Abstract
Bone tissue has a remarkable ability to regenerate and heal itself. However, large bone defects and complex fractures still present a significant challenge to the medical community. Current treatments center on metal implants for structural and mechanical support and auto- or allo-grafts to substitute long bone defects. Metal implants are associated with several complications such as implant loosening and infections. Bone grafts suffer from donor site morbidity, reduced bioactivity, and risk of pathogen transmission. Surgical implants can be modified to provide vital biological cues, growth factors and cells in order to improve osseointegration and repair of bone defects. Here we review strategies and technologies to engineer metal surfaces to promote osseointegration with the host tissue. We also discuss strategies for modifying implants for cell adhesion and bone growth via integrin signaling and growth factor and cytokine delivery for bone defect repair.
The need for improved orthopaedic implants
With increased life expectancy and an aging world population, there is a rapid increase in musculoskeletal conditions and diseases such as fractures, osteoporosis and bone metastases. As a result, bone-related medical treatments and costs are on the rise. For example, over 1 million total hip and knee replacements are performed annually in the U.S.A. at cost of over $25 billion [1]. Current methods for surgical treatments for fractures and joint arthroplasties primarily use metal implants. However, such implants can fail due to implant motion, inflammation and bone resorption and osteolysis due to implant loosening, wear and improper loading [2, 3]. Bone grafting is needed for more than 600,000 cases in the U.S.A. caused by cancer and traumatic injuries and costs around $2.5 billion [4]. Auto/allo bone grafts are used to treat such long bone defects. However, there are still shortcomings associated with bone grafting. Autografts, which are considered the gold standard in the field [5, 6], require long surgery times as the bone has to be harvested from the patient’s healthy tissue and then re-implanted at the diseased site [7, 8]. Bone is primarily harvested from the iliac crest or pelvis, and this can lead to donor site morbidity and often there is limited tissue to harvest. Allografts are tissues obtained from genetically distinct sources such as human cadavers and donors. They suffer from immune rejection, reduced bioactivity and the risk of pathogen transmission. The limited osseointegration of surgical metal implants and regeneration potential of auto/allo-grafts have prompted interest in other alternatives for bone tissue engineering. In this review, we will discuss major research strategies that hold promise for the future clinical translation with main emphasis on metal and synthetic polymer implants.
Bone repair biology and current challenges
Osteoblast and osteoclast are the main cells involved in remodeling of bone – osteoblasts lay down bone matrix while osteoclasts are involved in resorbing the bone tissue. Bone formation takes place via two major pathways: intramembranous and endochondral ossification. For detailed readings on these processes, readers are referred to other reviews [9–12]. For most fractures, bone tissue heals itself; however, for complex fractures and diseases, outside intervention is often required for complete healing to take place. It is important to understand the mechanism(s) involved in this healing process in order to develop technologies to facilitate treatment. There are two major mechanisms of bone healing: direct bone growth and indirect bone growth after callous formation.
Direct bone healing involves the growth of bone from the broken ends at fracture site without any intermediate fibrous tissue formation. Osseointegration of surgical implants mostly utilize this mode of bone healing. Osseointegration is defined as bonding of living bone tissue with surgical implants such that implants can replace bone and perform load bearing functions. An implant is considered osseointegrated if there is no relative motion between the implant and bone. There are 3 major stages which are involved in direct healing: woven bone formation, adaptation of bone mass to load and, adaptation of bone structure to load (bone remodeling) [13].
Indirect bone healing involves inflammation leading to callous formation via intra-membranous ossification. This is followed by endochondral ossification and resorption of the callous [14]. This process requires a coordinated functioning of progenitor cells. The source of these progenitor cells for bone fracture and defect healing is believed to come from the mesenchymal stem cell niche (bone marrow, periosteal and endosteal envelopes) in the surrounding region [15] and the vasculature disrupted during the fracture. Blood from the vasculature fills up the defect/fracture site and results in a clot, through inflammatory biomolecules and cells initiate the healing process. However, if the defect size is large enough then the bone cannot heal itself. The minimum length of the bone, where if left untreated, there will be no union is termed critical sized bone defect and can be different depending on the location of injury or the animal model used [16]. Such defects lack sufficient blood supply for callous formation and need surgical intervention to provide mechanical support and promote bone repair.
These healing processes involve osteogenic progenitor cells, which are recruited by various cytokines and growth factors. These cells actively participate in osseointegration and bone repair. The timing and concentration of these cytokines and growth factors are critical considerations in designing biomaterial scaffolds. Various growth factors have been shown to play a part in bone repair, and currently bone morphogenetic protein (BMP) (BMP-2 and BMP-7) are clinically approved for use in patients. Other growth factors and cytokines, including BMP-4, transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), insulin-like growth factor (IGF), vascular growth factor (VEGF), platelet derived growth factor (PDGF), and stromal derived growth factor (SDF1), are important to bone formation [14, 17–19]. Although use of BMP-2 and BMP-7 have shown positive results, there are concerns about adverse effects on humans due to use of supraphysiological doses, inflammatory side effects, and low retention times of these growth factors at the injury site [20]. Progenitor cells can also be delivered at the site of injury to facilitate healing. Once the cells are delivered/recruited at the site of fracture, it is important to provide the necessary biological cues so that they promote bone formation. A major class of cell membrane proteins that cells use to interact with their surrounding matrix is integrin. Integrins are transmembrane receptor proteins that interact with extracellular matrix components and regulate diverse cellular processes. The integrin heterodimer, consisting of alpha and beta subunits, binds to various extracellular matrix (ECM) proteins like fibronectin, collagen, vitronectin, laminin, osteopontin, and bone sialoprotein. Integrin signaling is an important part of the cell niche in bone tissue formation and healing [21–23] and can be used to engineer material surfaces to direct the appropriate cell response. Such engineered biomaterials can also be used to deliver cells and allow timely release of various growth factors and cytokines.
Orthopaedic Implant Requirements
The main function of orthopaedic implants such as joint prostheses, plates, and screws is to provide mechanical and structural support, integrate with the damaged tissue and provide biological cues to promote healing. The materials used for implants should be biocompatible, non-immunogenic and be able to integrate well with the host tissue. Metal implants are used for applications where mechanical support is essential such as weight bearing long bones (femur, tibia etc.) and long bone and vertebral fractures. The main aim of such implants is to bind strongly to bones such that there is minimal movement between implant and host tissue and provide physiological load bearing functionality to the implant site [24]. Polymeric implants are proposed for applications pertaining to osseointegration as well as bone growth and regeneration due to their ability to control key material properties and deliver bioactive agents. Bone growth takes place from the two ends of the defect and can be enhanced by use of implants by means of release of essential growth factors and proteins, biological signals to cells in a spatio-temporal manner as discussed in the previous section. Here, we will discuss various types of metal and polymer implants used for bone integration and regeneration. For other type of substrates such as ceramics, demineralized bone matrix and calcium phosphate cements, we refer readers to other excellent reviews and papers in the field [25–30].
Metal Implants
Metal substrates, in the form of screws and plates, are widely used when there is immediate need to provide stability and structural support. Considerable research has focused on titanium alloys (i.e. Ti-6Al-4V) and stainless steel. Stainless steel has been used for over 100 years as bone implant material. Most commonly used stainless steel surgical implants are made up of type 316L due to its excellent mechanical properties and resistance to corrosion due to presence of high chromium content (16%), molybdenum (2%) and low carbon content (<0.03%). In recent years, titanium and its alloys have also gained significant interest. It has similar strength to stainless steel but is much lighter in comparison. Titanium alloy has higher resistance to repeated stress loading than commercially pure titanium and hence is ideal for load-bearing orthopaedic applications. It also has lower modulus of elasticity and is more conducive to minimize stress at interfaces. Albrektsson et al. showed that titanium has closer contact with the bone tissue compared to other metal implants like stainless steel and zirconium [31, 32]. This closer interaction of titanium surfaces is attributed to a stable oxide layer (TiO2) that is formed on titanium surfaces and prevents corrosion. Although these metal implants are biocompatible, they can become loose and hence there is a need to modify these implants such that they can form strong bonds with host tissue and provide necessary signals.
Depending on the location and application, implants can have different requirements. Fracture fixation plates and screws are fixed with bolts and are typically removed from the body following healing. Such implants have low to moderate osseointegration requirements. Other implants such as joint replacements and pedicle screws and rods used in spinal fusion surgeries are required to provide support for an entire lifetime and hence have high osseointegration requirements. Although the success rate of primary hip and knee arthroplasties exceeds 90% at 15 years [33], there is a significant need to extend the finite lifetime of current orthopaedic implants and improve implant function, particularly in challenging cases such as revisions, younger/more active patients, and conditions with compromised bone stock (e.g., osteoporosis) [33, 34]. It is important that the implants have strong adhesion with the host tissue as quickly as possible [35, 36] and begin healing process as delayed healing can result in fibrous tissue development [37, 38]. Stability of implants after surgery is very essential for successful osseointegration. Bragdon et al. showed that 20 μm of oscillating motion does not affect osseointegration, however, 40 and 150 μm of oscillating motion of implants did not support bone ingrowth when scaffolds were implanted in dog distal femurs [39]. Other groups have also shown that excessive implant motion results in minimal osseointegration [40]. Hence for successful osseointegration, primary stability of implants is desired. Various strategies to achieve osseointegration with metal implants are reviewed here (Figure 1, Table 1).
Figure 1.
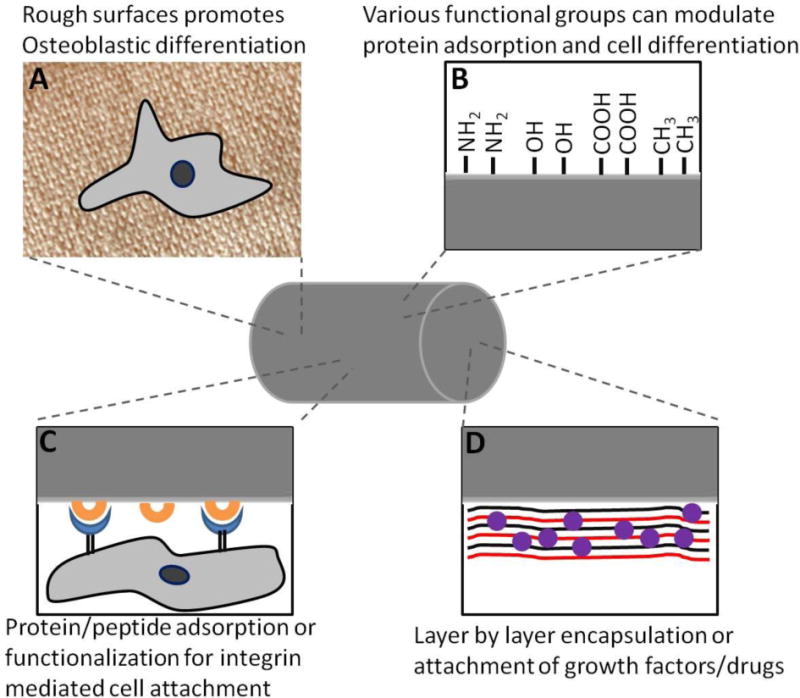
Metal Implants for bone tissue engineering. Osseointegration on metal implants can be mediated by A) rough surfaces that promote cell differentiation, B) surface functional groups that can promote adsorption of different proteins and cell ligands, C) adsorption of integrin ligands or D) immobilizing growth factors and drugs via layer by layer technique.
Table 1.
Strategies for bone tissue engineering using metal implants.
| Material | Strategies to increase Osseointegration | References |
|---|---|---|
| Titanium (Ti-6Al-4V) Stainless Steel (316 Grade) | Increasing Surface Roughness | [41, 42], [43], [44], [45], [46], [47], [48] |
| NH2 and OH functional groups on the surface | [51], [52] | |
| Making surface hydrophilic and increasing its wettability | [49], [50] | |
| CaP/HA coatings | [53], [54], [55], [56, 57] | |
| Engaging Integrins via RGD, collagen and Fibronectin sequences adsorbed or conjugated on metal surfaces | [67], [68], [69–71], [72], [73], [74] | |
| Integrin Clustering | [75] | |
| Growth Factor Release | [76], [77], [78], [79], [81] [89], [90] | |
| Other therapeutic release approaches (bisphosphonates, antibiotics, other small molecules) | [82], [83], [84], [85–88], [91], [92], [93] |
Surface Characteristics
Since the surface is the only region in contact with host bone tissue, many attempts have been made to modify the surface properties such as surface roughness and chemistry to enhance host tissue integration and mechanical fixation. Surface roughness plays an important part in cell attachment and differentiation. Increasing surface roughness can lead to increase in available area for host proteins and cells to interact with implants. Smooth surfaces promote osteoblast cell attachment and proliferation while rough surfaces promote differentiation and mineralization in vitro [41, 42]. Culturing osteoblast cell lines on rough surfaces led to an increase in production of alkaline phosphatase (ALP) and osteocalcin which are cell markers for osteoblastic differentiation [43]. Protein kinase A and phospholipase A2 were found to be involved in effects of surface roughness on osteoblasts [44]. Phospholipase A2 catalyzes prostaglandin E2 production and makes osteoblasts responsive to systemic hormones such as 1,25-(OH)2D3. It was further shown that α2β1 integrin signaling mediates osteoblastic differentiation on rough titanium surfaces [45]. α2 knockout cell lines blocked any surface dependent differentiation in MG63 human osteoblast cells [45]. Klokkevold et al. showed that acid etched titanium screws enhanced endosseous integration [46]. These screws were implanted in rabbit distal femurs and torque was measured to unscrew the implants. Dual acid etched screws and titanium plasma sprayed screws were found to significantly enhance endosseous integration compared to machined screws. Roughness values of Ra around 1–2 μm seems to be ideal for bone-implant interactions [47]. However, there is wide heterogeneity among studies due to difference in instruments and techniques used and it is not possible to conclude what specific surface roughness is needed for various kinds of implants [48].
Surface chemical groups on implants can also have a significant influence in bone growth and differentiation. There is evidence that hydroxylation of titanium surfaces increases its wettability and can promote cell attachment, however, this needs to be tested in vivo to see if hydroxylation leads to faster osseointegration of such implants [49]. Similarly, surface microtexture and chemistry can be modified via electrochemical anodization in strong acids at high potential which also results in thickening of titanium oxide and promotes bone formation when compared to untreated surfaces [50]. Keselowsky et al. used self-assembled monolayers of alkanethiols on gold surface presenting terminal CH3, OH, COOH and NH2 groups [51]. Different functional groups modulated the conformation of adsorbed proteins. It was shown that fibronectin had most active conformation on OH followed by COOH and NH2 groups which also resulted in higher cell adhesion to these surfaces via α5β1 integrin. Cells on OH and NH2 surfaces used α5β1 integrin while those on COOH surfaces utilized both α5β1 and αvβ3 for adhesion. This integrin specificity resulted in differential effects on osteoblast differentiation. Cells that were cultured on fibronectin adsorbed onto OH and NH2 surfaces had significantly more ALP expression and mineralization compared to COOH and CH3 surfaces [52]. However, this strategy relied on pre-coating fibronectin on implant surfaces before cells were cultured. It remains to be seen how bone formation would be affected in vivo in response to different surface functional groups pre-coated with protein of interest.
Surface Coatings
Calcium phosphate (CaP), including hydroxyapatite (HA), coatings have been proposed to enhance bone integration of metal implants. Soballe et al. showed that HA coated implants in femoral condyles of dogs can help in bridging initial gaps as large as 1–2 mm between implants and bone while uncoated titanium implants had significantly less bone growth [53]. Cook et al. showed that HA coated implants in canine femoral transcortical model had significantly higher bone ingrowth and interface attachment strength at 6, 8, 12, 18 and 26 week time points [54]. CaP coatings applied on titanium implants showed significantly higher bone contact compared to uncoated implants in goat femoral diaphysis at 6, 12 and 24 weeks [55]. Although these coatings are promising, they have shown variable results in patients [56, 57]. For detailed analysis of CaP/HA coatings, we encourage readers to refer to other reviews in the field [58–60].
An approach to significantly improve the mechanical fixation of implants to bone is to modify the surfaces with bioadhesive coatings. Typically, ECM proteins that contain cell binding domains can be adsorbed/functionalized on implant surfaces to mediate cell attachment and differentiation. However, ECM proteins can be large and surface adsorption may render the cell binding domains inaccessible. Peptide sequences containing the minimal cell binding domains have shown to be active both in vitro and in vivo [61–64]. Such peptides are more resistant and retain their activity after harsh treatments than compared to their full length counterparts [65]. It is also critical that the coating is compatible with sterilization and packaging techniques needed for bioimplants. Coatings that can be applied in the operation theatre would be ideal as it would allow surgeon to choose the implant type/size and apply the coating depending on the state of treatment site [66]. Few strategies have been explored in this regard. Ferris et al. coated titanium implants with RGD peptide and showed increased bone formation at 2 and 4 weeks [67]. RGD containing sequences bind to various types of integrins but are not integrin selective [68]. Several studies with RGD peptides have given only marginal improvement in osseointegration [69–71]. To increase the specificity of the signal, other more specific integrin binding sequences can be used. Reyes et al. showed that a triple helical peptide, GFOGER, derived from α1 (I) chain of type I collagen, mediates cell attachment to surfaces passively coated with this peptide. The cells attached using α2β1 integrin in a dose dependent manner and exhibited cell adhesion and spreading comparable to those cultured on type I collagen [72]. It was further shown that bone marrow stromal cells cultured on titanium surfaces coated with GFOGER had higher ALP activity and calcium content compared to uncoated surfaces [73]. These titanium implants had higher bone-implant contact when implanted in rat tibia and had significantly higher pull out force compared to uncoated and collagen coated implants after 4 weeks. Similarly, titanium samples coated with a recombinant fibronectin type III 7–10th fragment(FN7–10) that binds α5β1 integrin showed enhanced ALP activity, calcium content and mineralization and were also found to increase bone apposition and pull-out force compared to uncoated and full length fibronectin coated surfaces [64]. These enhancements in cell differentiation and osseointegration were attributed to selective α5β1 integrin binding for FN7–10 compared to α5β3 and other integrins for uncoated surfaces.
In order to control the presentation of bioligands from implant surfaces, titanium surfaces have been chemically modified to grow a non-fouling oligo (ethylene glycol methacrylate) (OEGMA) polymer which was then chemically conjugated to cell adhesive ligands such as RGD and FN7–10. Rat bone marrow stromal cells cultured on such surfaces showed higher Runx2, osteocalcin, bone sialoprotein, ALP activity and calcium content on FN7–10-functionalized surfaces compared to RGD and non-functionalized surfaces [74]. Implants presenting FN7–10 exhibited higher bone-implant contact and pull-out force when implanted in rat tibial metaphysis [74]. Such OEGMA-modified implant surfaces can be used for clustering and enhancing integrin signals. Petrie et al. used OEGMA brushes on titanium implants and functionalized monomer, dimer, trimer and pentamer of FN7–10 and showed that trimers and pentamer induce clustering of integrin receptors [75]. Trimers and pentamer induced higher osteogenic signaling and differentiation of human mesenchymal stem cells in vitro and exhibited bone formation and higher pull-out force when implanted in rat tibial metaphysis for 4 and 12 weeks [75]. It remains to be seen if such bioadhesive coatings can enhance osseointegration under diseased states such as osteoporosis.
Therapeutic Release Strategies to Enhance Osseointegration
Although surface functionalization strategies have potential to improve osseointegration, there is still a significant need to further augment these technologies especially in challenging diseased states. To address this challenge, research has focused on incorporating release of therapeutic biomolecules and drugs to enhance osseointegration. Most approaches have focused on adsorption and presentation of therapeutics on implant surfaces. Thorey et al. coated titanium implants with BMP-2 which resulted in improved bone volume compared to uncoated implants [76]. Ramazanoglu et al. co-delivered BMP-2 and VEGF on titanium implants coated with calcium phosphate and showed enhanced bone mineral density around implants than compared to all other groups demonstrating synergism between the two growth factors [77]. Although promising, immobilization of cytokines and growth factors on implant surfaces often leads to decrease in biological activity. BMP-2 is a fairly hydrophobic protein and adheres strongly to material surfaces and often denatures in the process losing its activity [78]. A sacrificial layer between implant and therapeutics has been proposed to overcome this issue [78–80]. Kashiwagi et al. constructed an artificial protein containing 3 reversible material binding motifs for titanium and fused it with the N-terminal of BMP-2 [78]. BMP-2 immobilized without the reversible binding motifs was unable to bind to its receptor, bone morphogenetic protein receptor type 1A (BMPR-1A) and was less active. Addition of reversible binding motif made it accessible to the receptor and more biologically active [78]. Such reversible and directional binding can allow immobilization and release of more active protein. Yuasa et al. later showed that the titanium implants adsorbed with this artificial fusion protein were active in vivo [79]. Collagen scaffolds coated with titanium and adsorbed with fusion protein when implanted in abdominal muscle promoted cartilage formation and mRNA of ALP and bone sialoprotein were increased by significant amount compared to scaffolds without the fusion protein [79]. Lu et al. developed a modular bone morphogenetic peptide (mBMP) which had a high affinity for hydroxyapatite [81]. This peptide was incorporated onto the titanium surfaces coated with hydroxyapatite and resulted in significant increase in implant push-out force in a sheep femoral condyle model [81]. Although promising, there are concerns of adverse immune reactions associated with use of such modified fusion proteins and peptides.
N-bisphosphonate coatings over implants have also been proposed to improve osseointegration. Tengvall et al. coated stainless steel screws with N-bisphosphonate (pamidronate and ibandronate) and implanted in rat tibia had significantly higher pull out force and energy compared to uncoated screws after 2 weeks [82]. Bisphosphonates act on osteoclast and reduce resorption of bone that is present in diseases such as osteoporosis and early stages of surgery due to trauma caused by implantation [83]. Such local delivery to implantation sites can reduce systemic side effects. Wermilin et al. further showed that such coatings lead to increase in pull-out force for up to 8 weeks [84]. Similar results were demonstrated with stainless steel and titanium implants coated with bisphosphonates [85–88]. Although promising, drugs and cytokines adsorbed on the surfaces exhibit burst release and can lead to adverse side effects.
Macdonald et al. used a layer by layer technique to increase the amount of adsorbed BMP-2 on implants [89]. Alternate coatings of BMP-2 and charged polymer allowed microgram quantities of BMP-2 to be loaded on the surface which had low burst release. They showed that such implants when implanted intramuscularly, initiated bone growth and maturation [89]. Shah et al. developed a self-assembled, polymer based osteogenic coating to improve mechanical fixation of implants [90]. The base coating consisted of positively charged chitosan and hydroxyl apatite alternated with negatively charged poly(acrylic acid) (PAA). The second component of this coating had hydrolytically degradable cationic poly(β-amino ester) alternated with PAA and BMP-2. BMP-2 release from titanium scaffolds was controlled and sustained for over 15 days and implants showed significant increase in pull out force [90]. Song et al. developed polymer nanofiber coatings of polycaprolactone (blended with hydroxyapatite) and polyvinyl alcohol (blended with collagen) (PCLCol/PVAHA) using co-axial electrospinning technique [91]. These nanofibers were then coated on titanium implants and found to be stable when tested on a porcine ex-vivo implantation model. Doxycline (anti-bacterial agent) and dexamethasone (osteoblast differentiation inducing agent) were incorporated in polymer nanofibers and enhanced proliferation and adhesion of pre-osteoblastic MC3T3-E1 cells in vitro [91]. Such polymer coatings can allow sustained and controllable release of therapeutics. However, effects of such coatings on implant osseointegration are yet to be examined.
Various other methods such as polymer and hydrogel coatings to trap biomolecules have been tried and offer some promising results [92]. Pauly et al. used a polymer coating of poly(lactic-co-glycolic acid) (PLGA) carrying simvastatin on titanium Kirschner wires and showed significantly enhanced torsional stiffness and maximum load after 42 days of implantation in rat tibia [93]. However, statins are associated with many side effects and further characterization is needed to ensure complete safety of such strategies. Microbial infection on implant surface is a serious concern and results in bone resorption. Infections are one of the major causes of implant failure. Release of antibiotics from the implant surface is proposed as a solution to tackle this challenge. For detailed discussion, readers are referred to other reviews in the field [94].
Although all these release strategies have shown promise, there is a need to characterize the release kinetics of each biomolecule, particularly in vivo, to better understand its effects on bone growth and osseointegration. There is very little literature on release kinetics and what is ideal release profile for various growth factors and cytokines to maximize osseointegration. In vivo release of growth factors and cytokines can be measured by labeling them with near infra-red dyes and monitoring its change over time non-invasively [95]. Other approaches such as characterizing amounts left on implants at various terminal points can also be used. There is also need to combine some of these therapeutic release technologies with material technologies described in previous sections to evaluate synergism in disease models like osteoporosis.
Polymeric implants for screws and plates
Polymeric screws have been developed and provide certain advantages such as radiolucency and ability to tune the elastic modulus in range of bone (~18 GPa) to avoid modulus mismatch. Polyaryletherketones (PAEKs) polymers have been widely used for applications such as to fill bone defects, spinal cages and joint revision surgery. However these polymers are non-degradable and require reinforcing agents such as carbon fiber to match the elastic modulus of bone and modification with bioactive molecules such as HA for increased osseointegration [97]. We refer readers to other papers and reviews for detailed discussion on PAEK polymers [97–99]. Other polymers that can degrade over time have also been proposed. Materials such as poly-L-lactic acid (PLA), polyglycolic acid and poly lactic-co-glycolic acid have been used to make resorbable screws and plates [100–102]. Although such resorbable screws have shown promise as an alternative to metal implants [103], there is concern surrounding the build-up of an acidic environment upon their degradation leading to inflammation [104]. Recently Perrone et al. made screws out of silk and showed that these screws are biocompatible and promote bone remodeling for the period of 4 and 8 weeks [105]. Such screws have some advantages over other polymer screws such as ease of machining and low swelling properties, however, whether their degradation causes inflammation over time remains to be seen. An argument against use of polymers for structural support is that although, metal implants are non-degradable, they have been shown to remain in the body for decades without any complications. Technology has further enhanced adhesion of metal implants with host tissue, and hence it is considered safe to leave the implants in the body and there is no need to perform a second surgery to remove them from the body [96].
Polymer Scaffolds
Although metal implants have shown excellent performance in orthopaedic applications and are essential for mechanical and structural support, their non-degradability and high rigidity are not optimal for bone regeneration. For optimal bone regeneration, scaffolds should degrade over time and be replaced by host bone tissue which can then be remodeled according to load and function. The rate of degradation should be similar to rate of bone growth. The implant should be osteoconductive and be able to deliver growth factors, cytokines and biological cues in a local site specific manner. Scaffolds can also serve as a depot for various growth factors, cytokines and cell transplant. Although cell therapy has shown excellent results in clinical applications [106, 107], transplanted cells diffuse out of the injury site, have low repair potential and low viability [108]. Polymer substrates can alleviate this problem by providing a niche for transplanted cells to localize at the injury site and remain viable and active. The success of polymer implants depends on being able to provide single or multiple spatio-temporal cues to resident or transplanted cells at the site of injury [109]. One drawback of most polymer implants is that they are soft materials and hence need support of metal screws and plates to stabilize the fracture site in load bearing bones.
Various types of polymers have been used as implants for bone tissue engineering. Broadly, polymers can be divided into natural and synthetic polymers. Natural polymers are those that are derived from natural sources, such as collagen, fibrin, chitosan, hyaluronic acid, and alginate, and have been used for many years in tissue engineering [110, 111]. Such polymers are biocompatible and biologically active and hence promote cell adhesion and growth. However, natural materials are difficult to engineer and process due to their low mechanical strength and limited processing and manufacturing capabilities. Furthermore, they exhibit batch-to-batch variability and have some risk of pathogen transmission. Hence considerable research has focused on synthetic polymers. For this review we will focus on synthetic polymers. Various strategies to regenerate bone using polymer implants are reviewed here (Figure 2, Table 2). For detailed review of natural polymers for tissue engineering applications please refer to other reviews in the field [112, 113].
Figure 2.
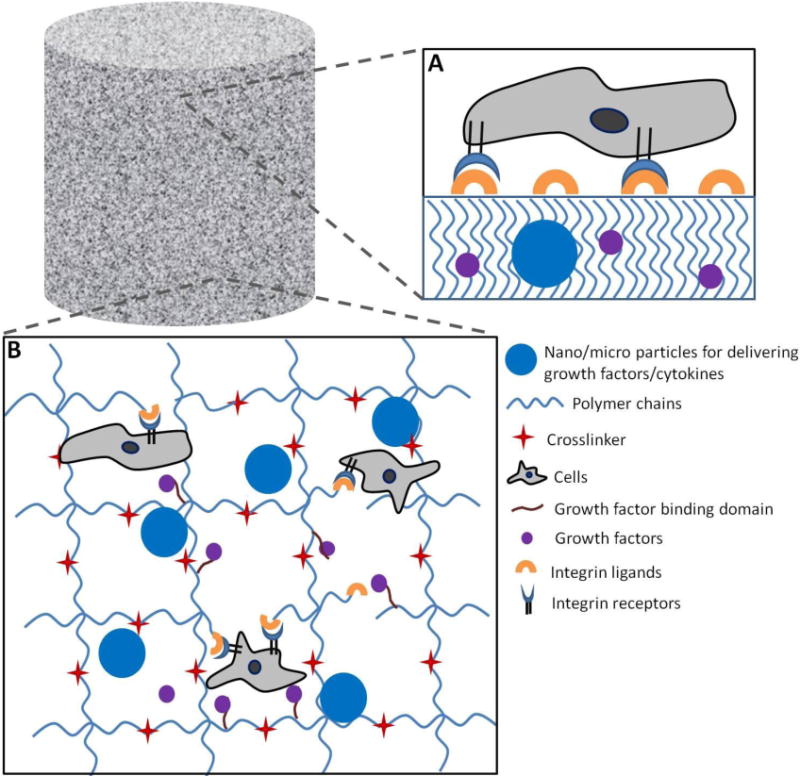
Polymer Implants for bone tissue engineering. A) Solid polymer surfaces or porous scaffolds can be adsorbed with integrin ligands for cell attachment for bone regeneration. Various growth factors can also be encapsulated during the fabrication process. B) Strategies for bone regeneration using hydrogel based polymer implants.
Table 2.
Strategies for bone tissue engineering using polymer scaffolds.
| Material | Strategies to increase Bone Formation | References |
|---|---|---|
| Hydrogels (PEG, fibrin, alginate etc.), PLGA, PCL etc. | Growth factor and cytokine encapsulation/binding | [121], [95], [152] |
| Encapsulating Particles within scaffolds for release of growth factors | [122–124], [125, 126], [126] | |
| Osteoprogenitor Cell Encapsulation | [135], [134], [136], [137] | |
| Co-encapsulation of Osteoprogenitor with Endothelial cells | [138] | |
| Integrin Ligand functionalization of scaffold to promote cell attachment and bone differentiation | [140], [141], [142], [143], [144] | |
| Growth Factor sequestering to scaffolds | [145, 146], [149] |
Synthetic polymers have many advantages such as defined chemistry and formulations, easy processing and tunability. However, unlike natural polymers, synthetic materials are not bioactive and can elicit undesirable inflammatory responses. To overcome this challenge, strategies have been developed to attach biomolecules to provide necessary biological signals as well as to make inert synthetic polymers to avoid immune response. Polymers such as poly(α-hydroxy acids), polyanhydrides [114–116], polycarbonates [117], polyfumarates [118, 119], poly(ethylene oxide) [120] have been investigated for bone tissue engineering. Among these polymers, synthetic hydrogels have gained significant interest as a promising material for tissue engineering. Hydrogels are network of hydrophilic polymers that associates with large volume of water. They mimic the hydrophilic networked structure present in the tissue. Due to high permeability, hydrogels allow transport of nutrients and biomolecules thereby act as good materials to encapsulate cells. In this review we will primarily focus on key development in synthetic polymers with a major focus on hydrogels.
Growth Factors and Cytokines Release
Mariner et al. used a poly(ethylene glycol) (PEG) based light cross-linkable synthetic polymer to encapsulate and deliver BMP-2 in critical sized calvaria bone defect in rat and showed significantly increased bone formation compared to collagen sponges [121]. Boerckel et al. used a hybrid nanofiber mesh/alginate delivery system functionalized with RGD to deliver BMP-2 and showed a dose dependent bone growth [95]. This hybrid system outperformed clinically used collagen sponge to deliver BMP-2, and this effect was attributed to greater retention and hence slower release of BMP-2 from the hybrid polymer system [95].
Another strategy that can be used to engineer release of growth factors and cytokines at the injury site is encapsulating nanoparticles/microparticles containing osteoinductive factors within the scaffold [122–124]. This approach provides for control over the release of multiple factors independently of each other which may include sequential or sustained release [125, 126]. Yilgor et al. used PLGA nanocapsules and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanocapsules to encapsulate BMP-2 and BMP-7 respectively [126]. This resulted in early release of BMP-2 and sustained long term release of BMP-7. When rat MSCs were cultured in the collagen scaffolds with these nanoparticles, they produced much higher ALP activity compared to simultaneous delivery of both growth factors highlighting the importance of sequential delivery. Such a system can be envisioned to deliver multiple growth factors in a time dependent manner.
Cell Encapsulation
Cell encapsulation strategies have also shown promise [127–133]. Sonnet et al. used PEGDA hydrogel microcapsules (50–350 μm in diameter) to encapsulate BMP-2 producing cell lines (Ad5BMP2) and were implanted in critical sized femoral defects in rats [134]. The BMP-2 expression from these cell lines were at relatively low levels (96 ng/day) and hence had minimal side effects associated with supra-physiological concentrations of growth factors. These authors found that there was rapid bone formation and biomechanical testing showed the torsional strength and stiffness was at 79% and 93% of that of healthy bone within 3 weeks. However, the shape of new bones formed were considerably different compared to original femur and may be a result of non-degradable nature of such particles or due to continued overproduction of BMP-2 from the encapsulated cells. Although hydrogels provide an ideal platform for cell encapsulation and in vivo delivery, other polymeric foam type materials have also shown promising results with cell seeding and delivery. Huang et al. used PLGA scaffolds to deliver VEGF, human bone marrow stromal cells and plasmid DNA encoding BMP-4 subcutaneously in SCID mice and showed significant bone formation in implants containing all the 3 factors compared to any other combination of implant [135]. Reichert et al. used medical grade polycaprolactone-tri calcium phosphate (mPCL-TCP) scaffolds in combination with autologous mesenchymal stem cells or BMP-7 in large defects (3 cm) in tibia of sheep [136]. This was compared with autologous bone graft, a gold standard in field. The group found that although synthetic scaffolds with mesenchymal stem cells had not much effect on bone growth, the scaffolds carrying BMP-7 had similar effects as autologous bone implants when tested for bone volume, torsional moment and torsional stiffness and act as a viable alternative to autologous bone transplants [136]. Wojtowicz et al. seeded genetically engineered bone marrow stromal cells that constitutively express Runx2 in PCL scaffolds loaded with type I collagen meshes [137]. This scaffold showed significant bone formation compared to scaffolds with control stromal cells and empty vehicle [137].
There has been growing evidence suggesting direct involvement of endothelial cells in bone regulation. Osteogenic cells are found to be close contact with endothelial cells. Kaigler et al. co-cultured endothelial cells and bone marrow stromal cells and found that when stromal cells were in direct contact with endothelial cells their osteogenic differentiation was enhanced compared to stromal cells cultured on endothelial cell-conditioned media [138]. Endothelial cells were found to express BMP-2 when in contact with bone marrow stromal cells. When marrow stromal cells and endothelial cells were co-transplanted in PLGA scaffolds subcutaneously in immunocompromised mice, it led to greater bone formation than compared with scaffolds containing only bone marrow stromal cells [138].
Surface Groups and Bioadhesive Cues
Surface functional groups and charge of polymer can also play a significant part. Gandavarapu et al. used PEG hydrogels functionalized with phosphate groups and showed that serum proteins that adsorb to such gels can promote osteogenic cell attachment and spreading [139]. Gels pre-incubated with serum were able to support human MSCs even in serum free medium. Blocking assays with antibodies attributed this effect to collagen and fibronectin related proteins on the surfaces and β1 and β3 integrins on cells [139].
RGD sequences are often used to make polymer implants conducive for cell attachment. Alsberg et al. used alginate hydrogels functionalized with RGD sequences and encapsulating chondrocytes and osteoblasts subcutaneously in immunocompromised mice and showed formation of structures analogous to endochronal ossification [140]. They found structures resembling growth plates at the interface of bony and cartilaginous regions. However, efficacy of such implants in immunocompetent mice at the site of bone injury remains to be seen. Hsu et al. used a cathepsin K-sensitive peptide as a crosslinker to form PEGDA hydrogels that were also functionalized with RGD for cell attachment [141]. They found that when osteoblasts and osteoclasts are cultured over such gels, the gel degrades in response to osteoclast attachment (RAW 264.7) and not for osteoblasts (MC3T3-E1). Such gels can thus be responsive to bone resorption activity. However, the in vivo efficacy needs to be evaluated. Lutolf et al. used PEG based hydrogels crosslinked with matrix metalloproteinase- degradable peptide to form gels [142]. Cell adhesion molecules (RGDSP) were tethered to the gel to allow cells to adhere and spread and recombinant human BMP-2 was encapsulated to allow slow release over time. This gel was used in critical sized defects in rat crania and resulted in complete infiltration of cells and bone regeneration within 5 weeks. Both the BMP-2 release as well as protease-sensitivity was essential for bone ingrowth and was minimal without either one [142].
RGD containing sequences are promiscuous in their binding to various types of integrins [68]. RGD sequences can be replaced by other specific sequencing known to induce osteogenic differentiation. Wojtowicz et al. used GFOGER (which binds to cells via α2β1 integrin) to coat polycaprolactone scaffolds to promote bone growth in critical sized segmental defects and showed that in absence of any exogenous growth factor, passively adsorbed GFOGER over the scaffolds resulted in significant difference in bone formation compared to uncoated scaffolds [143]. Shekaran et al. used PEG based hydrogels to enhance bone regeneration by using α2β1 integrin signaling with BMP-2 delivery [144]. Protease degradable PEG hydrogels were engineered and functionalized with GFOGER. The group showed significant bone ingrowth compared to clinical carriers at significantly lower BMP-2 doses. These hydrogels resulted in increased osteoprogenitor cell localization at the fracture site and resulted in bridging of critical sized bone defects with equivalent torsion strength as native bone [144].
Growth Factor Immobilization
Another approach that has been gaining lot of attention is immobilization of growth factors and cytokines to the scaffold by mimicking their presence in ECM via sequestering [145, 146]. This strategy allows the growth factors to be present on demand for cells and not diffuse out quickly over time which has been the major cause of failure of such therapies in clinical trials [20, 147]. Martino et al. engineered fibrin scaffolds with a modified multi-functional fibronectin motif where this motif had a factor XIIIa fibrin binding sequence which allows sequesteration of growth factors to the scaffold, FNIII9–10 domain which contains the α5β1 integrin binding site and FNIII12–14 domain which has been shown to bind growth factors like PDGF-BB and BMP-2 promiscuously [145, 148]. They showed that this multi-functional fibronectin motif resulted in significant reduction in growth factor dose needed in rat critical size bone defect when compared to scaffolds without this motif [145]. This shows that integrin signaling can significantly enhance the effects of growth factor in tissue engineering by synergistic effects.
Martino et al. established an even more elegant strategy where the growth factors were engineered to have a promiscuous binding site for ECM proteins [149]. They engineered growth factors like PDGF-BB and BMP-2 with a placenta growth factor 2 domain (PlGF-2123–144) which binds to various ECM proteins like fibronectin, vitronectin, tenascin C, osteopontin, collagen etc. These modified growth factors were then delivered in a fibrin matrix and resulted in significant bone tissue deposition (96% coverage) in a critical size calvarial defect in rats when compared to just fibrin matrix (50% coverage) demonstrating the effect of increased residence time of these growth factors and allowing reduction in dose of growth factors. Interestingly, when growth factors were delivered without any scaffold, it also led to significant bone deposition (74% coverage) when compared to untreated bone defect showing their ability to bind to endogenous ECM proteins and use the ECM as a scaffold [149].
Gene therapy and RNA therapeutics are also upcoming technology which has been used to enhance bone tissue regeneration. For a detailed review of these fields, readers are encouraged to read other reviews [150, 151].
Concluding Remarks
Biomaterials have significantly enhanced our understanding of osseointegration and bone tissue regeneration. Synthetic materials provide a starting framework for creating new technologies to engineer tissue interfaces. Functionalizing the surface of metal implants or bulk polymer implants with integrin ligands to improve osseointegration and direct bone differentiation has shown a lot of promise. When coupled with release of growth factors and cell encapsulation, it has resulted in synergistic effect on bone growth. Although few studies have looked at effects of multiple growth factors and ligands on osseointegration and bone growth, there is a need to evaluate effect of spatio-temporal presentation of multiple integrin ligands and growth factors in high throughput screening. At the same time, it is also important to translate promising mice and rat studies to higher animals such as dogs, sheep and pigs to establish the translational potential of these approaches. Bone tissue growth and regeneration is a dynamic process. It is hence required that biomaterial scaffolds be responsive to changing requirements of growing bone tissue. This would require a concerted effort from biologists to unravel the intricate mechanisms and details, biomedical engineers to mimic the process through biomaterials and implants and finally medical doctors to translate it to clinics.
Acknowledgments
Funding was provided by the National Institutes of Health (R01 AR062920).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee K, Goodman SB. Current state and future of joint replacements in the hip and knee. Expert Review of Medical Devices. 2008;5:383–393. doi: 10.1586/17434440.5.3.383. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Amer Y, Darwech I, Clohisy J. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Research & Therapy. 2007;9:1–7. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer TW, Schils J. The pathology of total joint arthroplasty. Skeletal Radiol. 1999;28:423–432. doi: 10.1007/s002560050541. [DOI] [PubMed] [Google Scholar]
- 4.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Review of Medical Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 5.DeCoster TA, Gehlert RJ, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. The Journal of the American Academy of Orthopaedic Surgeons. 2004;12:28–38. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Garbuz DS, Masri BA, Czitrom AA. Biology of allografting. The Orthopedic clinics of North America. 1998;29:199–204. doi: 10.1016/s0030-5898(05)70318-7. [DOI] [PubMed] [Google Scholar]
- 7.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 8.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to Autogenous Bone Graft: Efficacy and Indications. The Journal of the American Academy of Orthopaedic Surgeons. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609–619. doi: 10.1007/s00256-005-0958-z. [DOI] [PubMed] [Google Scholar]
- 10.Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends in Cell Biology. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddi AH. Cell Biology and Biochemistry of Endochondral Bone Development. Collagen and Related Research. 1981;1:209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- 12.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Schenk RK, Buser D. Osseointegration: a reality. Periodontology 2000. 1998;17:22–35. doi: 10.1111/j.1600-0757.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced Drug Delivery Reviews. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JS, Bemenderfer TB, Wessel AR, Kacena MA. A review of mouse critical size defect models in weight bearing bones. Bone. 2013;55:241–247. doi: 10.1016/j.bone.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92:161–168. doi: 10.1007/s12306-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman JR, Daluiski A, Einhorn TA. The Role of Growth Factors in the Repair of Bone. 2002 doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis & Rheumatism. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 20.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The spine journal: official journal of the North American Spine Society. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. Journal of Bone and Mineral Research. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Hashimoto R, Matsumoto A, Jahan E, Rafiq AM, Udagawa J, Hatta T, Otani H. In Vivo Analysis of Arg-Gly-Asp Sequence/Integrin α5β1-Mediated Signal Involvement in Embryonic Enchondral Ossification by Exo Utero Development System. Journal of Bone and Mineral Research. 2014;29:1554–1563. doi: 10.1002/jbmr.2166. [DOI] [PubMed] [Google Scholar]
- 23.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol. 2011;13:1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 24.Ramazanoglu M, Y.O.c.i.d.t.P.D.H. Özger, a.w.o.w.i.t.r.o. oncology Osseointegration and Bioscience of Implant Surfaces – Current Concepts at Bone-Implant Interface. 2011 [Google Scholar]
- 25.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64:1063–1077. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginebra MP, Canal C, Espanol M, Pastorino D, Montufar EB. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Holt DJ, Grainger DW. Demineralized bone matrix as a vehicle for delivering endogenous and exogenous therapeutics in bone repair. Adv Drug Deliv Rev. 2012;64:1123–1128. doi: 10.1016/j.addr.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 28.LeGeros RZ. Calcium Phosphate-Based Osteoinductive Materials. Chemical Reviews. 2008;108:4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 29.Salinas AJ, Vallet-Regi M. Bioactive ceramics: from bone grafts to tissue engineering. RSC Advances. 2013;3:11116–11131. [Google Scholar]
- 30.Lee K, Weir MD, Lippens E, Mehta M, Wang P, Duda GN, Kim WS, Mooney DJ, Xu HHK. Bone regeneration via novel macroporous CPC scaffolds in critical-sized cranial defects in rats. Dental materials: official publication of the Academy of Dental Materials. 2014;30:e199–e207. doi: 10.1016/j.dental.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985;6:97–101. doi: 10.1016/0142-9612(85)90070-5. [DOI] [PubMed] [Google Scholar]
- 32.Albrektsson T, Jansson T, Lekholm U. Osseointegrated dental implants. Dental clinics of North America. 1986;30:151–174. [PubMed] [Google Scholar]
- 33.Lee K, Goodman SB. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices. 2008;5:383–393. doi: 10.1586/17434440.5.3.383. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9(Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mjoberg B. The theory of early loosening of hip prostheses. Orthopedics. 1997;20:1169–1175. doi: 10.3928/0147-7447-19971201-12. [DOI] [PubMed] [Google Scholar]
- 36.Karrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4–7-year stereoradiographic follow-up of 84 cemented prostheses. Journal of Bone & Joint Surgery, British. 1994;76-B:912–917. [PubMed] [Google Scholar]
- 37.Pap T, Claus A, Ohtsu S, Hummel K, Schwartz P, Drynda S, Pap G, Machner A, Stein B, George M, Gay R, Neumann W, Gay S, Aicher W. Osteoclast-independent bone resorption by fibroblast-like cells. Arthritis Res Ther. 2003;5:R163–R173. doi: 10.1186/ar752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vale FM, Castro M, Monteiro J, Couto FS, Pinto R, Rico JMGT. Acrylic bone cement induces the production of free radicals by cultured human fibroblasts. Biomaterials. 1997;18:1133–1135. doi: 10.1016/s0142-9612(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 39.Bragdon CR, Burke D, Lowenstein JD, O’Connor DO, Ramamurti B, Jasty M, Harris WH. Differences in stiffness of the interface between a cementless porous implant and cancellous bone in vivo in dogs due to varying amounts of implant motion. The Journal of Arthroplasty. 11:945–951. doi: 10.1016/s0883-5403(96)80136-7. [DOI] [PubMed] [Google Scholar]
- 40.Maniatopoulos C, Pilliar RM, Smith DC. Threaded versus porous-surfaced designs for implant stabilization in bone-endodontic implant model. J Biomed Mater Res. 1986;20:1309–1333. doi: 10.1002/jbm.820200907. [DOI] [PubMed] [Google Scholar]
- 41.Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomaterialia. 2005;1:211–222. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Novaes AB, Jr, de Souza SL, de Barros RR, Pereira KK, Iezzi G, Piattelli A. Influence of implant surfaces on osseointegration. Brazilian dental journal. 2010;21:471–481. doi: 10.1590/s0103-64402010000600001. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant Surface Characteristics Modulate Differentiation Behavior of Cells in the Osteoblastic Lineage. Advances in Dental Research. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- 44.Boyan BD, Sylvia VL, Liu Y, Sagun R, Cochran DL, Lohmann CH, Dean DD, Schwartz Z. Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials. 1999;20:2305–2310. doi: 10.1016/s0142-9612(99)00159-3. [DOI] [PubMed] [Google Scholar]
- 45.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin α2β1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proceedings of the National Academy of Sciences. 2008;105:15767–15772. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klokkevold PR, Johnson P, Dadgostari S, Davies JE, Caputo A, Nishimura RD. Early endosseous integration enhanced by dual acid etching of titanium: a torque removal study in the rabbit. Clinical Oral Implants Research. 2001;12:350–357. doi: 10.1034/j.1600-0501.2001.012004350.x. [DOI] [PubMed] [Google Scholar]
- 47.Wennerberg A, Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. The International journal of oral & maxillofacial implants. 2000;15:331–344. [PubMed] [Google Scholar]
- 48.Shalabi MM, Gortemaker A, Hof MAVt, Jansen JA, Creugers NHJ. Implant Surface Roughness and Bone Healing: a Systematic Review. Journal of Dental Research. 2006;85:496–500. doi: 10.1177/154405910608500603. [DOI] [PubMed] [Google Scholar]
- 49.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. Journal of Biomedical Materials Research Part A. 2006;76A:323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 50.Sul YT, Johansson CB, Jeong Y, Wennerberg A, Albrektsson T. Resonance frequency and removal torque analysis of implants with turned and anodized surface oxides. Clinical Oral Implants Research. 2002;13:252–259. doi: 10.1034/j.1600-0501.2002.130304.x. [DOI] [PubMed] [Google Scholar]
- 51.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 52.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta orthopaedica Scandinavica. 1993;255:1–58. doi: 10.3109/17453679309155636. Supplementum. [DOI] [PubMed] [Google Scholar]
- 54.Cook SD, Thomas KA, Dalton JE, Volkman TK, Whitecloud TS, 3rd, Kay JF. Hydroxylapatite coating of porous implants improves bone ingrowth and interface attachment strength. J Biomed Mater Res. 1992;26:989–1001. doi: 10.1002/jbm.820260803. [DOI] [PubMed] [Google Scholar]
- 55.Barrère F, van der Valk CM, Meijer G, Dalmeijer RAJ, de Groot K, Layrolle P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2003;67B:655–665. doi: 10.1002/jbm.b.10057. [DOI] [PubMed] [Google Scholar]
- 56.Lindner T, Kanakaris NK, Marx B, Cockbain A, Kontakis G, Giannoudis PV. Fractures of the hip and osteoporosis: THE ROLE OF BONE SUBSTITUTES. Journal of Bone & Joint Surgery, British Volume. 2009;91-B:294–303. doi: 10.1302/0301-620X.91B3.21273. [DOI] [PubMed] [Google Scholar]
- 57.Moroni A, Hoang-Kim A, Lio V, Giannini S. Current augmentation fixation techniques for the osteoporotic patient. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2006;95:103–109. doi: 10.1177/145749690609500205. [DOI] [PubMed] [Google Scholar]
- 58.Valentin MI. Coatings based on calcium phosphates for metallic medical implants. Russian Chemical Reviews. 2013;82:131. [Google Scholar]
- 59.Elyada A, Garti N, Furedi-Milhofer H. Polyelectrolyte multilayer – calcium phosphate composite coatings for metal implants. Biomacromolecules. 2014 doi: 10.1021/bm5006245. [DOI] [PubMed] [Google Scholar]
- 60.de Groot K, Wolke JG, Jansen JA. Calcium phosphate coatings for medical implants. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 1998;212:137–147. doi: 10.1243/0954411981533917. [DOI] [PubMed] [Google Scholar]
- 61.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J Biomed Mater Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 62.Rezania A, Healy KE. Biomimetic Peptide Surfaces That Regulate Adhesion, Spreading, Cytoskeletal Organization, and Mineralization of the Matrix Deposited by Osteoblast-like Cells. Biotechnology Progress. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 63.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrie TA, Reyes CD, Burns KL, Garcia AJ. Simple application of fibronectin-mimetic coating enhances osseointegration of titanium implants. J Cell Mol Med. 2009;13:2602–2612. doi: 10.1111/j.1582-4934.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kariolis MS, Kapur S, Cochran JR. Beyond antibodies: using biological principles to guide the development of next-generation protein therapeutics. Current Opinion in Biotechnology. 2013;24:1072–1077. doi: 10.1016/j.copbio.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Trajkovski B, Petersen A, Strube P, Mehta M, Duda GN. Intra-operatively customized implant coating strategies for local and controlled drug delivery to bone. Advanced Drug Delivery Reviews. 2012;64:1142–1151. doi: 10.1016/j.addr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Ferris DM, Moodie GD, Dimond PM, Giorani CWD, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20:2323–2331. doi: 10.1016/s0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 68.García AJ. Get a grip: integrins in cell–biomaterial interactions. Biomaterials. 2005;26:7525–7529. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 69.Schliephake H, Scharnweber D, Dard M, Rößler S, Sewing A, Meyer J, Hoogestraat D. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. Clinical Oral Implants Research. 2002;13:312–319. doi: 10.1034/j.1600-0501.2002.130312.x. [DOI] [PubMed] [Google Scholar]
- 70.Hennessy KM, Clem WC, Phipps MC, Sawyer AA, Shaikh FM, Bellis SL. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials. 2008;29:3075–3083. doi: 10.1016/j.biomaterials.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(AAM-co-EG/AAC) interpenetrating polymer network-coated titanium implants. Journal of Biomedical Materials Research Part A. 2007;80A:306–320. doi: 10.1002/jbm.a.30927. [DOI] [PubMed] [Google Scholar]
- 72.Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65:511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 73.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrie TA, Raynor JE, Reyes CD, Burns KL, Collard DM, Garcia AJ. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29:2849–2857. doi: 10.1016/j.biomaterials.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrie TA, Raynor JE, Dumbauld DW, Lee TT, Jagtap S, Templeman KL, Collard DM, Garcia AJ. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci Transl Med. 2010;2:45ra60. doi: 10.1126/scitranslmed.3001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thorey F, Menzel H, Lorenz C, Gross G, Hoffmann A, Windhagen H. Osseointegration by bone morphogenetic protein-2 and transforming growth factor beta2 coated titanium implants in femora of New Zealand white rabbits. Indian journal of orthopaedics. 2011;45:57–62. doi: 10.4103/0019-5413.73659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramazanoglu M, Lutz R, Rusche P, Trabzon L, Kose GT, Prechtl C, Schlegel KA. Bone response to biomimetic implants delivering BMP-2 and VEGF: An immunohistochemical study. Journal of Craniomaxillofacial Surgery. 41:826–835. doi: 10.1016/j.jcms.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 78.Kashiwagi K, Tsuji T, Shiba K. Directional BMP-2 for functionalization of titanium surfaces. Biomaterials. 2009;30:1166–1175. doi: 10.1016/j.biomaterials.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 79.Yuasa K, Kokubu E, Kokubun K, Matsuzaka K, Shiba K, Kashiwagi K, Inoue T. An artificial fusion protein between bone morphogenetic protein 2 and titanium-binding peptide is functional in vivo. Journal of Biomedical Materials Research Part A. 2014;102:1180–1186. doi: 10.1002/jbm.a.34765. [DOI] [PubMed] [Google Scholar]
- 80.Ceylan H, Kocabey S, Tekinay AB, Guler MO. Surface-adhesive and osteogenic self-assembled peptide nanofibers for bioinspired functionalization of titanium surfaces. Soft Matter. 2012;8:3929–3937. [Google Scholar]
- 81.Lu Y, Lee JS, Nemke B, Graf BK, Royalty K, Illgen R, III, Vanderby R, Jr, Markel MD, Murphy WL. Coating with a Modular Bone Morphogenetic Peptide Promotes Healing of a Bone-Implant Gap in an Ovine Model. PLoS ONE. 2012;7:e50378. doi: 10.1371/journal.pone.0050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tengvall P, Skoglund B, Askendal A, Aspenberg P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials. 2004;25:2133–2138. doi: 10.1016/j.biomaterials.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 83.Skoglund B, Holmertz J, Aspenberg P. Systemic and local ibandronate enhance screw fixation. Journal of Orthopaedic Research. 2004;22:1108–1113. doi: 10.1016/j.orthres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 84.Wermelin K, Tengvall P, Aspenberg P. Surface-bound bisphosphonates enhance screw fixation in rats–increasing effect up to 8 weeks after insertion. Acta Orthop. 2007;78:385–392. doi: 10.1080/17453670710013979. [DOI] [PubMed] [Google Scholar]
- 85.Andersson T, Agholme F, Aspenberg P, Tengvall P. Surface immobilized zoledronate improves screw fixation in rat bone: a new method for the coating of metal implants. J Mater Sci Mater Med. 2010;21:3029–3037. doi: 10.1007/s10856-010-4154-x. [DOI] [PubMed] [Google Scholar]
- 86.Wermelin K, Suska F, Tengvall P, Thomsen P, Aspenberg P. Stainless steel screws coated with bisphosphonates gave stronger fixation and more surrounding bone. Histomorphometry in rats. Bone. 2008;42:365–371. doi: 10.1016/j.bone.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Wermelin K, Aspenberg P, Linderbäck P, Tengvall P. Bisphosphonate coating on titanium screws increases mechanical fixation in rat tibia after two weeks. Journal of Biomedical Materials Research Part A. 2008;86A:220–227. doi: 10.1002/jbm.a.31583. [DOI] [PubMed] [Google Scholar]
- 88.Abtahi J, Tengvall P, Aspenberg P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone. 50:1148–1151. doi: 10.1016/j.bone.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32:1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah NJ, Hyder MN, Moskowitz JS, Quadir MA, Morton SW, Seeherman HJ, Padera RF, Spector M, Hammond PT. Surface-Mediated Bone Tissue Morphogenesis from Tunable Nanolayered Implant Coatings. Science Translational Medicine. 2013;5:191ra183. doi: 10.1126/scitranslmed.3005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song W, Yu X, Markel DC, Shi T, Ren W. Coaxial PCL/PVA electrospun nanofibers: osseointegration enhancer and controlled drug release device. Biofabrication. 2013;5:035006. doi: 10.1088/1758-5082/5/3/035006. [DOI] [PubMed] [Google Scholar]
- 92.Choi J, Konno T, Takai M, Ishihara K. Controlled drug release from multilayered phospholipid polymer hydrogel on titanium alloy surface. Biomaterials. 2009;30:5201–5208. doi: 10.1016/j.biomaterials.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Pauly S, Luttosch F, Morawski M, Haas NP, Schmidmaier G, Wildemann B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone. 2009;45:505–511. doi: 10.1016/j.bone.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Advanced Drug Delivery Reviews. 2012;64:1165–1176. doi: 10.1016/j.addr.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, García AJ, Guldberg RE. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32:5241–5251. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aspenberg P. Bone: Silk, metal and bone: why take implants out? Nat Rev Rheumatol. 2014;10:386–387. doi: 10.1038/nrrheum.2014.57. [DOI] [PubMed] [Google Scholar]
- 97.Liao JC, Niu CC, Chen WJ, Chen LH. Polyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusion. International Orthopaedics (SICO) 2008;32:643–648. doi: 10.1007/s00264-007-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toth JM, Wang M, Estes BT, Scifert JL, Seim HB, Iii, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27:324–334. doi: 10.1016/j.biomaterials.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 100.Eppley BL. Use of resorbable plates and screws in pediatric facial fractures. Journal of Oral and Maxillofacial Surgery. 63:385–391. doi: 10.1016/j.joms.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Böstman OM. Absorbable implants for the fixation of fractures. The Journal of Bone & Joint Surgery. 1991;73:148–153. [PubMed] [Google Scholar]
- 102.Dumont C, Fuchs M, Burchhardt H, Appelt D, Bohr S, Stürmer KM. Clinical Results of Absorbable Plates for Displaced Metacarpal Fractures. Journal of Hand Surgery. 32:491–496. doi: 10.1016/j.jhsa.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Ebraheim N, Lausé GE, Xiao B, Xu R. A comparison of absorbable screws and metallic plates in treating calcaneal fractures: A prospective randomized trial. Journal of Trauma and Acute Care Surgery. 2012;72:E106–E110. doi: 10.1097/ta.0b013e3182231811. 110.1097/TA.1090b1013e3182231811. [DOI] [PubMed] [Google Scholar]
- 104.Böstman OM, Laitinen OM, Tynninen O, Salminen ST, Pihlajamäki HK. Tissue restoration after resorption of polyglycolide and poly-laevo-lactic acid screws. Journal of Bone & Joint Surgery, British. 2005;87-B:1575–1580. doi: 10.1302/0301-620X.87B11.16520. [DOI] [PubMed] [Google Scholar]
- 105.Perrone GS, Leisk GG, Lo TJ, Moreau JE, Haas DS, Papenburg BJ, Golden EB, Partlow BP, Fox SE, Ibrahim AMS, Lin SJ, Kaplan DL. The use of silk-based devices for fracture fixation. Nat Commun. 2014;5 doi: 10.1038/ncomms4385. [DOI] [PubMed] [Google Scholar]
- 106.Jager M, Hernigou P, Zilkens C, Herten M, Li X, Fischer J, Krauspe R. Cell therapy in bone healing disorders. Orthopedic reviews. 2010;2:e20. doi: 10.4081/or.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim SJ, Shin YW, Yang KH, Kim SB, Yoo MJ, Han SK, Im SA, Won YD, Sung YB, Jeon TS, Chang CH, Jang JD, Lee SB, Kim HC, Lee SY. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(OssronTM) injection to treat fractures. BMC Musculoskeletal Disorders. 2009;10:20. doi: 10.1186/1471-2474-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mooney DJ, Vandenburgh H. Cell Delivery Mechanisms for Tissue Repair. Cell Stem Cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Guldberg RE. Spatiotemporal Delivery Strategies for Promoting Musculoskeletal Tissue Regeneration. Journal of Bone and Mineral Research. 2009;24:1507–1511. doi: 10.1359/jbmr.090801. [DOI] [PubMed] [Google Scholar]
- 110.Mizuno M, Shindo M, Kobayashi D, Tsuruga E, Amemiya A, Kuboki Y. Osteogenesis by bone marrow stromal cells maintained on type I collagen matrix gels in vivo. Bone. 1997;20:101–107. doi: 10.1016/s8756-3282(96)00349-3. [DOI] [PubMed] [Google Scholar]
- 111.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. Journal of Orthopaedic Research. 1999;17:205–213. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 112.Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. International Journal of Biological Macromolecules. 2010;47:1–4. doi: 10.1016/j.ijbiomac.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 113.Stevens MM. Biomaterials for bone tissue engineering. Materials Today. 2008;11:18–25. [Google Scholar]
- 114.Hanes J, Chiba M, Langer R. Degradation of porous poly(anhydride-co-imide) microspheres and implications for controlled macromolecule delivery. Biomaterials. 1998;19:163–172. doi: 10.1016/s0142-9612(97)00221-4. [DOI] [PubMed] [Google Scholar]
- 115.Ibim SM, Uhrich KE, Bronson R, El-Amin SF, Langer RS, Laurencin CT. Poly(anhydride-co-imides): in vivo biocompatibility in a rat model. Biomaterials. 1998;19:941–951. doi: 10.1016/s0142-9612(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 116.Uhrich KE, Gupta A, Thomas TT, Laurencin CT, Langer R. Synthesis and Characterization of Degradable Poly(anhydride-co-imides) Macromolecules. 1995;28:2184–2193. [Google Scholar]
- 117.Choueka J, Charvet JL, Koval KJ, Alexander H, James KS, Hooper KA, Kohn J. Canine bone response to tyrosine-derived polycarbonates and poly(L-lactic acid) Journal of Biomedical Materials Research. 1996;31:35–41. doi: 10.1002/(SICI)1097-4636(199605)31:1<35::AID-JBM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 118.Peter SJ, Yaszemski MJ, Suggs LJ, Payne RG, Langer R, Hayes WC, Unroe MR, Alemany LB, Engel PS, Mikos AG. Characterization of partially saturated poly(propylene fumarate) for orthopaedic application. Journal of Biomaterials Science, Polymer Edition. 1997;8:893–904. doi: 10.1163/156856297x00074. [DOI] [PubMed] [Google Scholar]
- 119.Yaszemski MJ, Payne RG, Hayes WC, Langer RS, Aufdemorte TB, Mikos AG. The ingrowth of new bone tissue and initial mechanical properties of a degrading polymeric composite scaffold. Tissue Eng. 1995;1:41–52. doi: 10.1089/ten.1995.1.41. [DOI] [PubMed] [Google Scholar]
- 120.Deschamps AA, Claase MB, Sleijster WJ, de Bruijn JD, Grijpma DW, Feijen J. Design of segmented poly(ether ester) materials and structures for the tissue engineering of bone. Journal of Controlled Release. 2002;78:175–186. doi: 10.1016/s0168-3659(01)00497-7. [DOI] [PubMed] [Google Scholar]
- 121.Mariner PD, Wudel JM, Miller DE, Genova EE, Streubel SO, Anseth KS. Synthetic hydrogel scaffold is an effective vehicle for delivery of INFUSE (rhBMP2) to critical-sized calvaria bone defects in rats. Journal of Orthopaedic Research. 2013;31:401–406. doi: 10.1002/jor.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santo VE, Frias AM, Carida M, Cancedda R, Gomes ME, Mano JF, Reis RL. Carrageenan-Based Hydrogels for the Controlled Delivery of PDGF-BB in Bone Tissue Engineering Applications. Biomacromolecules. 2009;10:1392–1401. doi: 10.1021/bm8014973. [DOI] [PubMed] [Google Scholar]
- 123.Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30:5215–5224. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 124.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yilgor P, Hasirci N, Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. Journal of Biomedical Materials Research Part A. 2010;93A:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 126.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30:3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 127.Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135–1150. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- 128.Hutmacher DW, Garcia AJ. Scaffold-based bone engineering by using genetically modified cells. Gene. 2005;347:1–10. doi: 10.1016/j.gene.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 129.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) Journal of Cellular and Molecular Medicine. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caplan AI. New era of cell-based orthopedic therapies. Tissue engineering Part B, Reviews. 2009;15:195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic. Experiments in rats. Acta orthopaedica Scandinavica. 1989;60:334–339. doi: 10.3109/17453678909149289. [DOI] [PubMed] [Google Scholar]
- 132.Horwitz EM, Prockop DJ, Gordon PL, Koo WWK, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. 2001 doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 133.Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: Major challenges. Journal of Cellular Biochemistry. 2008;105:1352–1360. doi: 10.1002/jcb.21957. [DOI] [PubMed] [Google Scholar]
- 134.Sonnet C, Simpson CL, Olabisi RM, Sullivan K, Lazard Z, Gugala Z, Peroni JF, Weh JM, Davis AR, West JL, Olmsted-Davis EA. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. Journal of Orthopaedic Research. 2013;31:1597–1604. doi: 10.1002/jor.22407. [DOI] [PubMed] [Google Scholar]
- 135.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined Angiogenic and Osteogenic Factor Delivery Enhances Bone Marrow Stromal Cell-Driven Bone Regeneration. Journal of Bone and Mineral Research. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 136.Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, Schuetz MA, Duda GN, Hutmacher DW. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4:141ra193. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 137.Wojtowicz AM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ. Runx2 overexpression in bone marrow stromal cells accelerates bone formation in critical-sized femoral defects. Tissue Eng Part A. 2010;16:2795–2808. doi: 10.1089/ten.tea.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. The FASEB Journal. 2005 doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 139.Gandavarapu NR, Mariner PD, Schwartz MP, Anseth KS. Extracellular matrix protein adsorption to phosphate-functionalized gels from serum promotes osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2013;9:4525–4534. doi: 10.1016/j.actbio.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proceedings of the National Academy of Sciences. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hsu CW, Olabisi RM, Olmsted-Davis EA, Davis AR, West JL. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. Journal of Biomedical Materials Research Part A. 2011;98A:53–62. doi: 10.1002/jbm.a.33076. [DOI] [PubMed] [Google Scholar]
- 142.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 143.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31:2574–2582. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shekaran A, García JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, García AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35:5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra189. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 146.Lu H, Kawazoe N, Kitajima T, Myoken Y, Tomita M, Umezawa A, Chen G, Ito Y. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33:6140–6146. doi: 10.1016/j.biomaterials.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 147.Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, Song F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health technology assessment. 2007;11:1–150. iii–iv. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 148.Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 149.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Grainger DW. RNA therapeutics targeting osteoclast-mediated excessive bone resorption. Advanced Drug Delivery Reviews. 2012;64:1341–1357. doi: 10.1016/j.addr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Evans CH. Gene delivery to bone. Advanced Drug Delivery Reviews. 2012;64:1331–1340. doi: 10.1016/j.addr.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suárez-González D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials. 2012;33:713–721. doi: 10.1016/j.biomaterials.2011.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]


