding factors make it difficult to determine the efficacy of these agents using observational data, we were able to correlate the patterns of hemostatic agent use with ecologic trends in transfusion. For orthopedic procedures, as well as several general surgical procedures including liver resection, gastrectomy, and pancreatectomy, the increased use over time of hemostatic agents was accompanied by decreased transfusion requirements. For some procedures, including hysterectomy, colectomy and nephrectomy transfusion rates increased over time in association with increased use of hemostatic agents. While a number of factors including the increased use of minimally invasive surgery, more stringent transfusion triggers, and changing patterns of surgical practice influence these trends, the widespread use of hemostatic agents raises concern. These concerns are highlighted by thyroidectomy where the annual rate of transfusion is <2% but use of hemostatic agents has more than doubled to nearly 35% over a decade.
Despite the inclusion of a large number of patients, we recognize a number of important limitations. We captured hemostatic agents using billing codes collected by hospitals and submitted to Perspective. Although this methodology has been validated in a number of outcomes studies, we cannot exclude the possibility that a small number of patients who received hemostatic agents were not captured.14,35,43 Secondly, a large number of hemostatic agents are now commercially available. As many of these agents include various combinations of fibrin and/or thrombin, separating the analysis into specific hemostatic agents would have been imprecise. We therefore analyzed a composite of any use of a hemostatic agent. A priori we chose to examine transfusion rates over time. While transfusion requirements are an important endpoint and a surrogate for hemostasis, we recognize that there are other potential benefits of hemostatic agent use, such as lower blood loss, earlier return to activity, and decreased wound complications, that we are unable to measure using administrative data. Finally, we recognize that a number of patient, procedure, and surgeon-specific factors and preferences that are impossible to measure may have contributed to the decision to use a hemostatic agent. As these unmeasured factors limit our ability to associate hemostat use with transfusion, we chose to provide a descriptive analysis of patterns of use and to report transfusion trends over time.
The major question raised by our findings is how to rationally utilize hemostatic agents in clinical practice. Although these agents have clearly shown efficacy, data from high-quality, randomized trials remain limited.3 While many agents are approved for surgical hemostasis, clearly the benefits and comparative effectiveness of these agents is highly procedure specific. As the use of hemostatic agents continues to increase, further comparative effectiveness studies to define specific procedures and clinical scenarios in which these agents are most beneficial are needed to maximize clinical outcomes and decrease costs.
Footnotes
Disclosure: The authors have no disclosures or conflicts of interest.
References
- 1.Karkouti K, Dattilo KM. Perioperative hemostasis and thrombosis. Can J Anaesth. 2006;53:1260–2. doi: 10.1007/BF03021588. [DOI] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453–62. doi: 10.1111/j.1537-2995.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- 3.Carless PA, Henry DA, Anthony DM. Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2003:CD004171. doi: 10.1002/14651858.CD004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasley PB, Lave JR, Hanusa BH, et al. Variation in the use of red blood cell transfusions. A study of four common medical and surgical conditions. Med Care. 1995;33:1145–60. [PubMed] [Google Scholar]
- 5.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 6.Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010:CD002042. doi: 10.1002/14651858.CD002042.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. Jama. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 8.Spotnitz WD, Burks S. State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clin Appl Thromb Hemost. 2010;16:497–514. doi: 10.1177/1076029610363589. [DOI] [PubMed] [Google Scholar]
- 9.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48:1502–16. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 10.Hong YM, Loughlin KR. The use of hemostatic agents and sealants in urology. J Urol. 2006;176:2367–74. doi: 10.1016/j.juro.2006.07.128. [DOI] [PubMed] [Google Scholar]
- 11.Carless PA, Anthony DM, Henry DA. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. Br J Surg. 2002;89:695–703. doi: 10.1046/j.1365-2168.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- 12.Lagu T, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Lindenauer PK. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171:292–9. doi: 10.1001/archinternmed.2011.12. [DOI] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 14.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. Jama. 2010;303:2359–67. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 15.Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama. 2013;309:689–98. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA internal medicine. 2013;173:559–68. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871–5. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–83. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carless PA, Henry DA, Moxey AJ, et al. Desmopressin for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2004:CD001884. doi: 10.1002/14651858.CD001884.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632–44. doi: 10.1111/j.1537-2995.2004.03353.x. [DOI] [PubMed] [Google Scholar]
- 23.Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010:CD001888. doi: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011:CD001886. doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Figueras J, Llado L, Miro M, et al. Application of fibrin glue sealant after hepatectomy does not seem justified: results of a randomized study in 300 patients. Ann Surg. 2007;245:536–42. doi: 10.1097/01.sla.0000245846.37046.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mawatari M, Higo T, Tsutsumi Y, Shigematsu M, Hotokebuchi T. Effectiveness of autologous fibrin tissue adhesive in reducing postoperative blood loss during total hip arthroplasty: a prospective randomised study of 100 cases. J Orthop Surg (Hong Kong) 2006;14:117–21. doi: 10.1177/230949900601400202. [DOI] [PubMed] [Google Scholar]
- 27.Taylor LM, Jr, Mueller-Velten G, Koslow A, Hunter G, Naslund T, Kline R. Prospective randomized multicenter trial of fibrin sealant versus thrombin-soaked gelatin sponge for suture- or needle-hole bleeding from polytetrafluoroethylene femoral artery grafts. J Vasc Surg. 2003;38:766–71. doi: 10.1016/s0741-5214(03)00474-9. [DOI] [PubMed] [Google Scholar]
- 28.Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg. 2003;75:1587–92. doi: 10.1016/s0003-4975(02)04994-9. [DOI] [PubMed] [Google Scholar]
- 29.Doria C, Fischer CP, Wood CG, Li PM, Marra S, Hart J. Phase 3, randomized, double-blind study of plasma-derived human thrombin versus bovine thrombin in achieving hemostasis in patients undergoing surgery. Curr Med Res Opin. 2008;24:785–94. doi: 10.1185/030079908X273426. [DOI] [PubMed] [Google Scholar]
- 30.Henry DA, Henderson KM, Fryer JL, Treloar CJ, McGrath KM, Deveridge SF. Use of interventions to minimise perioperative allogeneic blood transfusion in Australia. A survey by the International Study of Perioperative Transfusion (ISPOT) Study Group. Med J Aust. 2000;172:365–9. doi: 10.5694/j.1326-5377.2000.tb124007.x. [DOI] [PubMed] [Google Scholar]
- 31.Fergusson D, Blair A, Henry D, et al. Technologies to minimize blood transfusion in cardiac and orthopedic surgery. Results of a practice variation survey in nine countries. International Study of Peri-operative Transfusion (ISPOT) Investigators. Int J Technol Assess Health Care. 1999;15:717–28. [PubMed] [Google Scholar]
- 32.Graham ID, Fergusson D, McAuley L, Laupacis A. The use of technologies to minimize exposure to perioperative allogeneic blood transfusion in elective surgery. A survey of Canadian hospitals. Int J Technol Assess Health Care. 2000;16:228–41. doi: 10.1017/s0266462300161197. [DOI] [PubMed] [Google Scholar]
- 33.Katz E, Gaitini L, Samri M, Egoz N, Fergusson D, Laupacis A. The use of technologies to decrease peri-operative allogenic blood transfusion: results of practice variation in Israel. Isr Med Assoc J. 2001;3:809–12. [PubMed] [Google Scholar]
- 34.Hutchinson AB, Fergusson D, Graham ID, Laupacis A, Herrin J, Hillyer CD. Utilization of technologies to reduce allogeneic blood transfusion in the United States. Transfus Med. 2001;11:79–85. doi: 10.1046/j.1365-3148.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 35.Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–22. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw AD, Stafford-Smith M, White WD, et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784–93. doi: 10.1056/NEJMoa0707768. [DOI] [PubMed] [Google Scholar]
- 37.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 38.Avorn J, Kesselheim A. A hemorrhage of off-label use. Ann Intern Med. 2011;154:566–7. doi: 10.7326/0003-4819-154-8-201104190-00010. [DOI] [PubMed] [Google Scholar]
- 39.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021–6. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 40.Leveque D. Off-label use of anticancer drugs. Lancet Oncol. 2008;9:1102–7. doi: 10.1016/S1470-2045(08)70280-8. [DOI] [PubMed] [Google Scholar]
- 41.Wright JD, Neugut AI, Wilde ET, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–18. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–40. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Jama. 2010;303:2035–42. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]