Abstract
Objective
Real-life safety and efficacy of sorafenib in advanced renal cell carcinoma in a nationwide patient population were evaluated by post-marketing all-patient surveillance.
Methods
All patients with unresectable or metastatic renal cell carcinoma in Japan who started sorafenib therapy from February 2008 to September 2009 were registered and followed for up to 12 months. Baseline characteristics, treatment status, tumor response, survival and safety data were recorded by the prescribing physicians.
Results
Safety and efficacy were evaluated in 3255 and 3171 patients, respectively. The initial daily dose was 800 mg in 78.2% of patients. Median duration of treatment was 6.7 months and the mean relative dose intensity was 68.4%. Overall, 2227 patients (68.4%) discontinued the treatment by 12 months, half of which (52.0% of discontinued patients) were due to adverse events. The most common adverse drug reactions were hand–foot skin reaction (59%), hypertension (36%), rash (25%) and increase in lipase/amylase (23%). The median progression-free survival was 7.3 months (95% confidence intervals: 6.7–8.1), and the overall survival rate at 1 year was 75.4% (73.5–77.1). Prognostic factors for overall survival were mostly consistent with those in previous clinical trials in the univariate analysis and largely similar to those for progression-free survival and duration of treatment in the multivariate analysis.
Conclusions
Sorafenib for the treatment of advanced renal cell carcinoma under the labeled dose was feasible in daily medical practice, for its acceptable toxicity profile and favorable clinical benefit that were consistent with those in clinical trials.
Keywords: molecularly targeted therapy, post-marketing surveillance, renal cell carcinoma, sorafenib tosylate
Introduction
Renal cell carcinoma (RCC) accounts for ∼3% of adult malignancies. The majority (∼70%) shows clear-cell histology that is frequently associated with inactivation of the von Hippel–Lindau (VHL) tumor suppressor gene (1–6). Increased production of vascular endothelial growth factors (VEGFs), platelet-derived growth factors (PDGFs) and hypoxia-inducible proteins due to inactivation of the VHL gene are considered to be involved in tumor growth and neoangiogenesis of clear-cell RCC (7,8). Up to 30% of patients with RCC present with advanced metastatic disease, which is often refractory to chemotherapy and has a very poor prognosis (1,3,9). Cytokine regimens with interferon-α (IFN-α) and/or interleukin-2 (IL-2) have been used as first-line treatment for advanced metastatic RCC, but cytokine therapy shows modest success (response rate of 10–20%), and is associated with significant toxicity (10,11). Recent understanding of the molecular mechanism responsible for tumor initiation and progression has led to the development of molecularly targeted agents, which provide a useful option for the treatment of advanced refractory cancer.
Sorafenib tosylate (sorafenib) is an orally active multikinase inhibitor that blocks the VEGF receptor 2 and 3 kinases, PDGF receptor β kinase, Raf kinase (RAF-1), FMS-like tyrosine kinase 3 (Flt-3), c-Kit protein and RET receptor tyrosine kinases (12,13). In a Phase II, randomized discontinuation trial, sorafenib prolonged progression-free survival (PFS) as compared with placebo, in patients with metastatic RCC in whom previous treatment with sorafenib had resulted in stable disease with <25% changes in bi-dimensional tumor measurements (14). The Phase III multicenter Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET), a randomized, double-blind, placebo-controlled study demonstrated that treatment with sorafenib significantly prolonged PFS as compared with placebo, in patients with advanced clear-cell RCC who had failed previous therapy (15). The results of secondary analysis of the TARGET study censoring placebo-assigned patients who crossed over to sorafenib showed significant overall survival (OS) benefit of sorafenib (16).
Based on these clinical results, sorafenib was approved by the US Food and Drug Administration in 2005 and the European Medicines Agency in 2006 for the treatment of advanced RCC. Sorafenib has become the first tyrosine kinase inhibitor indicated for use in patients with advanced RCC. Subsequently, six other molecular targeted agents had been approved for the treatment of advanced RCC (17–22).
In Japan, interim analysis of a Japanese Phase II, open-label study demonstrated acceptable tolerability of sorafenib with evidence of disease control in patients with advanced metastatic RCC who had undergone nephrectomy and failed treatment with at least one cytokine-containing therapy (23). Based on the results of the Phase II trial and the global Phase III TARGET study, sorafenib received marketing approval with indications for unresectable or metastatic RCC in 2008. As a condition for approval, the Pharmaceuticals and Medical Devices Agency in Japan mandated the manufacturer to implement a specific drug-use investigation in the form of all-patient post-marketing surveillance (PMS) to confirm the safety and efficacy of sorafenib in patients in the clinical settings. Therefore, an all-patient PMS study was conducted by the company targeting all patients with unresectable or metastatic RCC who were treated with sorafenib. The primary objective of this PMS study was to evaluate the real-life safety and efficacy of sorafenib in a large patient population who received long-term treatment with sorafenib under daily practice conditions. Since this study covered all of the >3200 consecutive patients who were treated with sorafenib at all hospitals in Japan, the obtained results provide a non-biased whole picture of the treatment of advanced RCC under daily medical practice, which is potentially different from the results of clinical trials because it is a more heterogeneous population and the protocol is not as strict.
Patients and methods
Patients
All patients with histologically or cytologically confirmed unresectable or metastatic RCC in Japan who started sorafenib treatment between February 2008 and September 2009 were eligible for this PMS study. One hundred thirteen patients who were enrolled to compassionate-use program prior to the launch in April 2008 were also included. Eligible patients were enrolled through a central registration system. Completeness of the all-patient registration was ensured by mandating registration for delivery of the drug product to the pharmacy. Sorafenib was orally administered at the labeled dose/regimen (400 mg twice daily on a continuous basis). Dose modification (interruption/dose reduction) and discontinuation were performed at the physicians' discretion.
Study design
The study was conducted as a specific drug-use investigation (all-patient post-marketing surveillance) of sorafenib, as mandated by the Japanese health authorities. The primary objective of this PMS study was to evaluate and confirm real-life safety and efficacy of long-term treatment with sorafenib in a large patient population under daily practice conditions. The follow-up observation continued for 12 months after the start of treatment or up to 30 days after discontinuation. Case report forms (CRFs) were filled out by the prescribing physician, and were collected by the company. Patients' outcome beyond the individual survey period was reflected when obtained during the process of CRF finalization. Safety and efficacy evaluations were performed in patients whose final CRF was collected and data were locked. This study was conducted in accordance with the Good Post-marketing Surveillance Practice at 724 investigational sites in Japan from February 2008 through March 2011.
Investigation items
Major investigated items included baseline characteristics (patient demographic and clinical characteristics, primary disease, treatment history, laboratory values and concomitant drugs) at the start of treatment, and post-treatment data obtained at 1, 3, 6, 9 and 12 months after the start of treatment; the status (continued or discontinued) of treatment, tumor assessment the presence or absence of metastases, patient outcome, laboratory test results, concomitant drugs, adverse events (AEs) and adverse drug reactions (ADRs). Among the baseline characteristics, Memorial Sloan Kettering Cancer Center (MSKCC) risk for the all-treatment line (24) and for the pre-treatment setting (25), MSKCC (1999) and MSKCC (2004) hereinafter, respectively, had not been included in the CRF and were determined in a post hoc manner.
Safety evaluation
Safety data were obtained from the findings of clinical signs/symptoms, physical examinations, vital signs and laboratory test results during the individual survey period. Safety evaluation included all patients who received at least one dose of sorafenib (safety analysis set). AEs and ADRs were summarized based on the Medical dictionary for regulatory activities (MedDRA), version 15.0 terminology, and classified into serious and non-serious according to the seriousness criteria defined in International Conference on Harmonization Guideline E2A. The most common ADRs requiring particular attention, a priority item for investigation, were tabulated by combining similar ADRs together.
Efficacy evaluation
Efficacy valuables included objective response rate [defined as the proportion of patients with complete response (CR) and partial response (PR), disease control rate (DCR) (the proportion of patients who had a best response rate of CR, PR or no change (NC)], OS (the time from initiation of treatment to death), PFS (the time from initiation of treatment to the first date when disease progression was objectively documented or death), time to response and duration of treatment (DOT).
Tumor response was assessed based on daily medical practice (i.e. the schedule was not defined in the protocol). CRFs were collected at 1, 3, 6, 9 and 12 months after the start of sorafenib therapy. In each CRF, the result of single assessment (assumed to be the latest available data in most cases) before the CRF collection was recorded with the observation date. Tumor response was recorded according to the criteria for non-invasive evaluation of therapeutic effectiveness in the Japanese Urological Association's General Rules for Clinical and Pathological Studies of Renal Cell Carcinoma, Third Edition [Japanese Urological Association (JUA) criteria], where tumor response was evaluated based on changes in either uni- or bi-dimensional tumor measurements or both (26).
Statistical analyses
After collection of CRF and subsequent inquiry by the pharmaceutical company, CRF were sent to an external data center and were input into a database. Resultant clean database was sent to another external contract research organization, where statistical analyses (except multivariate analysis) were conducted based on the statistical analysis plan provided by the pharmaceutical company. Multivariate analyses were conducted internally by a statistician in the pharmaceutical company. Interpretation of the analyses was supervised by the Proper Use Advisory Committee of Nexavar.
For efficacy valuables, point estimates and their 95% confidence intervals (CIs) were calculated. Survival analyses were performed using the Kaplan–Meier method. Univariate analyses were performed using Cox proportional hazards regression models to identify the prognostic factors for OS, PFS or DOT. In multivariate analysis, all baseline variables used in univariate analysis were evaluated irrespective of their significance, except for variables (A) that included >10% missing data, (B) that were unbalanced with >95% data at 1 level and (C) that were of redundant hierarchy, which were removed to avoid loss of comprehensiveness due to decrease of analyzed data. Hazard ratio (HR) was calculated for the variables adopted in the model optimized after stepwise selection.
To assess the relationship between ADRs and survival outcome, landmark analysis (27) was conducted to minimize the confounding bias (guarantee-time bias; longer survival gives a greater chance of ADRs). Patients who survived longer than 30 days after the start of treatment were stratified by the presence or absence of specified ADRs (ADRs or ADR groups with ≥5% incidence) on Day 30, and the survival thereafter were compared by Kaplan–Meier analysis. Statistical significance was tested by the log-rank test and generalized Wilcoxon test. For all statistical analyses, SAS version 9.1 or higher (SAS Institute Inc.) was utilized.
Results
Study population
Out of 3422 patients registered during the enrolment period, CRFs of 3335 patients were collected and finalized. The safety analysis set included 3255 patients, after excluding 80 for the following reasons: 4 failed to return to the hospital, 11 to whom sorafenib was not administered, 2 duplicate registrations and 63 whose records before and after hospital change were integrated. The efficacy analysis set consisted of 3171 patients, after excluding 84: of these 39 did not apply to the indication of sorafenib, 29 had previous sorafenib treatment and 16 had concomitant use with similarly acting drugs (e.g. sunitinib).
Table 1 summarizes the baseline characteristics of patients in the safety analysis set. Most of the patients had Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 (95.0%); unresectable or metastatic RCC (98.8%); Stage IV (97.7%) rated based on the TNM classification. Among 97.0% of all metastases, the major involved organs were the lungs (70.9%), including 25.9% of lungs only, bone (31.1%), liver (15.2%) and other sites (43.3%), including the lymph nodes. Prior surgery (83.4%) and prior systemic anticancer therapy (79.4%) were common, the latter of which consisted mostly of cytokine therapy with IFN-α and/or IL-2 (75.9%) and less of sunitinib malate (3.8%). Most patients had clear-cell type RCC (69.2%), and MSKCC 1999 and 2004 intermediate risk scores of 56.4 and 51.6%, respectively.
Table 1.
Baseline demographic and disease characteristics in patients
| Safety analysis set (n = 3255) |
||
|---|---|---|
| n | % | |
| Gender | ||
| Male/female | 2450/805 | 75.3/24.7 |
| Age (years) | ||
| Median | 67 | |
| <65/≥65 to <75/≥75 | 1399/1139/703 | 43.0/35.0/21.6 |
| Weight (kg) | ||
| Median | 58.5 (male 60.9, female 50.0) | |
| ECOG PS | ||
| 0/1/≥2 | 2093/1000/162 | 64.3/30.7/5.0 |
| Stage (TNM classification) | ||
| I–III/IV | 71/3180 | 2.1/97.7 |
| Prior surgery | ||
| Yes/no | 2716/539 | 83.4/16.6 |
| Time from surgery (years) | ||
| Median | 2.6 | |
| <1/≥1 to <5/≥5 | 697/1139/821 | 21.4/35.0/25.2 |
| Prior systemic anticancer therapya | ||
| Any | 2584 | 79.4 |
| Prior cytokine therapy | 2472 | 75.9 |
| IFN-α | 2419 | 74.3 |
| IL-2 | 886 | 27.2 |
| Sunitinib malate | 125 | 3.8 |
| Others | 332 | 10.2 |
| Primary diseasea | ||
| Unresectable/metastatic RCC | 3216 | 98.8 |
| Others | 47 | 1.4 |
| Subtype | ||
| Clear-cell carcinoma only | 2254 | 69.2 |
| Including non-clear-cell RCC | 442 | 13.6 |
| Metastatic sitea | ||
| Any | 3158 | 97.0 |
| Bone | 1013 | 31.1 |
| Brain | 169 | 5.2 |
| Liver | 496 | 15.2 |
| Lung/lung only | 2308/842 | 70.9/25.9 |
| Kidney | 237 | 7.3 |
| Others (including lymph nodes) | 1410 | 43.3 |
| CRP (mg/dl) | ||
| Median | 0.08 | |
| <0.1/≥0.1 to <0.3/≥0.3 | 1294/452/694 | 39.8/13.9/21.3 |
| MSKCC risk (1999)b | ||
| Favorable/intermediate/poor | 542/1836/179 | 16.7/56.4/5.5 |
| MSKCC risk (2004)c | ||
| Favorable/intermediate/poor | 585/1333/206 | 22.6/51.6/8.0 |
| Comorbiditya | ||
| Cardiac, yes/no | 548/2662 | 16.8/81.8 |
| Hepatic, yes/no | 152/3058 | 4.7/94.0 |
| Pulmonary, yes/no | 113/3097 | 3.5/95.2 |
| Renal, yes/no | 239/2971 | 7.3/91.3 |
| Starting daily dose | ||
| 800 mg/<800 mg | 2547/708 | 78.2/21.8 |
| Concomitant use of cytokines | ||
| Yes/no | 133/3122 | 4.1/95.9 |
ECOG PS, Eastern Cooperative Oncology Group performance status; IFN-α, interferon-alfa; IL-2, interleukin-2; RCC, renal cell carcinoma; CRP, C-reactive protein; MSKCC, Memorial Sloan Kettering Cancer Center.
aIncluding multiple choices.
bPatients with any line of therapy.
cPatients with prior systemic therapy.
The study included patients with ECOG PS of 2 or greater (5%), those with Stages I–III (2.1%), those without prior surgery (16.6%) and those receiving sorafenib as the first-line systemic therapy (20.6%). Meanwhile, in the Japanese Phase II study, all patients had ECOG PS of 0 or 1 and Stage IV malignancy, and received sorafenib as second-line therapy, and 86.3% of patients had clear-cell type carcinoma (23,28).
In this survey, the starting dose was 800 mg/day in 78.2% of patients, and concomitant use of cytokine was limited (4.1%).
Treatment status and drug exposure
Table 2 summarizes the status of treatment and drug exposure during the treatment period. While 1028 patients (31.6%) were still continuing the treatment at the end of the 12-month survey, 2227 patients (68.4%) had discontinued the treatment due to AEs (52.0% of discontinued patients), ineffectiveness (31.3%) and others or a combination of reasons (16.7%). The reasons shifted from AEs in the earlier period to ineffectiveness in the later period. For patients who discontinued due to AEs, major AEs during the 30 days before or after discontinuation were palmar-plantar erythrodysesthesia syndrome (hand–foot skin reaction), renal cell carcinoma (progression of primary disease) and abnormal hepatic function.
Table 2.
Status of treatment continuation/discontinuation and drug exposure (safety analysis set)
| Treatment period | Entire period | Safety analysis set (n = 3255) |
||||
|---|---|---|---|---|---|---|
| Months | ||||||
| N, (%)a, [%]b, {%}c | First | Second–third | Fourth–sixth | Seventh–ninth | Tenth–twelfth | |
| Status of treatment | ||||||
| Continuation | 1028 (31.6) | 2691 (82.7) | 2176 (66.9) | 1695 (52.1) | 1352 (41.5) | 1028 (31.6) |
| Discontinuation | 2227 (68.4) | 564 (17.3) [17.3] | 515 (15.8) [19.1] | 481 (14.8) [22.1] | 343 (10.5) [20.2] | 324 (10.0) [24.0] |
| Reason for discontinuation | ||||||
| Adverse events | 1158 (35.6) {52.0} | 466 [14.3] | 310 [11.5] | 186 [8.5] | 94 [5.5] | 102 [7.5] |
| Ineffectiveness | 698 (21.4) {31.3} | 25 [0.8] | 113 [4.2] | 204 [9.4] | 183 [10.8] | 173 [12.8] |
| Other reasons or combination | 371 (11.4) {16.7} | 73 [2.2] | 92 [3.4] | 91 [4.2] | 66 [3.9] | 49 [3.6] |
| Relative dose intensity (%)d | 68.4 | 76.4 | 63.3 | 62.4 | 62.1 | 62.0 |
| Entire period | ||||||
| Dose modification | ||||||
| Dose interruption | 1356 (41.7) | |||||
| Dose reduction | 975 (30.0) | |||||
| Interruption/reduction | 1918 (58.9) | |||||
| Median duration of treatment | 6.7 (95% CI: 6.2–7.0) | |||||
Days of interruption were included in the denominator, but days after discontinuation were not.
a(%), Percent of starting 3255 patients.
b[%], Percent of patients who completed the previous period.
c{%}, Percent of discontinuations.
dRelative dose intensity = actual total dose during the period (mg)/hypothetical total dose (800 mg × days of treatment).
Relative dose intensity (RDI), the percent of the actual total dose during the period relative to the hypothetical total dose (800 mg × days from the start to the end of treatment), was 76.4% during the first month, declined to 63.3% during the next 2 months, and remained at a similar level through 12 months, resulting in overall RDI of 68.4%. Dose interruption or reduction occurred in 1918 patients (58.9%). The incidence of dose interruption/reduction was higher in patients continuing treatment for 12 months than in those who discontinued earlier (76.2 vs. 51.0%). The median DOT was 6.7 months (95% CI: 6.2–7.0).
Safety
Of the 3255 patients in the safety analysis set, 3028 patients (93.0%) experienced at least one ADR. Table 3 summarizes the most common ADRs (single preferred term in MedDRA) or grouped ADRs (similar preferred terms were combined for the ADRs of special interest) occurring in 5% or more of patients. Corresponding data of the Japanese Phase II study, which were recoded from common terminology criteria for adverse events (CTCAE) into MedDRA, are shown for comparison. The most common ADRs or grouped ADRs were hand–foot skin reaction (HFSR) (59%), hypertension (36%), rash (25%), increase in lipase/amylase (23%) and diarrhea (21%). Serious ADRs occurring in 5% or more of patients were rash, liver dysfunction, hemorrhagic events and HFSR.
Table 3.
Most common ADRs (safety analysis set)
| PMS study (N = 3255) |
Japanese Phase II study (N = 131) |
||||
|---|---|---|---|---|---|
| Overall ADRs | Serious ADRs | Time to reach to 80% of the final incidencea (days) | Overall ADRs | Serious ADRs | |
| Hand–foot skin reactionb | 59% | 5% | 44 | 41% | |
| Hypertensionb | 36% | 2% | 68 | 27% | |
| Rashb | 25% | 7% | 23 | 44% | 1% |
| Increase in lipase/amylaseb | 23% | 1% | 45 | 60% | 2% |
| Diarrhea | 21% | 1% | 204 | 34% | 1% |
| Alopeciab | 18% | 0% | 99 | 39% | |
| Liver dysfunctionb | 17% | 7% | 78 | 17% | 2% |
| Cytopeniab | 12% | 4% | 160 | 10% | 2% |
| Hemorrhagic eventsb | 9% | 5% | 211 | 5% | |
| Decreased appetite | 8% | 1% | 161 | 14% | 2% |
| Stomatitis | 8% | 0% | 95 | 6% | |
| Hypophosphatemiab | 8% | 0% | 134 | 2% | |
| Malaise | 7% | 1% | 202 | 6% | |
| Dysphonia | 7% | 0% | 69 | 12% | |
| Pyrexia | 5% | 2% | 34 | 5% | |
Values represent number (%) of patients.
PMS, post-marketing surveillance; ADR, adverse drug reaction; MedDRA, medical dictionary for regulatory activities.
aTime to reach to the incidence corresponding to 80% of the final incidence at Day 365.
bIncluding multiple preferred terms in MedDRA (version 15.0) that correspond to ADR of special interest.
To describe the temporal tendency of the first onset of an ADR, the time to reach 80% of the incidence at Day 365 by Kaplan–Meier method was also shown. Most early-onset ADRs included rash (23 days), pyrexia (34 days), HFSR (44 days) and increase in lipase/amylase (45 days), while those with slow-onset included hemorrhagic events (211 days), diarrhea (204 days) and malaise (202 days).
In total, 110 patients (3.4%) died as a result of ADRs (152 events). The most common cause of death was RCC (progression of the primary disease) in 29 cases, followed by hemorrhagic events in 17 cases (8 intracranial, 6 gastrointestinal, 2 respiratory and 1 tumor hemorrhage), liver dysfunction in 9 cases, cardiac failure in 7 cases and gastrointestinal perforation, interstitial lung disease, renal dysfunction and pneumonia in 5 cases each.
We conducted multivariate analysis to identify the risk factors for the occurrence of serious ADRs of interest. Among serious AEs with an incidence of ≥5%, HFSR was chosen as the most common reason for discontinuation, as well as liver dysfunction and hemorrhagic events, which were potentially life-threatening. To minimize the potential effect of prognostic factors on the incidence of ADRs through modulation of the DOT, Cox proportional hazard model was utilized to detect factors affecting the time to first occurrence of serious HFSR, liver dysfunction and hemorrhagic ADRs (Table 4). Factors affecting risk by >2-fold included a starting dose of <800 mg for decreased risk of serious HFSR (HR = 0.28, 95% CI: 0.14–0.55) and serious hemorrhagic events (HR = 0.50, 95% CI: 0.29–0.85), ECOG PS ≥ 2 for increased risk of serious hemorrhagic events (HR = 2.84, 95% CI: 1.49–5.43) and renal comorbidity for increased risk of hemorrhagic events (HR = 2.01, 95% CI: 1.19–3.38).
Table 4.
Factors that altered the time to first occurrence of serious ADRs of interest
| ADR | Factor | HR | 95% CI | P value |
|---|---|---|---|---|
| Serious hand–foot skin reaction | Initial daily dose | |||
| 800 mg | 1 | |||
| <800 mg | 0.28 | 0.14–0.55 | 0.0002 | |
| Sex | ||||
| Male | 1 | |||
| Female | 1.63 | 1.14–2.33 | 0.0075 | |
| Age | ||||
| <65 | 1 | |||
| ≥65, <75 | 0.67 | 0.46–0.97 | 0.0343 | |
| Body weight | ||||
| <Median | 1 | |||
| ≥Median | 0.66 | 0.47–0.93 | 0.0161 | |
| ECOG PS | ||||
| 0 | 1 | |||
| 1 | 0.58 | 0.37–0.89 | 0.0137 | |
| Bone metastasis | ||||
| No | 1 | |||
| Yes | 0.55 | 0.36–0.85 | 0.0066 | |
| Serious hepatic dysfunction | Initial daily dose | |||
| 800 mg | 1 | |||
| <800 mg | 0.50 | 0.29–0.85 | 0.01 | |
| Hemoglobin | ||||
| <Median | 1 | |||
| ≥Median | 1.81 | 1.28–2.54 | 0.0007 | |
| Serious hemorrhagic events | ECOG PS | |||
| 0 | 1 | |||
| 1 | 1.85 | 1.26–2.71 | 0.0018 | |
| ≥2 | 2.84 | 1.49–5.43 | 0.0016 | |
| Hemoglobin | ||||
| <Median | 1 | |||
| ≥Median | 0.62 | 0.42–0.91 | 0.0159 | |
| Renal comorbidity | ||||
| No | 1 | |||
| Yes | 2.01 | 1.19–3.38 | 0.0090 | |
| Prior surgery | ||||
| No | 1 | |||
| Yes | 0.62 | 0.41–0.96 | 0.032 | |
The following factors were used for multivariate analysis: initial daily dose, sex, age (<65, ≥65 to <75, ≥75), body weight (cut-off, male/female 60.9/50.0 kg), ECOG PS, metastatic status (lung only vs. others), metastases in bone, brain, liver, lung, kidney and others, ALT (17.0 IU/l), AST (20.5 IU/l), platelets (216 000/μl), creatinine (1.04 mg/dl), hemoglobin (11.6 g/dl), cardiac, hepatic, pulmonary and renal comorbidity, allergy, prior surgery and prior IFN-α.
CI, confidence interval.
Tumor response
Table 5 summarizes the best tumor response evaluated according to the JUA criteria, as well as those in the Japanese Phase II study as a reference. It should be noted that the former was evaluated based on changes in either uni- or bi-dimensional tumor measurements or both, and cannot be fairly compared with the latter which was based on the Response Evaluation Criteria in Solid Tumors (RECIST). Overall, 1.4% achieved CR; 24.1% had PR; 52.6% had NC; and 13.5% had disease progression. The objective response rate and DCR were 25.4 and 78.1%, respectively, while these were 19.4 and 73.6% based on RECIST in the Japanese Phase II study. Response varied depending on the metastatic organ; the objective response rate was highest for lung lesions (31.2%), but lower in bone (11.4%) and brain (12.7%); in terms of disease control rate, bone disease (76.7%) was comparable to the overall results (78.1%), whereas brain disease (57.6%) was the poorest. When stratified by the treatment line, tumor response in first-line patients (CR/PR/NC/progressive disease [PD] = 0.9/19.9/49.9/17.4%, respectively) was slightly worse than that in ≥second-line patients (CR/PR/NC/PD = 1.4/25.2/53.3/12.5%, respectively). The median time to response and the median duration of response were 1.9 and 5.8 months, respectively, whereas those in the Japanese Phase II study were 2.8 and 13.8 months, respectively.
Table 5.
Best response (efficacy analysis set)
| Study | PMS studya (JUA criteria) |
Japanese Phase II studyb (RECIST) | |||||
|---|---|---|---|---|---|---|---|
| Response | Overall | Metastatic organc |
Overall | ||||
| Lung | Kidneyd | Bone | Liver | Brain | |||
| n = 3171 | n = 2260 | n = 1021 | n = 988 | n = 477 | n = 165 | n = 129 | |
| CR | 43 (1.4) | 83 (3.7) | 10 (1.0) | 12 (1.2) | 3 (0.6) | 2 (1.2) | 0 (0) |
| PR | 764 (24.1) | 622 (27.5) | 151 (14.8) | 101 (10.2) | 79 (16.6) | 19 (11.5) | 25 (19.4) |
| NC/SD | 1669 (52.6) | 1198 (53.0) | 784 (76.8) | 645 (65.3) | 245 (51.4) | 74 (44.8) | 87 (67.4) |
| Disease progression | 429 (13.5) | 169 (7.5) | 76 (7.4) | 113 (11.4) | 87 (18.2) | 22 (13.3) | 13 (10.1) |
| Not evaluable | 266 (8.4) | 180 (8.3) | 0 (0) | 117 (11.8) | 63 (13.2) | 48 (29.1) | 4 (3.1) |
| Objective response | 807 (25.4) | 705 (31.2) | 161 (15.8) | 113 (11.4) | 82 (17.2) | 21 (12.7) | 25 (19.4) |
| Overall disease control | 2476 (78.1) | 1903 (84.2) | 945 (92.6) | 758 (76.7) | 327 (68.6) | 95 (57.6) | 95 (73.6) |
| Median time to response | 1.9 months | Not available | 2.8 months | ||||
| Median duration of response | 5.8 months | 13.8 months | |||||
Values represent number (%) of patients.
PMS, post-marketing surveillance; JUA, Japanese Urological Association; RECIST, response evaluation criteria in solid tumor; CR, complete response; PR, partial response; NC, no change; SD, stable disease.
aThe best overall response according to the Criteria for Non-invasive Evaluation of Therapeutic Effectiveness in the Japanese Urological Association's General Rules for Clinical and Pathological Studies of Renal Cell Carcinoma.
bThe best overall response according to RECIST.
cResponse of metastatic disease in each organ among the patients who had metastases in the organ at baseline, except for the response in kidney disease which was differently defined below (d).
dResponse of kidney disease was compiled among all patients who had tumor evaluation data for kidney disease regardless of primary or metastatic lesion.
Survival outcome and prognostic factors
The median PFS was 7.3 months (95% CI: 6.7–8.1) and median OS was not reached. PFS and OS rates at 1 year were 34.0% (95% CI: 32.1–35.8) and 75.4% (73.5–77.1), respectively. In first-line patients, median PFS and OS rates at 1 year were 6.0 months (5.7–6.5) and 63.9% (59.2–68.3), respectively, compared with 8.1 months (7.2–8.7) and 78.1% (76.1–79.9) in ≥second-line patients. As a reference, corresponding data of the Japanese Phase II study (23,28) were as follows: median PFS, 7.9 months (95%CI 6.4–10.8); PFS rate at 1 year, 38.2% (29.7–46.6); median OS, 25.3 months (19.0–32.0); OS rate at 1 year, 69.9% (61.9–78.0).
In univariate analysis to identify baseline factors affecting clinical outcome, adverse prognostic factors for OS included MSKCC risk (intermediate, poor), ECOG PS (≥2,1), C-reactive protein (CRP) (≥0.1 mg/dl), number of metastatic organs, metastasis in non-lung organs, liver, bone, brain and others, platelet count (≥median value), pulmonary comorbidity and the presence of non-clear-cell histology, whereas favorable prognostic factors included albumin ≥3.8 g/dl (median), prior surgery, hemoglobin ≥11.6 g/dl, time from surgery ≥1 year, prior systemic therapy with IFN-α, any prior systemic therapy, body weight ≥60.9/50.0 kg (median of male/female), kidney metastases, total bilirubin ≥0.5 mg/dl and creatinine ≥1.04 mg/dl (Supplementary data, Table S1).
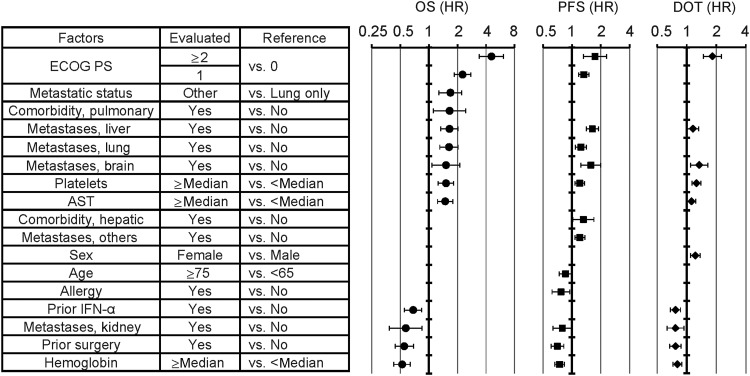
Among the 13 factors that were significant in the univariate analysis and were included in the multivariate analysis, 4 factors (body weight, metastases in bone and in other organs and creatinine) lost significance in the multivariate analysis. In addition, two other factors (lung metastases and aspartate aminotransferase [AST]) newly became significant in the multivariate analysis. However, several factors that had been highly prognostic in the univariate analysis, such as MSKCC risk, CRP, albumin, time from surgery and bilirubin, were not included in the multivariate analysis due to missing data (>10%). The results of the multivariate analyses of OS, PFS and DOT are shown in parallel in Fig. 1, which showed a generally similar trend for the three outcomes.
Figure 1.
Multivariate analysis of factors affecting overall survival, progression-free survival and duration of treatment. Factors were sorted by HR (in univariate analysis) for OS. Error bars indicate lower and upper confidence intervals, and symbols indicate point estimates. Only the values in the model optimized by stepwise method are shown because values do not exist for non-significant factors. Factors that were used in the multivariate analysis, but were not significant for any parameters were not shown for clarity. They include body weight, bone metastasis, ALT, creatinine, renal comorbidity, cardiac comorbidity and starting dose. The median value was used as the cutoff value for laboratory test values. ECOG PS, Eastern Cooperative Oncology Group performance status; AST, aspartate aminotransferase; IFN-α, interferon-alfa; OS, overall survival; HR, hazard ratio; PFS, progression-free survival; DOT, duration of treatment; ALT, alanine aminotransferase.
ADR and efficacy
To elucidate the association between ADRs and OS, exploratory landmark analyses (27) were performed in which patients were grouped based on the presence or absence of specified ADRs within 30 days, and their survival thereafter was analyzed. In these analyses, 3038 patients with an OS period of ≥30 days (96% of 3171 in the efficacy set) were included, and ADRs or grouped ADRs with an incidence of ≥5% were chosen for the analysis. OS was statistically longer in patients who were presented with any of the following five ADRs within 30 days; HFSR (1 year OS with and without ADRs, 84.3 vs. 69.9%, n = 1401 and 1637, P <0.0001 by the log-rank test), hypertension (83.4 vs. 74.2%, n = 839 and 2199, P < 0.0001), increase in lipase/amylase (84.2 vs. 75.1%, n = 553 and 2485, P < 0.0001), dysphonia (90.5 vs. 76.1%, n = 144 and 2894, P = 0.0001) and alopecia (85.2 vs. 76.2%, n = 218 and 2820, P = 0.0015). When categorized by the total numbers of the five ADRs (0, 1 and ≥2, n = 1004, 1181 and 853), patients who experienced more ADRs within 30 days showed longer OS (Supplementary data, Fig. S2). A similar association was also observed with regard to PFS and six ADRs, i.e. the five associated with OS plus diarrhea; HFSR (median PFS with and without ADR, 9.0 vs. 6.5 months, P < 0.0001 by the log-rank test), hypertension (8.9 vs. 7.1, P = 0.0006), increase in lipase/amylase (9.0 vs. 7.2, P = 0.0003), diarrhea (9.5 vs. 7.4, n = 195 and 2843, P = 0.0034), dysphonia (12.2 vs. 7.4, P = 0.0002) and alopecia (9.2 vs. 7.4, P = 0.014). In contrast, OS was shorter in patients who showed either of the two ADRs within 30 days; hypophosphatemia ≥Grade 2 (1 year OS with or without ADRs, 75.6 vs. 81.4%, n = 505 and 599, P = 0.0374 by the log-rank test, among 1104 patients whose phosphate data were available and with a baseline value of ≤G1), and hemorrhagic events (68.8 vs. 77.1, n = 86 vs. 2952, P = 0.0602 by the log-rank test and P = 0.0228 by a generalized Wilcoxon test). Neither hypophosphatemia (8.7 vs. 7.0, P = 0.4070 by the log-rank test) nor hemorrhagic events (7.8 vs. 7.7, P = 0.8969) were associated with a shorter PFS. PFS was statistically shorter only in patients who were presented with anorexia (5.8 vs. 7.9, n = 103 and 2935, P = 0.0269).
Discussion
Sorafenib is the first commercially available tyrosine kinase inhibitor for the treatment of unresectable/metastatic RCC in Japan. Since domestic experience with its use had been limited (131 patients in the Phase II study), all-patient post-marketing surveillance was requested as a condition for its approval by the health authorities to accumulate safety and efficacy data. It is noteworthy that the number of patients for this survey amounted to as many as >3200, and even more importantly, that they were consecutively collected from all hospitals in Japan where patients were treated with this drug. The results of a clinical study may not always predict the outcome in the real-world setting, because of such limitations as strict eligibility criteria and bias based on the selected study centers. In this respect, this PMS provides good nationwide real-world data under daily medical practice, and is quite valuable.
The difference in the patient profile between this survey and the Japanese Phase II study reflects the strict eligibility in the latter; this survey consisted of a higher percentage of elderly patients (i.e. ≥75 years old; 21.6% in this survey vs. 8.4% in the Japanese Phase II study, Akaza et al., unpublished data), those with worse PS (i.e. PS ≥ 1; 35.7 vs. 22.1%), and of less MSKCC favorable risk patients (16.7 vs. 40.5%). Patients without prior surgery (16.7%) and without prior systemic therapy (20.6%) were only included in this surveillance.
Adherence to the treatment in this survey was moderately lower than in the clinical study. In 22% of patients, treatment was started with reduced dose (mostly 400 mg, 19%) at the discretion of treating physicians, which is common in daily medical practice. At the end of the 12-month survey, 31.6% of patients had been continuing the treatment. Half of the permanent discontinuations (35.6% of the starting patients) were due to AEs, a ratio was higher than in the Phase II study (22.1% of the starting patients). Meanwhile, the frequency of dose interruptions (PMS, 41.7% vs. Phase II, 42%) and dose reduction (30.0 vs. 34%) were similar between the two studies. The median DOT (6.7 months) and average RDI (68.4%) were moderately worse than in the clinical study (7.7 months and 86.4%, respectively). Nevertheless, drug exposure was relatively well maintained under daily medical practice despite the poorer patient background.
In general, the overall ADR profiles of sorafenib were consistent with those observed in previous clinical trials. ADRs occurred in 93.0% of patients; the most common ADRs were HFSR, hypertension, rash, increase in lipase/amylase and diarrhea. Except for diarrhea, ADRs with a higher incidence tended to occur in the early stage of the treatment, taking 23–68 days to reach 80% of the final incidence. It is in line with the temporal change in the reason for discontinuations (AEs in the earlier period and PD in the later period), and a drop in the RDI between the first month and the second—third months. These findings support the importance of careful monitoring of ADRs and immediate or proactive management, especially in the earlier period. However, ADRs with a lower incidence still included those with an early onset such as dysphonia and pyrexia. Some of the major ADRs had a very different incidence than in the Japanese Phase II study. Increase in lipase/amylase had a lower incidence in this survey. Since the increase in the pancreatic enzymes was transient with peak plasma levels at 1 week after the start of treatment (29), the lower incidence in this survey may be due to its short time window. On the contrary, HFSR was higher in this survey. Actually, the incidence of HFSR in the Phase II study had been 58% when based on CTCAE (version 3.0) (28). It is possible that re-coding into MedDRA had artificially underestimated the incidence of HFSR.
Special attention should be paid to some ADRs that could become serious, such as rash, liver dysfunction, hemorrhagic events and HFSR. Regarding serious HFSR, the trend for a higher incidence in females and ECOG PS 0 patients, which had been suggested in the Japanese Phase II study (30), was confirmed in the present study. The lower incidence in elderly patients and those with bone metastases may be explained by less physical activity, as in patients with a worse PS. As for hemorrhagic events, risk factors in the present analysis were mostly related to poor prognosis (poor PS, low hemoglobin, no prior surgery), although their clinical relevance is uncertain. Renal comorbidity could predispose patients to bleeding independent of sorafenib, because it has been reported that even mild levels of renal impairment are associated with increased risk of post-operative bleeding after coronary artery bypass surgery (31). Baseline factors such as AST and alanine aminotransferase (ALT), expected to be involved in hepatic dysfunction, and platelets, expected to be involved in hemorrhagic events, were not included in the risk factors, whereas a median value was adopted to dichotomize the cut-off. It should be noted that attention should continually be paid to these two potentially life-threatening ADRs during the entire treatment period, because their incidence was not limited to the earlier period after the start of treatment. In addition, ADRs with a low overall incidence, such as cardiac failure, gastrointestinal perforation and interstitial lung disease (ILD) can be fatal. Not having been reported in the literature before launch in Japan, ILD was newly added to the Japanese package insert and the results of detailed analysis were reported elsewhere (32).
The overall efficacy results in this survey were generally comparable to those observed in previous clinical trials. The objective response rate and DCR according to JUA criteria were 25.4 and 78.1%, respectively, in this surveillance. Although it is difficult to directly compare these with the efficacy results of clinical trials evaluated through RECIST, it is likely that the efficacy of sorafenib is also maintained in a real-world setting. Additionally, the organ-specific objective response was the highest when limited to lung metastases. Median duration of response in this survey was 5.8 months, which was shorter than that in the Phase II study (13.8 months). A shorter observation period (up to 12 months) in the former may partly explain the difference.
As for survival, median PFS of 7.3 months was within the same range among 7.9 months of the Japanese Phase II study, 8.3 months (36 weeks) and 6.6 months of the expanded access programs in North America (NA-ARCCS) (33) and in Europe (EU-ARCCS) (34), although it was longer than 5.5 months in the TARGET trials. In addition, 1 year OS rate of 75.4% in the present study was numerically higher than the Phase II results (69.9%), which included more favorable patients (e.g. ECOG PS, prior surgery, clear-cell histology and MSKCC risk). Taken together, consistent efficacy in the real-world setting is also suggested in terms of survival. Moreover, when compared with the historical data, the 1-year OS rate of 1463 Japanese patients in the cytokine era (35) was 92.8, 76.6 and 44.1%, respectively, in the favorable, intermediate and poor risk groups, whereas the corresponding values in the present survey were 94.3% (95% CI: 91.4–96.2), 73.5% (71.0–75.9) and 27.2% (18.7–36.4), respectively. Taking the lead time of previous therapies into account, sorafenib is suggested to have substantial efficacy.
Prognostic factors identified in the univariate analysis were mostly consistent with previous findings (36). In addition, lower albumin and body weight are likely to reflect a worse PS. Pulmonary comorbidity could directly affect survival. Among the prior cytokines, only IFN-α positively affected survival, whereas IL-2 did not. Independent prognostic factors for OS, revealed by multivariate analysis, were generally similar with those for PFS and DOT. However, care should be taken with the interpretation of the results, because several factors that had been well known to be prognostic and/or those shown to be so in the present univariate analysis, such as MSKCC risk, CRP and albumin, were excluded from the multivariate analysis, because >10% of data were missing. Some of these excluded factors could have been independently prognostic, and the potential of these factors to be confounding cannot be eliminated.
The effect of the treatment line on efficacy should be considered carefully because it could be affected by potential confounding. Although prior IFN-α treatment remained significant after multivariate analysis and was identified as one of the favorable prognostic factors (HR = 0.68, 95% CI: 0.55–0.84), it does not necessarily mean that prior therapy improves the efficacy of sorafenib in later settings. Instead, a shorter PFS in the first line can, at least partially, be attributable to a worse condition in this population; distribution of MSKCC risk (favorable/intermediate/poor) was 17.5/57.7/3.5% for ≥second-line vs. 13.6/51.3/13.1% for first-line, median CRP was 0.07 mg/dl for ≥second-line vs. 0.18 mg/dl for first-line, and median time from surgery was 2.8 years for ≥second-line vs. 0.6 years for first-line. It is conceivable that, among the first-line patients, sorafenib was preferentially prescribed to those with a poor prognosis instead of IFN-α, which had been the standard first-line treatment at the beginning of this survey. Such imbalances leave the possibility of substantial activity of sorafenib in the first-line setting. In line with this, sorafenib also provided clinical benefit in the first-line setting in NA-ARCCS and EU-ARCCS (33,34).
This survey including these exploratory landmark analyses showed an association between longer survival (OS and PFS) and an early onset of specific ADRs. Among them, hypertension and dysphonia have been commonly reported in patients treated with VEGF signaling-pathway inhibitors (37,38). The correlation between hypertension and longer OS/PFS was shown for sorafenib and axitinib in the AXIS trial (39), and in a retrospective analysis of four clinical trials of sunitinib (40). It is intriguing to consider that the occurrence of specific ADRs may reflect the pharmacological effect of sorafenib and predict its future efficacy. In contrast, the onset of hypophosphatemia ≥Grade 2 and hemorrhagic events within 30 days were associated with a shorter OS, and not with PFS, suggesting that these ADRs are only prognostic and not predictive.
There are several limitations to this study. Because some analyses were introduced in a post hoc manner, some of the baseline factors contained considerable missing data, and these factors had to be removed from the multivariate analysis. Since AEs/ADRs were collected according to MedDRA, information on the grade of the AE/ADR was lacking. Judgment of causality regarding AEs/ADRs by the reporting physician may contain uncertainty; for example, progression of primary disease, which is more likely to be AE, was listed as the most common ADR causing death. Tumor evaluation was also not based on RECIST and could not be fairly compared with other clinical study data. Since the survey was non-interventional, the timing of response evaluation could not be defined despite the fixed timing of CRF collection, thus an earlier evaluated PD, if present, could have been missed and might have led to overestimation of PFS. In addition, the association between ADR and survival obtained from this landmark analysis remains exploratory. Although this method removes the guarantee-time bias, there are several drawbacks why a conclusive discussion is not possible: the post hoc nature of the analysis, lack of adjustment for prognostic factors as well as imbalances in sample size among subgroups.
In conclusion, this PMS study among a non-biased nationwide large population demonstrated that treatment of advanced RCC patients with sorafenib at 400 mg BID, the labeled dose, presented an acceptable toxicity profile and favorable clinical benefit, which were consistent with those observed in previous Japanese Phase II and global Phase III trial. The present results support sorafenib as a viable treatment option in patients with unresectable or metastatic RCC who failed cytokine treatment, and further suggest a possible therapeutic option as a first-line therapy.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Funding
This work was supported and funded by Bayer Yakuhin Ltd, the manufacturer of the Nexavar. Funding to pay the Open Access publication charges for this article was provided by Bayer Yakuhin, Ltd.
Conflict of interest statement
Masatoshi Adachi, Yutaka Okayama, Toshiyuki Sunaya and Lyo Inuyama are employees of Bayer Yakuhin, Ltd. Hideyuki Akaza, Mototsugu Oya, Masafumi Iijima, Ichinosuke Hyodo, Akihiro Gemma and Hiroshi Itoh are members of the Proper Use Advisory Committee of Nexavar. List of the companies with paid consulting (consulting fee, honoraria, speaking fee) is as follows: Hideyuki Akaza, Bayer, Pfizer, Novartis; Mototsugu Oya, Bayer, Pfizer, Novartis, GloxoSmithKline, Shionogi; Masafumi Iijima, Bayer, GlaxoSmithKline; Ichinosuke Hyodo, Bayer, Ohtsuka, Shionogi; Akihiro Gemma, Bayer; Hiroshi Itoh, TAkeda, MSD, Novartis, Daiichi-Sankyo, Mitsubishi-Tanabe, Pfizer, Astellas, SBI Pharmaceuticals, Nipro, Kyowa Hakko Kirin, AstraZeneca, Eli Lilly, Sanofi, Teijin.
Supplementary Material
Acknowledgements
The authors deeply appreciate the cooperation/contribution of all people who were involved in this survey including 2379 physicians at 724 hospitals/departments, and, above all, 3422 patients and their families.
References
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med 1996;335:865–75. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 3.Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev 2005;31:536–45. [DOI] [PubMed] [Google Scholar]
- 4.Gnarra JR, Tory K, Weng Y et al. . Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 1994;7:85–90. [DOI] [PubMed] [Google Scholar]
- 5.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci USA 1996;93:10595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell PH, Wiesener MS, Chang GW et al. . The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399:271–5. [DOI] [PubMed] [Google Scholar]
- 7.Cockman ME, Masson N, Mole DR et al. . Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel–Lindau tumor suppressor protein. J Biol Chem 2000;275:25733–41. [DOI] [PubMed] [Google Scholar]
- 8.Na X, Wu G, Ryan CK, Schoen SR, di'Santagnese PA, Messing EM. Overproduction of vascular endothelial growth factor related to von Hippel–Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 2003;170:588–92. [DOI] [PubMed] [Google Scholar]
- 9.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. [DOI] [PubMed] [Google Scholar]
- 10.Negrier S, Escudier B, Lasset C et al. . Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 1998;338:1272–8. [DOI] [PubMed] [Google Scholar]
- 11.Gitlitz BJ, Figlin RA. Cytokine-based therapy for metastatic renal cell cancer. Urol Clin North Am 2003;30:589–600. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm SM, Carter C, Tang L et al. . BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109. [DOI] [PubMed] [Google Scholar]
- 13.Carlomagno F, Anaganti S, Guida T et al. . BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst 2006;98:326–34. [DOI] [PubMed] [Google Scholar]
- 14.Ratain MJ, Eisen T, Stadler WM et al. . Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:2505–12. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM et al. . Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Eisen T, Stadler WM et al. . Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009;27:3312–8. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P et al. . Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 18.Hudes G, Carducci M, Tomczak P et al. . Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81. [DOI] [PubMed] [Google Scholar]
- 19.Escudier B, Pluzanska A, Koralewski P et al. . Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet 2007;370:2103–11. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Escudier B, Oudard S et al. . Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet 2008;372:449–56. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg CN, Davis ID, Mardiak J et al. . Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. [DOI] [PubMed] [Google Scholar]
- 22.Rini BI, Escudier B, Tomczak P et al. . Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- 23.Akaza H, Tsukamoto T, Murai M, Nakajima K, Naito S. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol 2007;37:755–62. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and Prognostic Stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Bacik J, Schwartz LH et al. . Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004;22:454–63. [DOI] [PubMed] [Google Scholar]
- 26.The Japanese Urological Association. General Rules for Clinical and Pathological Studies on Renal Cell Carcinoma. Tokyo: Kanehara & Co., Ltd; 1999. [Google Scholar]
- 27.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1:710–9. [DOI] [PubMed] [Google Scholar]
- 28.Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int 2011;108:1813–9. [DOI] [PubMed] [Google Scholar]
- 29.Hyodo I, Adachi M, Tsukamoto T, Murai M, Naito S, Akaza H. Serum pancreatic enzyme elevation under treatment with sorafenib. Gan to Kagaku Ryoho 2012;39:1651–6. (in Japanese). [PubMed] [Google Scholar]
- 30.Iijima M, Fukino K, Adachi M et al. . Sorafenib-associated hand-foot syndrome in Japanese patients. J Dermatol 2011;38:261–6. [DOI] [PubMed] [Google Scholar]
- 31.Winkelmayer WC, Levin R, Avorn J. Chronic kidney disease as a risk factor for bleeding complications after coronary artery bypass surgery. Am J Kidney Dis 2003;41:84–9. [DOI] [PubMed] [Google Scholar]
- 32.Horiuchi-Yamamoto Y, Gemma A, Taniguchi H et al. . Drug-induced lung injury associated with sorafenib: analysis of all-patient postmarketing surveillance in Japan. Int J Clin Oncol 2013;18:743–9. [DOI] [PubMed] [Google Scholar]
- 33.Stadler WM, Figlin RA, McDermott DF et al. . Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 2010;116:1272–80. [DOI] [PubMed] [Google Scholar]
- 34.Beck J, Procoppio G, Bajetta E et al. . Final results of the European advanced renal cell carcinoma sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol 2011;22:1812–23. [DOI] [PubMed] [Google Scholar]
- 35.Naito S, Yamamoto N, Takayama T et al. . Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol 2010;57:317–25. [DOI] [PubMed] [Google Scholar]
- 36.Furniss D, Harnden P, Ali N et al. . Prognostic factors for renal cell carcinoma. Cancer Treat Rev 2008;34:407–26. [DOI] [PubMed] [Google Scholar]
- 37.Di Lorenzo G, Porta C, Bellmunt J et al. . Toxicities of targeted therapy and their management in kidney cancer. Eur Urol 2011;59:526–40. [DOI] [PubMed] [Google Scholar]
- 38.Saavedra E, Hollebecque A, Soria J-C, Hartl DM. Dysphonia induced by anti-angiogenic compounds. Invest New Drugs 2014;32:774–82. [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Escudier B, Tomczak P et al. . Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomized phase 3 trial. Lancet Oncol 2013;14:552–62. [DOI] [PubMed] [Google Scholar]
- 40.Rini BI, Cohen DP, Lu DR et al. . Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.