Abstract
Indoxacarb and metaflumizone belong to a relatively new class of sodium channel blocker insecticides (SCBIs). Due to intensive use of indoxacarb, field-evolved indoxacarb resistance has been reported in several lepidopteran pests including the diamondback moth, Plutella xylostella, a serious pest of cruciferous crops. In particular, the BY12 population of P. xylostella, collected from Baiyun, Guangdong province of China in 2012, was 750-fold more resistant to indoxacarb and 70-fold more resistant to metaflumizone compared with the susceptible Roth strain. Comparison of cDNA sequences encoding the sodium channel genes of Roth and BY12 revealed two point mutations (F1845Y and V1848I) in the 6th segment of domain IV of the PxNav protein in the BY population. Both mutations are located within a highly conserved sequence region that is predicted to be involved in the binding sites of local anesthetics and SCBIs based on mammalian sodium channels. A significant correlation was observed among ten field-collected populations between the mutant allele (Y1845 or I1848) frequencies (1.7% to 52.5%) and resistance levels to both indoxacarb (34- to 870-fold) and metaflumizone (1- to 70-fold). The two mutant alleles were never found to co-exist in the same allele of PxNav, suggesting that they arose independently. This is the first time that sodium channel mutations have been associated with high levels of resistance to SCBIs. F1845Y and V1848I are molecular markers for resistance monitoring in the diamondback moth and possibly other insect pest species.
Keywords: indoxacarb, metaflumizone, mutation, Plutella xylostella, resistance, sodium channel
Introduction
Voltage-gated sodium channels are a group of integral transmembrane proteins that are responsible for the initiation and propagation of action potentials in almost all excitable cells (Catterall, 2012). Due to their crucial role in regulating cell excitability, sodium channels are the primary targets of several classes of chemical insecticides (Usherwood et al., 2007; Dong et al., 2014; Silver et al., 2014). DDT and pyrethroids are among the earliest synthetic compounds that were identified to target sodium channels and they prolong sodium channel opening resulting in repetitive nerve firing and membrane depolarization (Narahashi, 2000). Indoxacarb and metaflumizone, known as sodium channel blocker insecticides (SCBIs), represent a new class of sodium channel-targeting insecticides with a distinct mode of action from that of DDT and pyrethroids (Silver et al., 2010). SCBIs preferably bind to sodium channels in the slow-inactivated (non-conducting) state and block the channels (Wing et al., 2005; Silver et al., 2010).
Indoxacarb is metabolized by insect esterases or amidases to a decarbomethoxylated metabolite (DCJW), which is a more active sodium channel blocker than indoxacarb, leading to flaccid paralysis and death of insects (Wing et al., 1998, 2005). Metaflumizone is a novel semicarbazone insecticide, sharing a common mode of action with indoxacarb (Salgado & Hayashi, 2007). Both indoxacarb and metaflumizone have a broad spectrum of insecticidal efficacy against a wide range of pests, and with good mammalian safety (Harder et al., 1996; BASF, 2007).
Diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) is one of the most destructive global pests of cruciferous vegetables (Talekar & Shelton, 1993; Furlong et al., 2013). A conservative estimate of the global economy costs for this pest was around U.S.$ 4–5 billion annually (Zalucki et al., 2012). Control of P. xylostella has relied heavily on chemical insecticides and Bt sprays for decades. This pest has evolved different levels of resistance to as many as 92 active ingredients to date (APRD, 2015), and has become one of the most difficult and expensive insect pests in the world to control.
Due to intensive use, resistance to SCBIs has evolved in the field in at least three lepidopteran pests, including P. xylostella (Sayyed & Wright, 2006; Zhao et al., 2006; Santos et al., 2011; Khakame et al., 2013), Spodoptera litura (Shad et al., 2012; Tong et al., 2013) and Spodoptera exigua (Zhou et al., 2011; Che et al., 2013; Su & Sun, 2014). However, resistance mechanisms to SCBIs in these pests are poorly understood. Most of the evidence for resistance mechanisms has come from observations of synergistic effects of metabolic inhibitors on the toxicity of SCBIs (Ahmad & Hollingworth, 2004; Shono et al., 2004; Sayyed & Wright, 2006; Nehare et al., 2010; Pang et al., 2012), which suggests metabolic detoxification is involved in resistance to SCBIs. To our knowledge, target site resistance to SCBIs has not yet been reported.
In the present study, we report for the first time the identification of sodium channel mutations that are associated with high levels of resistance to SCBIs in P. xylostella. Two point mutations (F1845Y and V1848I) in the sixth transmembrane segment of domain IV of the sodium channel gene of P. xylostella (named PxNav) were first detected at high frequencies in the BY12 population, which is highly resistant to indoxacarb. Subsequent screening of the two mutations in ten field populations of P. xylostella collected from China showed a significant correlation between the mutated allele frequencies of PxNav and the resistance levels to SCBIs. Our results provide valuable information for mapping the receptor site of SCBIs and for developing molecular tools for SCBI resistance monitoring in P. xylostella and possibly in other insects.
Material and methods
Insects
The susceptible Roth strain of P. xylostella, which has been maintained in the laboratory for more than 20 years without exposure to any insecticide, was passed to our laboratory from Rothamsted Research (Herts, United Kingdom) in 2003.
Eleven field populations of P. xylostella were collected from Guangdong province (Baiyun, Zengcheng, Huizhou, Zhuhai and Shenzhen), Hainan province (Sanya), and Anhui province (Hefei) of China during 2012–2014 (Table 1). More than two hundred larvae or pupae were collected from each sampling site. Field-collected insects were mass mated and their F1 larvae were used for bioassay (F5 for HZ13) and DNA-based genotyping for PxNav.
Table 1.
Log-concentration probit-mortality regression data for indoxacarb and metaflumizone tested against a susceptible strain and ten field populations of P. xylostella.
| Population/Strain | Location of collection† | Time of collection | Indoxacarb
|
Metaflumizone
|
||||
|---|---|---|---|---|---|---|---|---|
| Slope (±SE) | LC50 (95%FL‡) (mg/L) | RR § | Slope (± SE) | LC50 (95%FL‡) (mg/L) | RR§ | |||
| Susceptible laboratory strain | ||||||||
| Roth | 1.8 ± 0.2 | 0.10 (0.08–0.13) | 2.5 ± 0.3 | 2.17 (1.48–3.07) | ||||
| Field-collected populations | ||||||||
| BY12 | Baiyun, GD | Nov. 2012 | 1.1 ± 0.2 | 74.96 (50.07–122.3) | 750 | 0.9 ± 0.2 | 152.8 (90.72–385.2) | 70 |
| ZH12 | Zhuhai, GD | Nov. 2012 | 0.9 ± 0.1 | 9.53 (5.97–15.16) | 95 | 1.1 ± 0.1 | 16.89 (11.32–25.13) | 8 |
| BY13 | Baiyun, GD | Nov. 2013 | 2.1 ± 0.6 | 25.40 (13.62–36.28) | 250 | 0.8 ± 0.2 | 22.56 (7.57–52.77) | 10 |
| ZC13 | Zengcheng, GD | Nov. 2013 | 0.6 ± 0.1 | 5.57 (1.73–12.33) | 56 | 1.0 ± 0.1 | 8.28 (4.33–13.66) | 4 |
| HZ13 | Huizhou, GD | Nov. 2013 | 1.0 ± 0.2 | 4.81 (2.49–8.17) | 48 | 1.0 ± 0.2 | 5.96 (3.35–9.85) | 3 |
| SY14 | Sanya, HN | Jan. 2014 | 1.0 ± 0.3 | 85.00 (42.66–177.8) | 850 | 1.0 ± 0.1 | 13.04 (4.98–27.52) | 6 |
| HF14 | Hefei, AH | May 2014 | 2.9 ± 0.4 | 12.81(9.626–17.62) | 128 | 3.7±0.5 | 5.555 (4.335–7.337) | 3 |
| BY14 | Baiyun, GD | Nov. 2014 | 1.2 ± 0.2 | 87.03 (44.80–226.3) | 870 | 1.3±0.2 | 106.8 (37.99–854.2) | 49 |
| HZ14 | Huizhou, GD | Nov. 2014 | 1.7 ± 0.2 | 35.31(21.85–60.91) | 350 | 1.7±0.2 | 12.01 (7.479–19.43) | 5 |
| ZC14 | Zengcheng, GD | Nov. 2014 | 1.5 ± 0.2 | 3.366 (1.897–5.602) | 34 | 1.8±0.3 | 1.918 (1.104–3.073) | 1 |
| SZ14 ¶ | Shenzhen, GD | Nov. 2014 | ||||||
GD: Guangdong province. HN: Hainan province. AH: Anhui province of China.
95% Fiducial limits.
RR (resistance ratio) = LC50 of field populations/LC50 of Roth.
Bioassay data was not available because not enough larvae were produced when reared in the laboratory.
Adults were fed on 10% (w/v) honey solution and allowed to lay eggs on radish seedlings (Raphanus sativus L.). Larvae were fed on radish seedlings. All stages were maintained at 25 ± 1°C, 60%–70% relative humidity (RH) and a photo period of 16 h light: 8 h dark.
Insecticides and chemicals
Formulated chemicals used for bioassay were metaflumizone (130 g/L EC, BASF Corporation, Research Triangle Park, NC), indoxacarb (50 g/L EC, IPP, Guangdong Academy of Agricultural Sciences, Guangzhou, China). The oxidase inhibitor piperonyl butoxide (PBO, 95%) was purchased from Endura (Ravenna, Italy), the esterase inhibitor S,S,S-tributyl phosphorothioate (DEF, 98%) was from Sigma (St. Louis, MO), and the glutathine S-transferase depletor diethyl maleate (DEM, 95%) was from the Shanghai Chemical Reagent Co. Ltd. (Shanghai, China).
Bioassay
The leaf dip method was used to determine the susceptibility of the third instar larvae of P. xylostella against indoxacarb and metaflumizone. The insecticides were diluted to generate five to seven serial dilutions with distilled water containing 0.1% Triton X-100 which facilitates uniform leaf disc coverage with the active ingredient. Cabbage (Brassica oleracea) leaf discs (diameter = 6.5 cm) were cut and dipped in an insecticide solution for 10 s. Control discs were treated with 0.1% Triton X-100 solution in water only. The leaf discs were dried at room temperature for 1–2 h. Each treated leaf disc with ten third instar larvae was placed in a separate plastic petri dish, then kept at 25 ± 1°C and an RH of 60%–70% with a photoperiod of 16 h light: 8 h dark. For each concentration, 3 replicates of 10 third instar larvae were treated. For evaluation of the synergistic effect of inhibitors on the insecticide, 100 mg/L of PBO, DEM, and DEF were added to separate aliquots of each dilution. The mortality was assessed after 48 h. Larvae were counted as dead if they could not be induced to move when touched with a probe. Control mortality was less than 10% in all bioassays. The PoloPlus program (LeOra Software, 2002) was used for probit analysis of concentration-response data.
Cloning and sequencing of PxNav cDNA
Total RNA was prepared from individual fourth instar larvae of P. xylostella with Trizol kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and was reverse transcribed with the Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Seven overlapping cDNA fragments were amplified covering domains IS1-IVS6 using the PCR primers and reaction conditions described by Sonoda et al. (2008). The cDNA fragments were cloned into a pGEM-T easy vector (Promega) and sequenced by Life Technologies (Shanghai, China). Sequence assembling and alignment were done using Geneious v7.1.4 Software (Biomatters Ltd., Auckland, New Zealand).
DNA-based genotyping assay for the F1845Y and V1848I mutations of PxNav
Genomic DNA was extracted from individual larvae of the susceptible Roth strain and eleven field populations of P. xylostella with the method described by Yuan et al. (2010). A pair of specific primers (forward: 5′-ATTTGGGATGTCCTTCTTC-3′; reverse: 5′-TGTACTTGTTGGGCTTGTG-3′) was used to amplify a genomic DNA fragment of 654 bp, which includes the location with two mutations (F1845Y and V1848I) of PxNav. The PCR reaction solution consisted of 12.5 μL 2×GC Buffer I (Takara, Japan), 1 μL of each primer (10 μmol/L), 1 μL of dNTP mixture (10 mmol/L), 1 μL template genomic DNA, 8.3 μL sterile distilled water and 0.2 μL LA Taq DNA polymerase (Takara, Japan) in a final volume of 25 μL. The PCR amplification was performed for 35 cycles (94 °C for 30 s, 47 °C for 30 s, and 72 °C for 1 min) with a final extension at 72 °C for 10 min. The PCR products were purified using an AxyPrep™ DNA Gel Extraction Kit (Axygen Biosciences, Union, CA), and directly sequenced with the reverse primer by Life Technologies (Shanghai, China). Genotypes of PxNav for individual larvae were identified according to their sequence chromatograms.
Results
Identification of two novel sodium channel mutations in a field-collected strain of P. xylostella (BY12) with high levels of resistance to SCBIs
The BY12 strain of P. xylostella, collected from Baiyun, Guangdong province of China in 2012, had developed 750- and 70-fold resistance to indoxacarb and metaflumizone, respectively (Table 1). Indoxacarb has been widely used to control P. xylostella, while use of metaflumizone is limited in the area where BY12 was sampled (Khakame et al., 2013), suggesting that selection of indoxacarb in the field may confer cross-resistance to metaflumizone in the BY12 population.
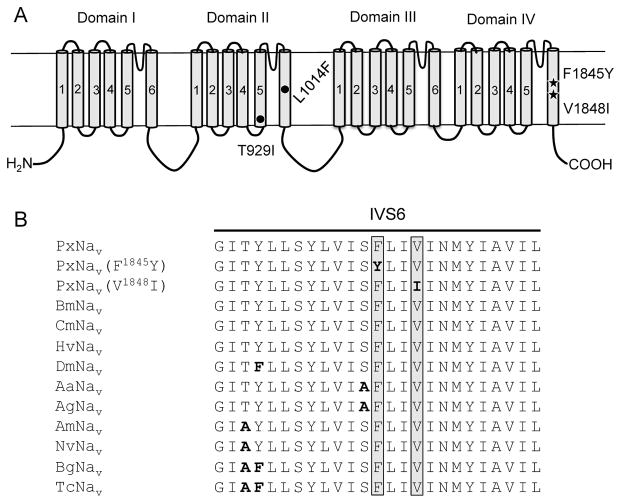
Because the two SCBIs, indoxacarb and metaflumizone, have a common target site (sodium channel) but distinct chemical structure, any cross-resistance between them would be most likely caused by target site alterations. To test this hypothesis, nearly full-length cDNA sequences (~1950 aa) encoding the sodium channel gene of P. xylostella (PxNav) were cloned from individual larvae of the susceptible reference Roth and BY12 populations, and their sequences were compared. The five individuals sequenced from the Roth strain all showed a consistent presence of an F at 1845 and a V at 1848 in PxNav. Positions are numbered according to the amino acid sequence of the PxNav sodium channel protein from Roth (GenBank accession no. KM027335). However, one of the five individuals from the BY12 strain had a heterozygous mutation at position 1845 (F/Y) and the other four individuals had a heterozygous mutation at position 1848 (V/I). Both F1845Y and V1848I mutations are located in the 6th segment of domain IV (IVS6) of PxNav, and the amino acid sequences in this region are highly conserved among sodium channel proteins from different insect species (Fig. 1). Besides the two mutations in IVS6, we also detected two additional mutations, L1014F in IIS6 and T929I in IIS5 (Fig. 1) in the BY12 population. Both mutations have previously been confirmed to confer resistance to pyrethroids in P. xylostella (Schuler et al., 1998; Sonoda et al., 2008).
Fig. 1.
Positions of sodium channel mutations in P. xylostella and sequence alignment of the IVS6 segment. A: The sodium channel consists of four main domains (I–IV) and six transmembrane segments (S1–S6) within each domain. The two mutations related to SCBI resistance are marked with solid pentacles, and the two mutations associated with pyrethroid resistance are marked with solid circles. The amino acid positions are numbered based on a PxNav sequence from the Roth strain of P. xylostella (GenBank accession no. KM027335). B: Sequence alignment of the IVS6 segment of sodium channels from 11 different insects. Two mutations sites (F1845Y and V1848I in grey) in PxNav were boxed. Polymorphic amino acids were in bold. PxNav: Plutella xylostella (GenBank accession no. KM027335); BmNav: Bombyx mori (NP_001136084.1); CmNav: Cnaphalocrocis medinalis (AGH70334.1); HvNav: Heliothis virescens (AAC26517.1); DmNav: Drosophila melanogaster (AAB59193.1); AaNav: Aedes aegypti (ACB37022.1); AgNav: Anopheles gambiae (CAM12801.1); AmNav: Apis mellifera (NP_001159377.1); NvNav: Nasonia vitripennis (NP_001128389.1); BgNav: Blattella germanica (AAC47484.1); TcNav: Tribolium castaneum (NP_001159380.1).
Association of the F1845Y and V1848I mutations with SCBI resistance among field populations of P. xylostella
A DNA-based genotyping assay for F1845Y and V1848I was developed through direct sequencing of the ~650 bp PCR product including IVS6 of PxNav. This assay allows identification of all three genotypes for each mutation in individual insects of P. xylostella (Fig. 2).
Fig. 2.
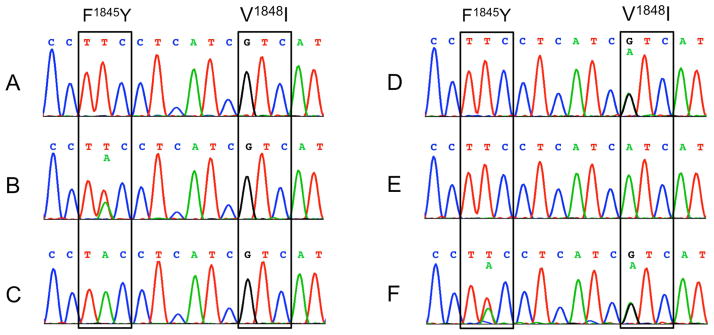
Chromatograms of the nucleotide sequences of a genomic DNA fragment including the F1845Y and V1848I mutation sites in the sodium channel gene of P. xylostella (PxNav). The triple codons corresponding to the F1845Y and V1848I mutations are boxed. A: the wild type homozygote (1845F, 1848V). B: heterozygous mutation at 1845 (F/Y). C: homozygous mutation at 1845 (Y). D: heterozygous mutation at 1848 (V/I). E: homozygous mutation at 1848 (I). F: heterozygous mutations at both 1845 (F/Y) and 1848 (V/I).
Ten field populations of P. xylostella, collected from three provinces of China during 2012–2014, developed high levels of resistance to indoxacarb (34- to 870-fold, Table 1), but low to medium levels of resistance to metaflumizone (1- to 70-fold, Table 1). Frequencies of PxNav alleles with either F1845Y or V1848I mutations ranged from 1.7% to 52.5% among the ten populations (Table 2). The V1848I mutation was detected from all ten populations, but the F1845Y mutation was only detected from five of the ten populations. Mutation frequencies for V1848I (1.7% to 42.5%) were much higher than for F1845Y (0 to 11.7%) among the ten populations (Table 2). It should be noted that the SZ14 population (for which there is no bioassay data) had the highest frequency of mutation (60%, including 23.3% F1845Y mutation and 36.7% V1848I mutation), and this population may be expected to possess high levels of resistance to indoxacarb and metaflumizone.
Table 2.
Frequencies of F1845Y and V1848I of PxNav in eleven field populations of P. xylostella.
| Population/Strain | N† | F1845Y
|
V1848I
|
Pooled frequency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F/F | F/Y | YY | Mutation frequency (%) | V/V | V/I | I/I | Mutation frequency (%) | |||
| Laboratory reference strain | ||||||||||
| Roth | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 |
| Field-collected populations | ||||||||||
| BY12 | 20 | 16 | 4 | 0 | 10 | 5 | 13 | 2 | 42.5 | 52.5 |
| ZH12 | 22 | 22 | 0 | 0 | 0 | 20 | 2 | 0 | 4.5 | 4.5 |
| BY13 | 15 | 14 | 1 | 0 | 3.3 | 11 | 4 | 0 | 13.3 | 16.6 |
| ZC13 | 27 | 27 | 0 | 0 | 0 | 25 | 1 | 1 | 5.6 | 5.6 |
| HZ13 | 20 | 20 | 0 | 0 | 0 | 15 | 5 | 0 | 12.5 | 12.5 |
| SY14 | 30 | 29 | 1 | 0 | 1.7 | 20 | 9 | 1 | 18.3 | 20 |
| HF14 | 28 | 26 | 2 | 0 | 3.6 | 26 | 2 | 0 | 3.6 | 7.2 |
| BY14 | 30 | 23 | 7 | 0 | 11.7 | 11 | 15 | 4 | 38.3 | 50 |
| HZ14 | 30 | 30 | 0 | 0 | 0 | 21 | 8 | 1 | 16.7 | 16.7 |
| ZC14 | 30 | 30 | 0 | 0 | 0 | 29 | 1 | 0 | 1.7 | 1.7 |
| SZ14 | 30 | 18 | 10 | 2 | 23.3 | 12 | 14 | 4 | 36.7 | 60 |
Number of insects genotyped.
Amongst mutation carrying individuals detected from the eleven populations, only 3 individuals from the BY14 population and 4 individuals from the SZ14 population had dual mutations, being heterozygous for both F1845Y and V1848I. Five clones of the ~650 bp genomic DNA fragment used for genotyping were sequenced from each of the seven individuals to ascertain whether the dual mutations were in a single allele or in different alleles. Sequencing results showed that there were no PxNav alleles with dual mutations.
The hypothesis that the level of resistance (resistance ratio, RR) is positively associated with the pooled frequency of the two mutations was tested using Pearson’s correlation analysis. The results show a significant association between RR and the pooled mutation frequency for both indoxacarb (r2 = 0.716, df = 8, P = 0.001, one-tailed) and metaflumizone (r2 = 0.886, df = 8, P < 0.0001, one-tailed). This indicates that the two mutations identified in this study are likely responsible for resistance to indoxacarb and metaflumizone in these P. xylostella.
Differential contribution of sodium channel mutations to SCBI resistance in the BY12 and SY14 strains of P. xylostella
It is interesting to note that both BY12 and SY14 strains were highly resistant to indoxacarb (750- and 850-fold, respectively), but differentially resistant to metaflumizone (70-fold in BY12, and only 6-fold in SY14). The proportions of individuals with one of the two sodium channel mutations were 95% in the BY12 strain, but only 36.7% in the SY14 strain (calculated from the data in Table 2). This may indicate that target site resistance mediated by sodium channel mutations may make a greater contribution to SCBI resistance in the BY12 than in SY14. In other words, metabolic resistance or other mechanisms may be more important in SY14 than in BY12.
To determine if metabolic resistance is involved, the synergistic effects of three synergists to indoxacarb were tested for SY14 and to metaflumizone for BY12. In the SY14 strain, the oxidase inhibitor PBO and the esterase inhibitor DEF significantly reduced resistance levels to indoxacarb (by 10- and 4-fold respectively), and the glutathine S-transferase depletor DEM had no effect on resistance (Table 3). In the BY12 strain, PBO and DEM had no effect on metaflumizone resistance and DEF had a very limited effect (SR 2.5-fold) (Table 3). These suggest that target site resistance is a major mechanism of resistance to metaflumizone in the BY12 strain, whereas enhanced metabolic detoxification by both oxidases and esterases may be important mechanisms of resistance to indoxacarb in the SY14 strain.
Table 3.
Synergism of PBO, DEM and DEF to metaflumizone and indoxacarb in the BY12 and SY14 populations of P. xylostella.
| Strain/Population | Insecticide | Slope (± SE) | LC50 (95%FL†) (mg/L) | RR‡ | SR§ |
|---|---|---|---|---|---|
| Roth | Metaflumizone | 2.5 ± 0.3 | 2.17 (1.48–3.07) | ||
| Indoxacarb | 1.8 ± 0.2 | 0.10 (0.08–0.13) | |||
| BY12 | Metaflumizone | 0.9 ± 0.2 | 152.8 (90.72–385.2) | 70 | |
| Metaflumizone+PBO | 0.9 ± 0.2 | 116.4 (72.99–201.1) | 54 | 1.3 | |
| Metaflumizone+DEM | 0.8 ± 0.1 | 112.7 (68.05–202.4) | 52 | 1.4 | |
| Metaflumizone+DEF | 0.8 ± 0.1 | 61.13 (33.66–100.9) | 28 | 2.5 | |
| SY14 | Indoxacarb | 1.0 ± 0.3 | 85.00 (42.66–177.8) | 850 | |
| Indoxacarb+PBO | 0.6 ± 0.2 | 21.26 (9.51–38.01) | 210 | 4 | |
| Indoxacarb+DEM | 0.9 ± 0.3 | 79.73 (50.08–277.1) | 800 | 1 | |
| Indoxacarb+DEF | 0.7 ± 0.2 | 8.63 (4.43–18.16) | 86 | 9.9 |
95% Fiducial limits.
RR (resistance ratio) = LC50 of BY12 or SY14/LC50 of Roth.
SR (synergistic ratio) = LC50 of insecticide alone/LC50 of insecticide with synergist.
Discussion
Metabolic mechanisms involved in resistance to SCBIs have been reported in several insect pest species. Resistance to indoxacarb in a field population of the oblique banded leafroller, Choristoneura rosaceana was reduced from 705- to 20-fold by PBO, suggesting enhanced detoxification mediated by oxidases is a major mechanism of resistance to indoxacarb (Ahmad et al., 2002; Ahmad & Hollingworth, 2004). A high level of resistance to indoxacarb (778-fold) in a field-derived population (Indoxa-DEL) of P. xylostella was largely inhibited by either PBO or a PBO analogue specific to the inhibition of esterases. The authors claimed that indoxacarb resistance in the Indoxa-SEL population was due to enhanced esterase activities (Sayyed & Wright, 2006). In a laboratory-selected strain of P. xylostella with a medium level of resistance to indoxacarb (31-fold), both synergistic suppression by metabolic inhibitors and increased metabolic enzyme activities were observed indicating that metabolic mechanisms were involved in resistance to indoxacarb in the selected strain (Nehare et al., 2010). Resistance to indoxacarb (118-fold) in a laboratory-selected strain (NYINDR) of Musca domestica was partially overcome by PBO, but not by DEF or DEM, indicating oxidase-based detoxification is involved (Shono et al., 2004).
In the current study, two novel mutations associated with SCBI resistance were identified in IVS6 of the PxNav channel from several field-collected populations of P. xylostella. To our knowledge, this study is the first one that documents target-site modification as a mechanism of SCBI resistance. The IVS6 region of sodium channel (which includes the F1845Y and V1848I mutations) is highly conserved among Lepidoptera, Diptera, Hymenoptera, Blattodea and Coleoptera (up to 97% identity). Further, the two mutations are in a region (IVS6) thought to be critical for SCBI binding in the mammalian sodium channel Nav1.4 (Silver and Soderlund, 2007). This led us to examine the effect of these two mutations on the sensitivity of a cockroach sodium channel to SCBIs. Indeed, we found that F1845Y and V1848I reduced the sensitivity of cockroach sodium channels to indoxacarb, DCJW and metaflumizone in Xenopus oocytes (Manuscript in preparation). In view of common and distinct sodium channel mutations that are involved in pyrethroid resistance (Dong et al., 2014), F1845Y and V1848I mutations could also be valuable molecular markers for screening homologous mutations in other insect pests. On the other hand, it is possible that additional new mutations may occur and confer resistance to SCBIs in P. xylostella and other pest species.
Field populations of P. xylostella collected from China during 2009–2011 had developed various levels of resistance to indoxacarb (5- to 110-fold), but no cross-resistance to metaflumizone (Khakame et al., 2013). In the present study, ten field populations collected from southern China during 2012–2014 had developed 34- to 870-fold resistance to indoxacarb, but only 1- to 70-fold resistance to metaflumizone. Resistance levels to both indoxacarb and metaflumizone were significantly correlated to frequencies of the two sodium channel mutations in the ten field populations. This is precisely the case for the BY12 and BY14 populations of P. xylostella, which have the highest mutant allele frequencies of PxNav (52.5% and 51.7%) and the highest resistance to metaflumizone (70- and 49-fold) among the ten populations screened. It suggests that sodium channel mutations may result in cross-resistance between the two SCBIs in P. xylostella.
Interestingly, one field population (HZ11) of S. exigua collected from Huizhou, Guangdong province of China in 2011 developed 942-fold resistance to metaflumizone, but only 16-fold resistance to indoxacarb (Su & Sun, 2014). Synergistic analysis with metabolic inhibitors suggested the role of detoxification was limited in metaflumizone resistance for the HZ11 population (Su & Sun, 2014). It will be intriguing to check if there are any sodium channel mutations associated with the high level resistance to metaflumizone seen in S. exigua.
Pyrethroids have been used as a major class of insecticides for decades to control diamondback moth, and serious resistance to pyrethroids has been evolved in field populations worldwide (Talekar & Shelton, 1993; Furlong et al., 2013). A number of mutations (L1014F, T929I and M918I) in the sodium channel gene (PxNav) conferring nerve insensitivity have been associated with pyrethroid resistance in P. xylostella (Schuler et al., 1998; Sonoda et al., 2006, 2008, 2010, 2012). The three field populations (BY14, HZ14 and ZC14) collected in 2014 from Guangdong province showed very high levels of resistance to cypermethrin (300- to 680-fold) compared with the susceptible Roth strain (unpublished data), and we found that L1014F and T929I mutations were fixed (100%), but the M918I mutation was detected at very low frequencies (0 in ZY14, and 6.7% in HZ14 and ZC14) (unpublished data). It shows the two mutations (1845Y and 1848I) associated with SCBI-resistance in P. xylostella occur on a 1014F-929I background, which confers resistance to pyrethroids. This is the first report that two sets of mutations are evolved sequentially in a single target gene (sodium channel) to cope with two different classes of insecticides (pyrethroids and SCBIs).
Acknowledgments
This work was supported by grants from the Ministry of Agriculture of China (No.201203038 to YW), the “111” project of the Ministry of Education of China (B07030 to YW) and the National Institutes of Health (GM057440 to KD).
References
- Ahmad M, Hollingworth RM, Wise JC. Broad-spectrum insecticide resistance in obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) from Michigan. Pest Management Science. 2002;58:834–838. doi: 10.1002/ps.531. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Hollingworth RM. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) Pest Management Science. 2004;60:465–473. doi: 10.1002/ps.829. [DOI] [PubMed] [Google Scholar]
- APRD. Arthropod pesticide resistance database. 2015 ( http://www.pesticideresistance.org/)
- BASF. BASF Agricultural products, Metaflumizone Worldwide Technical Brochure. 2007. [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. Journal of Physiology. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che W, Shi T, Wu Y, Yang Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. Journal of Economic Entomology. 2013;106:1855–1862. doi: 10.1603/ec13128. [DOI] [PubMed] [Google Scholar]
- Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochemistry and Molecular Biology. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong MJ, Wright DJ, Dosdall LM. Diamondback moth ecology and management: problems, progress, and prospects. Annual Review of Entomology. 2013;58:517–541. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- Harder HH, Riley SL, McCann SF, Irving SN. DPX-MP062: a novel broad-spectrum, environmentally soft, insect control compound. Proceedings of the 1996 Brighton Crop Protection Conference: Pests and Diseases; 1996. pp. 449–454. [Google Scholar]
- Khakame SK, Wang X, Wu Y. Baseline toxicity of metaflumizone and lack of cross resistance between indoxacarb and metaflumizone in diamondback moth (Lepidoptera: Plutellidae) Journal of Economic Entomology. 2013;106:1423–1429. doi: 10.1603/ec12494. [DOI] [PubMed] [Google Scholar]
- LeOra Software. Polo Plus, a User’s Guide to Probit or Logit Analysis. LeOra Software; Berkely, CA, USA: 2002. [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1–26. [PubMed] [Google Scholar]
- Nehare S, Moharil MP, Ghodki BS, Lande GK, Bisane KD, Thakare AS, Barkhade UP. Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. Journal of Asia-Pacific Entomology. 2010;13:91–95. [Google Scholar]
- Pang S, You W, Duan L, Song X, Li X, Wang C. Resistance selection and mechanisms of oriental tobacco budworm (Helicoverpa assulta Guenee) to indoxacarb. Pesticide Biochemistry and Physiology. 2012;103:219–223. [Google Scholar]
- Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Veterinary Parasitology. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Santos VC, de Siqueira HA, da Silva JE, de Farias MJ. Insecticide resistance in populations of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), from the state of Pernambuco, Brazil. Neotropical Entomology. 2011;40:264–270. doi: 10.1590/s1519-566x2011000200017. [DOI] [PubMed] [Google Scholar]
- Sayyed AH, Wright DJ. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae) Pest Management Science. 2006;62:1045–1051. doi: 10.1002/ps.1270. [DOI] [PubMed] [Google Scholar]
- Schuler TH, Martinez-Torres D, Thompson AJ, Denholm I, Devonshire AL, Duce IR, Williamson MS. Toxicological, electrophysiological, and molecular characterisation of knockdown resistance to pyrethroid insecticides in the diamondback moth, Plutella xylostella (L.) Pesticide Biochemistry and Physiology. 1998;59:169–182. [Google Scholar]
- Shad SA, Sayyed AH, Fazal S, Saleem MA, Zaka SM, Ali M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae) Journal of Pest Science. 2012;85:153–162. [Google Scholar]
- Shono T, Zhang L, Scott JG. Indoxacarb resistance in the house fly, Musca domestica. Pesticide Biochemistry and Physiology. 2004;80:106–112. [Google Scholar]
- Silver KS, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Silver KS, Nomura Y, Salgado VL, Dong K. Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel-blocker insecticides. Neurotoxicology. 2009;30:613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver KS, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pesticide Biochemistry and Physiology. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver KS, Du Y, Nomura Y, Oliveira EE, Salgado VL, Zhorov BS, Dong K. Voltage-gated sodium channels as insecticide targets. Advances in Insect Physiology. 2014;46:389–433. doi: 10.1016/B978-0-12-417010-0.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, Igaki C, Ashfaq M, Tsumuki H. Pyrethroid-resistant diamondback moth expresses alternatively spliced sodium channel transcripts with and without T929I mutation. Insect Biochemistry and Molecular Biology. 2006;36:904–910. doi: 10.1016/j.ibmb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tsukahara Y, Ashfaq M, Tsumuki H. Genomic organization of the para-sodium channel α-subunit genes from the pyrethroid-resistant and -susceptible strains of the diamondback moth. Archives of Insect Biochemistry and Physiolology. 2008;69:1–12. doi: 10.1002/arch.20246. [DOI] [PubMed] [Google Scholar]
- Sonoda S. Molecular analysis of pyrethroid resistance conferred by target insensitivity and increased metabolic detoxification in Plutella xylostella. Pest Management Science. 2010;66:572–575. doi: 10.1002/ps.1918. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Shi X, Song D, Zhang Y, Li J, Wu G, Liu Y, Li M, Liang P, Wari D, Matsumura M, Minakuchi C, Tanaka T, Miyata T, Gao X. Frequencies of the M918I mutation in the sodium channel of the diamondback moth in China, Thailand and Japan and its association with pyrethroid resistance. Pesticide Biochemistry and Physiology. 2012;102:142–145. [Google Scholar]
- Su JY, Sun XX. High level of metaflumizone resistance and multiple insecticide resistance in field populations of Spodoptera exigua (Lepidoptera: Noctuidae) in Guangdong Province, China. Crop Protection. 2014;61:58–63. [Google Scholar]
- Talekar NS, Shelton AM. Biology, ecology, and management of the diamondback moth. Annual Review of Entomology. 1993;38:275–301. doi: 10.1146/annurev-ento-010715-023622. [DOI] [PubMed] [Google Scholar]
- Tong H, Su Q, Zhou XM, Bai LY. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. Journal of Pest Science. 2013;86:599–609. doi: 10.1007/s10340-013-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood PN, Davies TG, Mellor IR, O’Reilly AO, Peng F, Vais H, Khambay BP, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-S5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Letters. 2007;581:5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Archives of Insect Biochemistry and Physiolology. 1998;37:91–103. [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology and biology in insects. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; New York: 2005. pp. 30–53. [Google Scholar]
- Yuan G, Gao W, Yang Y, Wu Y. Molecular cloning, genomic structure, and genetic mapping of two Rdl-orthologous genes of GABA receptors in the diamondback moth, Plutella xylostella. Archives of Insect Biochemistry and Physiolology. 2010;74:81–90. doi: 10.1002/arch.20361. [DOI] [PubMed] [Google Scholar]
- Zalucki MP, Shabbir A, Silva R, Adamson D, Shu-Sheng L, Furlong MJ. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? Journal of Economic Entomology. 2012;105:1115–1129. doi: 10.1603/EC12107. [DOI] [PubMed] [Google Scholar]
- Zhao JZ, Collins HL, Li YX, Mau RFL, Thompson GD, Hertlein M, Andaloro JT, Boykin R, Shelton AM. Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. Journal of Economic Entomology. 2006;99:176–181. doi: 10.1603/0022-0493(2006)099[0176:MODMLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhou C, Liu YQ, Yu WL, Deng ZR, Gao M, Liu F, Mu W. Resistance of Spodoptera exigua to ten insecticides in Shandong, China. Phytoparasitica. 2011;39:315–324. [Google Scholar]