Abstract
Introduction
Antibiotic irrigations are occasionally used during endoscopic sinus surgery when there is evidence of gross mucosal infection. These are thought to flush out pathogenic bacteria and decrease the bacterial load within the mucosal surfaces. However, this has not been studied in vivo and it is unknown whether antibiotic rinses produce a quantitative reduction in pathologic bacteria within the sinus mucosa. The objective of this study was to quantify the amount of S. aureus within the maxillary sinus and to determine the impact of intraoperative mupiricon irrigation on bacterial count.
Methods
Sixteen patients with symmetric maxillary chronic rhinosinusitis were prospectively enrolled. After bilateral maxillary antrostomies, biopsies were taken of the maxillary sinus mucosa on both sides. In each patient, the right side was irrigated with 240 cc of normal saline (NS) and the left side was irrigated with 240 cc of NS mixed with 60 mg mupirocin. Repeat maxillary sinus mucosal biopsies were taken from each side 7–10 days post surgery. Each biopsy was analyzed using quantitative polymerase chain reaction to determine the presence and amount of S. aureus.
Results
Mupiricon irrigations were found to significantly reduce the amount of S. aureus found within the maxillary sinus mucosa compared to NS alone. The average fold change between the pre and post-treatment biopsies on the right and left was 9.05 and 97.42 respectively (p < 0.01).
Conclusion
Intraoperative mupirion irrigations significantly reduce the amount of S. aureus detected within the diseased sinus mucosa at up to 10 days post-op.
Keywords: mupiricon, maxillary sinus, endoscopic sinus surgery, staphylococcus aureus, polymerase chain reaction, intraoperative irrigations, bacterial load
INTRODUCTION
Staphylococcus aureus is one of the most common bacteria present in patients with chronic rhinosinusitis (CRS)1. Its role in refractory CRS is continuing to be elucidated as more evidence identifies it as a key player in biofilm formation and superantigen production. Chronic rhinosinusitis is typically treated with oral antibiotics and in the majority of patients oral therapy is sufficient enough to clear the inciting disease. However, in recent years there has been increasing interest in the use of topical antibiotics in patients with recalcitrant CRS. With oral antibiotics, it is unclear what concentration of the antibiotic actually reaches the sinonasal mucosa. Topical antibiotics delivered directly to the sinus mucosa enables high concentration of the medication at the site of infection with a lower systemic absorption and decreased systemic complication rate. Topical antibiotics have the potential to reduce pathogenic bacteria existing in a free planktonic state as well as those in a biofilm formation. Desrosiers et al.(2007) demonstrated that topical antibiotics are effective at killing bacteria residing in a biofilm formation in patients with CRS 2.
The presence of S. aureus post sinus surgery has been associated with a worse postoperative outcome, slower recovery time and shorter time to recurrent infection. Patients with S. auerus biofilms typically have a poorer response to both surgery and medical therapy including long term oral antibiotics 3,4. Jervis-Brady et al.(Year?)-(missing in the reference) determined that in those patients with S. aureus cultured at the time of surgery had a poorer early post-surgery outcome with worse endoscopy and symptoms scores5. They concluded in this study that in patients with recalcitrant CRS thought to be due to S. aureus biofilm formation, early aggressive topical antibiotic therapy is critical.
Mupiricon is an antibiotic produced by Pseudomonas fluorescens and exerts its effect by binding to the enzyme isoleucyl-transfer RNA synthetase preventing isoleucine incorporation during bacterial protein synthesis 6,7. Mupiricon effectively inhibits bacterial synthesis and displays a high level of activity against S. aureus. Topical application of mupiricon whether by nasal lavage or nebulizer has been cited in the literature as a treatment option in those patients with recalcitrant CRS8. What is not know is whether intraoperative rinses of the antibiotic mupirocin is effective in reducing the load of S. aureus in patients with CRS. In a previous study of ours we reported that intraoperative saline rinses effectively reduce bacterial load within the treated sinus9. In this study our goal is to determine (1) if mupirocin rinses reduce the bacterial load of S. aureus within the maxillary sinus and (2) if the antibiotic rinse is more effective then saline irrigations without the added antibiotic.
MATERIALS AND METHODS
Patients
After approval from the Loma Linda University Institutional Review Board, patients with CRS were prospectively recruited from our tertiary rhinology practice. The diagnosis of CRS was made according to the signs and symptoms as outline by the Rhinosinusitis Task Force10. Only those patients who had failed medical management and were undergoing endoscopic sinus surgery were recruited for this study. Inclusion criteria included a Lund Mackay score of at least one in each maxillary sinus and nasal endoscopy demonstrating mucosal inflammation and/or infection. Only those patients with symmetric bilateral maxillary sinus disease as noted by computer tomography and endoscopy were included. Patients were excluded if they had fungal ball, antrochoanal polyp, tumor or asymmetric disease in the maxillary sinus.
Surgical Technique
During surgery, a standard maxillary antrostomy was created on both sides and enlarged so that the majority of the maxillary sinus could be visualized. Mucosal biopsies were taken along the posterior wall prior to any instrumentation except for removal of any nasal polyps obstructing visualization of the mucosal surface. In each patient, the right maxillary sinus was irrigated with 240 cc of normal saline using a 60 cc syringe and a curved olive tip suction. The left side was irrigated with 240 cc of mupiricon irrigation (60 gram of mupiricon placed in 240 cc of saline, ASL pharmacy, San Diego, CA) using the same 60 cc syringe and curved olive tip suction. Repeat maxillary sinus mucosal biopsies were then taken (in a location adjacent to the pre-treatment biopsy) at the first postoperative visit 7–10 days later. Patients were given instructions not to start any other topical therapy or rinses until after the postoperative biopsy was taken. No patients were given postoperative antibiotics. All four biopsies (2 pretreatment and 2 post-treatment) were processed using quantitative rPCR for detection of S. aureus.
Tissue Storage and DNA extraction
The biopsies were placed on ice then rapidly transferred to a −80 degree freezer. No additional freeze/thaw cycles occurred prior to processing. DNA extraction was carried out as per the manufacturers method of the DNA easy Quiagen kit (Quiagen Inc, Valencia CA). Briefly,1.8 ml of phosphate buffered saline (PBS) was added to the sample and centrifuged at 1800 × g for 5 minutes. The supernatant was decanted and resuspended in 180μl of PBS and incubated for 2 min at room temperature. 25 μl of proteinase K solution and 200 μl Buffer AL were then added. These were mixed thoroughly by vortexing, and incubated at 56°C for 10 min. 200 μl of ethanol (96–100%) was then added to the sample, and mixed thoroughly by vortexing. The mixture was then pipetted (including any precipitate) into the DNeasy Mini spin column and placed in a 2 ml collection tube. This was then centrifuged at ≥6000 × g (8000 rpm) for 1 min. The DNeasy Mini spin column was then placed in a new 2 ml collection tube, 500 μl Buffer AW1 was added, and the mixture centrifuged for 1 min at ≥6000 × g (8000 rpm). The flow through and collection tube were discarded and the DNeasy Mini spin column placed in a new 2 ml collection tube, 500 μl Buffer AW2 added, and centrifuged for 3 min at 20,000 × g (14,000 rpm) to dry the DNeasy membrane. The flow-through and collection tube were again discarded and the DNeasy Mini spin column again placed in a clean 1.7 ml microcentrifuge tube and 200 μl Buffer AE pipetted directly onto the DNeasy membrane. Incubation at room temperature for 1 min was allowed to occur, and then the fluid was centrifuged for 1 min at ≥6000 × g (8000 rpm) to elute.
Real Time Polymerase Chain Reactions
The QuantiTect SYBR Green I fluorescent dye detection system (Quigen Inc, Valencia, CA) was used to run the real time polymerase chain reaction with the following primer for Staphylococcus aureus:
F- 5′-AAGGGCGAAATAGAAGTGCCGGGC-3′
R-5-CACAAGCAACTGCAAGCAT-3.
The following were then mixed together on ice (Reagent volumes are for one 25uL reaction): 12.5 μl of 2X Master mix, 1 μl Forward Primer,1 μl Reverse Primer, 1 μl DNA template, 9.5 μl DNase/RNase free water for a total volume of for a total volume of 25.0 μl. Two PCR reactions were prepared for each sample. The SmartCycler qPCR device (Cepheid, Sunnyvale, CA) using the SYBR Green I fluorescent dye detection system was used and reactions were performed in a 25-μl final volume. Amplification conditions consisted of initial denaturation at 95°C for 10 m followed by 45 three-step cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The threshold line was set at 30 as an arbitrarily chosen point within the log-linear phase of amplification, significantly greater than the variability in background fluorescence and well before the reaction plateau. Baseline was set for each probe set. After completion of the PCR, a melting curve was obtained by heating the amplicon from 60 to 95°C at a rate of 0.2°Cs. Results were reported as fold change between the individual samples as compared with the treatment.
Statistical analysis
The difference between pre and post-treatment bacterial load was calculated using a paired t test and unpaired t test. Statistical analysis was carried out using GraphPad Prism (GraphPad Software, La Jolla, CA) and statistical significance was defined as P < 0.05.
RESULTS
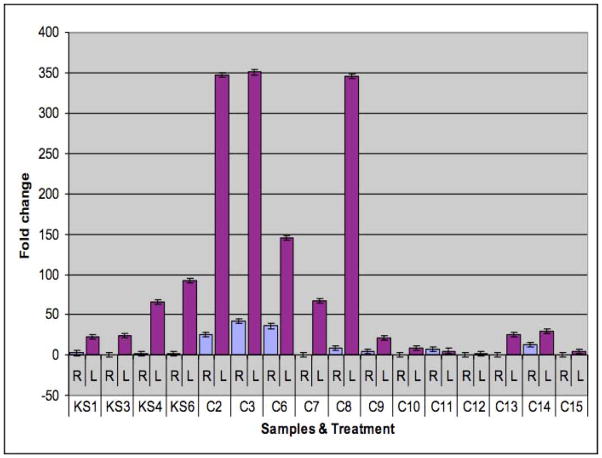
Twenty patients were recruited into this study; however, only 16 completed both the pre-treatment and post-treatment biopsies (table 1). Of the 16, 8 were females and 8 males with an average age of 45.6 years. Eight patients underwent revision surgery, all of them with CRS and nasal polyps. Eight patients underwent primary surgery, 2 of which had nasal polyps. The side with the mupirocin rinse showed a statistically significant reduction in bacterial load compared to normal saline irrigations as reflected in the fold change (table 1, figure 1 p< 0.01). In this analysis a higher fold change reflects a greater reduction in bacterial load. The average fold change in mupirocin side was 97.42 compared to the saline side where the average fold change was only 9.05. A significantly higher fold change in those patients who had CRS without polyps (197.1) compared to CRS with polyps (37.7). There was only one patient in which no difference found between the two sides.
TABLE 1.
patient characteristics and quantitative PCR results reported in fold change.
| PATIENT | DISEASE | FOLD CHANGE RIGHT | FOLD CHANGE LEFT | SURGERY |
|---|---|---|---|---|
| KS1 | CRS | 2.55 | 23 | Primary |
| KS3 | CRS | 0.18 | 23.81 | Primary |
| KS6 | CRS | 1.27 | 93 | Primary |
| C2 | CRS | 25.9 | 346.43 | Primary |
| C3 | CRS | 42.25 | 350.43 | Primary |
| C8 | CRS | 9 | 345.96 | Primary |
| C6** | POLYPS | 36 | 146 | Revision |
| C7** | POLYPS | 0.27 | 67.4 | Revision |
| C9 | POLYPS | 4.46 | 21.55 | Revision |
| C10** | POLYPS | 0.07 | 8.93 | Revision |
| C11 | POLYPS | 7.132 | 5.15 | Primary |
| C12 | POLYPS | 0.44 | 1.82 | Primary |
| C13* | POLYPS | 0.464 | 25.75 | Revison |
| C14 | POLYPS | 12.84 | 30 | Revison |
| C15 | POLYPS | 0.011 | 4.96 | Revison |
| KS4 | POLYPS | 2 | 65.61 | Revison |
patient with AFS,
patients with samter’s triad
Figure 1.
Fold change analysis between the left (mupirocin side) and right (normal saline side) in all of the samples
Fold change = 2 −ΔΔCt, where ΔΔCt = ΔCt of the sample − ΔCt of Reference.
DISCUSSION
We have established in previous studies that intraoperative saline irrigations are effective in reducing the load of pathogenic bacterial within the treated sinus9. Although at this point in time it is unclear whether this will have a clinical impact in the postoperative outcome of the patient, it is assumed that healing will be improved with a decrease in bacterial load within the sinus mucosa. In this study, we broaden our objectives to evaluate whether mupirocin irrigations given as a one time dose at the time of surgery would reduce bacterial load to a greater extent than saline irrigations alone. Our results demonstrate that intraoperative mupirocin irrigations effectively reduced the amount of S. aureus detected within the treated sinus mucosa at 7–10 days post-surgery. When compared to normal saline irrigations, mupirocin appears to have greater efficacy in reducing bacterial counts as detected by quantitative rPCR. Furthermore, mupirocin appears to be more effective in patients with CRS without polyps although larger numbers are needed to reach statistical significance.
Topical intranasal mupirocin has emerged as the agent of choice for eliminating S. aureus colonization and subsequent systemic S. aureus infection 11. In addition nasal lavage with mupirocin has been cited in the literature as an alternative to oral antibiotics in the treatment of CRS related to S. aureus colonization. Over the last several years there as been increased interest in the use of topical antibiotics over oral antibiotics in postoperative sinus patients. In many patients with recalcitrant CRS culture directed oral antibiotics fail to eradicate the pathogenic organism. This may be due to inadequate drug penetration or biofilm formation. It is hypothesized that topical antibiotics achieve a higher local concentrations at the target site with the added benefit of decreased systemic side effects. Once the sinus cavities have been opened topical therapy may be easily delivered to the sinus mucosa. In surgically recalcitrant CRS, nasal lavages containing 0.05% mupirocin for 3 weeks was shown to reduce symptoms and improve nasal endoscopy scores. Furthermore the treatment was well tolerated with no major adverse effects or systemic complications noted8. Repeat cultures for S. aureus were negative in 15 of the 16 treated patients. In this study mupirocin was given as a lavage in 200 ml of lactated ringers solution and a conclusion could not be made as to whether the results were due to the saline rinse or mupirocin alone. In our study we were able to directly compare the two sides and we found both to be effective with a greater reduction in bacterial load with the mupirocin side.
In patients with recalcitrant CRS thought to be due to biofilm formation topical therapy is often employed in hopes of disrupting the biofilm matrix. It is already well known that oral antibiotics have poor penetration into the biofilm matrix, which forms a protective barrier around the bacteria. Several studies have already shown that S. aureus biofilms are more resistant to antimicrobial therapy compared to their planktonic counterparts12,13. S. aureus has been associated with biofilm formation in CRS patients and associated with worse postoperative outcomes after endoscopic sinus surgery4. Ha et al demonstrated in vitro that mupirocin was capable of reducing biofilm mass by greater than 90% in all S. auerus isolates14. Although in our study we did not use a method for detecting biofilms specifically, quantitative PCR will detect the presence of bacteria whether in a biofilm formation or in the free planktonic state. Our results did show a trend toward a lesser degree of bacterial reduction in those patients with CRS with polyps, which may be indicative of biofilms. Tong et al. demonstrated in an in vitro sheep study that a single rinse of mupirocin effectively reduced biofilm mass at day 1 and day 8 but a substantial amount of the biofilm remained. A near complete eradication of the biofilm mass was found when mupirocin was used as twice daily irrigations15.
Future studies are needed to evaluate the clinical implications of our findings. Does a reduction of bacterial load created at the time of surgery imply that there will be a greater clinical response and more favorable postoperative outcome? It has been demonstrated in in vitro studies that a one time dose of mupirocin appears to disrupt biofilm formation but when the same tissue is studied 10 days later there appeared to be regrowth of the biofilm. In that study the most favorable results were found with twice daily mupirocin rinses for 3 weeks. We believe that topical application of mupirocin is beneficial in patients with persistent CRS due to S. aureus, however, at this time we cannot conclude as to the optimal dose and duration of the medication.
CONCLUSION
A one time dose of intraoperative mupirocin appears to be effective in reducing the amount of S. aureus detected within the treated sinus. Furthermore, mupirocin lavage was more effective than normal saline rinses in reducing bacterial load.
Footnotes
Conflict of interest- none
Financial disclosures- none
Bibliography
- 1.Brook I, Frazier EH. Correlation between microbiology and previous sinus surgery in patients with chronic maxillary sinusitis. Ann Otol Rhinol Laryngol. 2001;110:148–151. doi: 10.1177/000348940111000210. [DOI] [PubMed] [Google Scholar]
- 2.Desrosiers M, Bendouah Z, Barbeau J. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol. 2007;21:149–153. doi: 10.2500/ajr.2007.21.3007. [DOI] [PubMed] [Google Scholar]
- 3.Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol. 2008;22:1–6. doi: 10.2500/ajr.2008.22.3119. [DOI] [PubMed] [Google Scholar]
- 4.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134:991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Jervis-Bardy J, Foreman A, Boase S, Valentine R, Wormald PJ. What is the origin of Staphylococcus aureus in the early postoperative sinonasal cavity? Int Forum Allergy Rhinol. 1:308–312. doi: 10.1002/alr.20050. [DOI] [PubMed] [Google Scholar]
- 6.Boon RJ, Beale AS, Sutherland R. Efficacy of topical mupirocin against an experimental Staphylococcus aureus surgical wound infection. J Antimicrob Chemother. 1985;16:519–526. doi: 10.1093/jac/16.4.519. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985;27:495–498. doi: 10.1128/aac.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uren B, Psaltis A, Wormald PJ. Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope. 2008;118:1677–1680. doi: 10.1097/MLG.0b013e31817aec47. [DOI] [PubMed] [Google Scholar]
- 9.Seiberling KA, McHugh RK, Aruni W, Church CA. The impact of intraoperative saline irrigations on bacterial load within the maxillary sinus. Int Forum Allergy Rhinol. 2011;1:351–355. doi: 10.1002/alr.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert DNMR, Sande M. The Stanford guide to antimicrobial therapy. Hyde Park, VT: Antimicrobial Therapy Inc; 2002. [Google Scholar]
- 12.Amorena B, Gracia E, Monzon M, et al. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999;44:43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Melchior MB, Fink-Gremmels J, Gaastra W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J Vet Med B Infect Dis Vet Public Health. 2006;53:326–332. doi: 10.1111/j.1439-0450.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 14.Ha KR, Psaltis AJ, Butcher AR, Wormald PJ, Tan LW. In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope. 2008;118:535–540. doi: 10.1097/MLG.0b013e31815bf2e3. [DOI] [PubMed] [Google Scholar]
- 15.Le T, Psaltis A, Tan LW, Wormald PJ. The efficacy of topical antibiofilm agents in a sheep model of rhinosinusitis. Am J Rhinol. 2008;22:560–567. doi: 10.2500/ajr.2008.22.3232. [DOI] [PubMed] [Google Scholar]