Abstract
Background
Rates of contralateral prophylactic mastectomy (CPM) have increased in the United States, with younger women with breast cancer the most likely to have CPM.
Methods
As part of an ongoing cohort study of young women diagnosed with breast cancer at age ≤40 years, we conducted multinomial logistic regression of data from 560 women with unilateral Stage I-III disease to identify factors associated with: (1) CPM versus unilateral mastectomy (UM); (2) CPM versus breast-conserving surgery (BCS).
Results
Median age at diagnosis was 37 years; 66 % of women indicated that their doctor said that BCS was an option or was recommended. Of all women, 42.9 % had CPM, 26.8 % UM, and 30.4 % BCS. Among women who said the surgical decision was patient-driven, 59.9 % had CPM, 22.8 % BCS, and 17.3 % UM. Clinical characteristics associated with CPM versus BCS included HER2 positivity, nodal involvement, larger tumor size, lower BMI, parity, and testing positive for a BRCA mutation. Emotional and decisional factors associated with CPM versus UM and BCS included anxiety, less fear of recurrence, and reporting a patient-driven decision. Women who reported a physician-driven decision were less likely to have had CPM than both of the other surgeries, whereas higher confidence with the decision was associated with having CPM versus BCS.
Conclusions
Many young women with early-stage breast cancer are choosing CPM. The association between CPM and emotional and decisional factors suggest that improved communication together with better psychosocial support may improve the decision-making process.
Rates of contralateral prophylactic mastectomy (CPM) in early-stage breast cancer patients have dramatically increased in the United States during the past decade.1–6 Young women are the most likely to undergo CPM, with studies consistently identifying younger age at diagnosis as one of the strongest determinants of having CPM.1,3,5–7 Whereas CPM substantially reduces the risk of developing a contralateral breast cancer (CBC), most women (e.g., non-BRCA mutation carriers) with unilateral cancer have a relatively low risk of subsequently developing a CBC following their initial diagnosis.8,9 Furthermore, the risk of CBC in survivors has decreased in recent years, a reduction largely attributed to adjuvant treatment and there is insufficient evidence of any survival advantage conferred by CPM in most women with early-stage breast cancer, especially among those without a documented gene mutation.10–12 Many women who opt for CPM subsequently choose to have reconstructive surgery, resulting in a longer recovery time, increased surgical complications, and the potential for multiple surgical procedures, possibly delaying the time to initiation of adjuvant systemic therapy.13–15
Using a large, prospective cohort of women diagnosed with breast cancer at age 40 years or younger, we sought to characterize involvement and confidence with the decision about surgery and evaluate the association between clinical, decisional, and psychological factors and CPM versus other types of surgery. Understanding how women perceive surgical decisions along with how patient and disease characteristics relate to surgical choice can potentially identify targets for intervention to improve the decision-making process, ensuring that decisions are informed, patient-centered, and evidence-based.
METHODS
Study Population
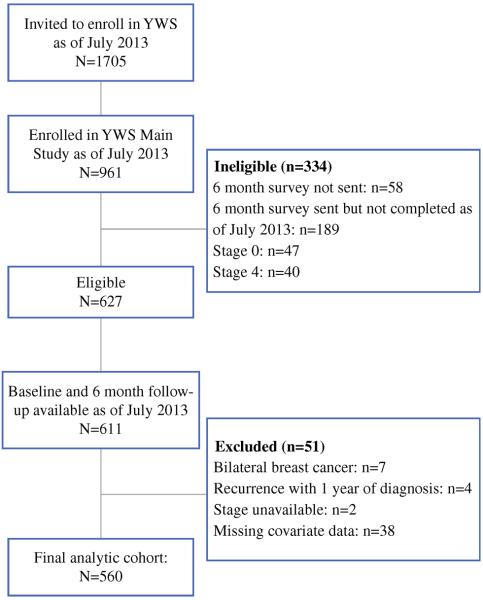
Helping Ourselves, Helping Others: The Young Women's Breast Cancer Study (YWS) is an ongoing, multicenter, prospective cohort established to explore biological, medical, and quality of life issues in young women with breast cancer. Following informed consent and enrollment, women are mailed a baseline survey (median of 4.6 months postdiagnosis) and then two additional surveys (at 6 months after baseline and 1 year postdiagnosis), for a total of three surveys during the first year following diagnosis. Women are then surveyed twice a year for the next 2 years, and then annually thereafter. Study sites include nine academic and community hospitals located in Massachusetts and academic sites in Denver, CO, and Toronto, Canada, although Toronto participants do not receive the full surveys and were not considered for this analysis. Eligibility criteria include being English-speaking and a diagnosis of breast cancer at age 40 years or younger. This analysis included 560 women with Stage I-III unilateral breast cancer who enrolled in the YWS from November 2006 to July 2013 and completed the baseline and initial follow-up surveys (Fig. 1). In most women, the timing of these assessments corresponded to the postsurgical period. The YWS is approved by the Institutional Review Board at the Dana-Farber/Harvard Cancer Center and other participating sites.
FIG. 1.
Study flow chart
Measurements
For this analysis, the following measures were utilized from the three surveys administered in the year after diagnosis as indicated given they had complementary content.
Study Population Characteristics
Sociodemographic characteristics were self-reported on the baseline survey. Race/ethnicity was dichotomized as white non-Hispanic (WNH) versus non-WNH; mixed racial/ethnic background was categorized as non-WNH. BMI was calculated from self-reported height and prediagnosis weight; if prediagnosis weight was missing, current weight was used as a proxy. Pathology reports and medical records were reviewed to ascertain stage, hormone receptor, and human epidermal growth factor receptor-2 (Her-2) status. Cases where Her-2 status was classified as “indeterminate” (n = 5) were categorized as Her-2 positive. Surgery was defined as the most definitive procedure within the year following diagnosis and was categorized as breast-conserving surgery (BCS), unilateral mastectomy (UM), and CPM. For example, a woman who initially had BCS but subsequently had a bilateral mastectomy within the year would be categorized as having CPM. Missing study population and treatment data were abstracted from the medical record when available.
Genetic Testing and Family History
Information about genetic testing status and results (BRCA 1/2 mutation carrier, mutation not known to contribute to breast cancer risk, not tested or unknown, no mutation) was self-reported on the survey administered 1 year following diagnosis. BRCA variants of unknown significance were categorized as negative test results. Family history of breast or ovarian cancer is asked on both the baseline and 1-year survey. The medical record was reviewed if family history data was missing or genetic testing results were missing or reported as “not tested.”
Psychological Factors
Symptoms of anxiety and depression were measured on the baseline survey using the Hospital Anxiety and Depression Scale (HADS). HADS scores range from 0 to 21; for each subscale, scores between 0 and 7 are classified as “normal,” scores between 8 and 10 are classified as “borderline abnormal,” and scores of 11 or greater are classified as “abnormal.”16 Fear of recurrence was evaluated with a single item on the baseline survey from the Lasry Fear of Recurrence Scale that measured concern about the cancer coming back (very much, moderately, a little, not at all).17 Responses were dichotomized, with responses of “very much” categorized as having a high degree of worry and all other responses categorized as moderate to low degrees of worry.
Surgical Decision-Making
Items pertaining to decision-making were measured on the initial follow-up survey. For each surgery type, women were asked whether: their doctor did not mention it; their doctor said they were not a candidate; their doctor said the surgery was an option; their doctor recommended it. A single question adapted from the Control Preferences Scale asked women to recall whether the surgical decision was mainly their own, shared between themselves and their doctor, or mainly their doctor's decision.18 Confidence with the decision was evaluated with a single item: “On a scale, from 0 to 10, where 0 means not at all confident and 10 means extremely confident, how confident are you that the decision about surgery for breast cancer was the right one?”19 Cutoffs were chosen a priori, with ratings between 0 and 6 categorized as “low to moderate” confidence, ratings between 7 and 9 as “very confident” and a rating of 10 as “extremely confident.”
Statistical Analysis
We calculated χ2 statistics to examine whether there were differences by surgery type in decisional involvement and confidence with the surgical decision. We used multinomial logistic regression to evaluate factors associated with: (1) CPM versus BCS; (2) CPM versus UM. We chose, a priori, to assess all psychological factors in the multivariate analysis. All other independent variables that were significant at the p ≤ 0.15 level in univariate analyses were included in the final multivariate models. To control for potential confounding by extent of disease (e.g., women with larger tumors for whom lumpectomy is not an option), we conducted a subset analysis that only included women who said their physicians recommended breast conserving surgery and/or for whom BCS was an option (n = 367). To explore whether results differed when excluding BRCA carries, we fit an additional model that included only women who tested negative for a BRCA mutation or who were not tested (n = 499). In the multivariate analyses, confidence with the decision was dichotomized as “high” (ratings of 7–10) versus “low-moderate” (ratings of 0–6). Additionally, given the small numbers, we grouped women with an unknown familial breast/ovarian cancer history (n = 12) with women who did not have a first-degree relative with breast or ovarian cancer.
RESULTS
Study Population Characteristics
Patient, treatment, and disease characteristics of the study population are presented in Table 1. Median age at diagnosis was 37 (range 17–40) years, most women (87.3%) had Stage I or Stage II breast cancer, and approximately 11% of women were carriers of a BRCA mutation.
TABLE 1.
Patient, disease, and treatment characteristics (n = 560)
| Median age at diagnosis (range) | 37 (17–40) |
|
|---|---|---|
| N | % | |
| White non-Hispanic | ||
| Yes | 483 | 86.3 |
| No | 77 | 13.8 |
| Married/living as married | ||
| Yes | 430 | 76.8 |
| No | 130 | 23.2 |
| Children | ||
| Yes | 361 | 64.5 |
| No | 199 | 35.5 |
| College education | ||
| Yes | 471 | 84.1 |
| No | 85 | 15.2 |
| Unknown | 4 | 0.7 |
| Stage | ||
| 1 | 225 | 40.2 |
| 2 | 264 | 47.1 |
| 3 | 71 | 12.7 |
| Tumor size | ||
| T2 and larger | 256 | 45.7 |
| T1 | 304 | 54.3 |
| Nodal involvement | ||
| Any | 229 | 40.9 |
| None | 331 | 59.1 |
| Estrogen receptor status | ||
| Positive | 386 | 68.9 |
| Negative | 174 | 31.1 |
| Her-2 neu status | ||
| Positive | 172 | 30.7 |
| Negative | 388 | 69.3 |
| BRCA 1/2 mutation status | ||
| Positive | 61 | 10.9 |
| Negative | 439 | 78.4 |
| Not tested/unknown | 60 | 10.7 |
| First-degree relative with breast or ovarian cancer | ||
| Yes | 85 | 15.2 |
| No | 463 | 82.7 |
| Unknown | 12 | 2.1 |
| Anxiety | ||
| Normal (HADS <8) | 307 | 54.8 |
| Borderline abnormal (HADS 8–10) | 123 | 22.0 |
| Abnormal (HADS ≥11) | 130 | 23.2 |
| Depression | ||
| Normal (HADS <8) | 436 | 77.9 |
| Borderline abnormal (HADS 8–10) | 83 | 14.8 |
| Abnormal (HADS ≥11) | 41 | 7.3 |
| Fear of recurrence | ||
| Very concerned about cancer coming back | 186 | 33.2 |
| Moderately/a little//not at all concerned | 374 | 66.8 |
HADS Hospital Anxiety and Depression Scale
Breast Cancer Surgery
Approximately 30% of women had BCS, 26.8% had UM, and 42.9% had CPM as their definitive surgery (Table 2). When we excluded BRCA mutation carriers and women with a positive family history, the proportion of women who had CPM was slightly lower. Approximately two-thirds of women indicated that their doctor had said that BCS was either an option or was recommended. Among these women, 36% had CPM. Of all women who had any type of mastectomy (n = 390), 79.7% had reconstructive surgery during the year after diagnosis (among women who had UM, 61.3%; among women who had CPM, 91.3%, p < 0.0001).
TABLE 2.
Breast cancer surgery by subgroup
| All women (n = 560) | BCS an option or recommended (n = 367) | BCS not option or not recommended (n = 193) | BRCA negative or not tested (n = 499) | No first degree (or unknown) family history of breast or ovarian cancer (n = 475) | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| BCS | 170 (30.4) | 168 (45.8) | 2 (1.0) | 164 (32.9) | 151 (31.8) |
| UM | 150 (26.8) | 67 (18.3) | 83 (43.0) | 147 (29.5) | 135 (28.4) |
| CPM | 240 (42.9) | 132 (36.0) | 108 (56.0) | 188 (37.7) | 189 (39.8) |
CPM contralateral prophylactic mastectomy, UM unilateral mastectomy, BCS breast-conserving surgery, HADS Hospital Anxiety and Depression Scale
Decision-Making
Regarding decisional involvement (Fig. 2), among women who said the decision was made on their own (n = 237), 59.9% had CPM, 22.8% had BCS and 17.3% had UM, whereas among women who said the decision was made by their doctor (n = 54), 5.6% had CPM, 48.2% had BCS, and 46.3% had UM (p < 0.0001). Confidence with the decision also differed by surgery type (p < 0.0001). Among women (n = 371) who were extremely confident with their decision, the proportion who had CPM (50.9%) was approximately double that of those who had BCS (24.0%) or UM (25.1%). While few women were not very or moderately confident about this decision (n = 35), among this group, more than half had BCS (54.3%), whereas 28.6% had UM and 17.1% had CPM.
FIG. 2.
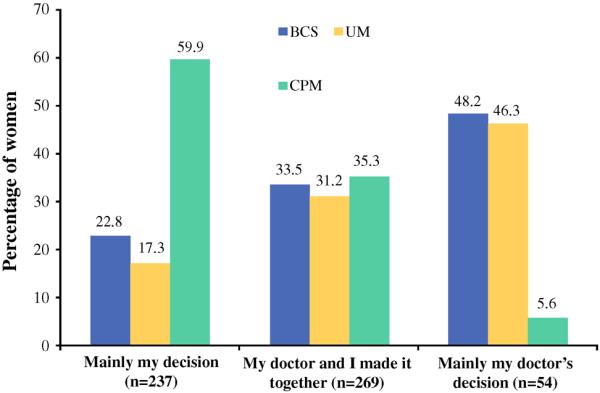
Patient reported involvement with the decision about surgery. BCS breast-conserving surgery, UM unilateral mastectomy, CPM contralateral prophylactic mastectomy
Factors Associated with Receipt of CPM
In the multivariate analysis (Table 3), receipt of CPM versus UM and versus BCS was associated with reporting a patient-driven surgical decision, testing positive for a BRCA mutation, anxiety, and less fear of recurrence. Women who reported a physician-driven decision were less likely to have had CPM than both of the other surgeries. HER-2 positivity, node-positive disease, larger tumor size, lower BMI, higher confidence with the decision, and parity were associated with CPM versus BCS. When we restricted the analysis to women who reported that their doctor told them that BCS was an option or was recommended (Table 3), decisional involvement was no longer significantly associated with CPM versus UM, whereas confidence with the decision, Her2-status, and nodal involvement were no longer significantly associated with receipt of CPM versus BCS. When we excluded BRCA mutation carriers (data not shown), HADS scores <11 were no longer significantly associated with receipt of CPM versus either surgery, nodal involvement was not significantly associated with CPM versus BCS, and fear of recurrence was not significantly associated with CPM versus UM. Age, race/ethnicity, ER status, marital status, having a first-degree relative with breast or ovarian cancer, and depression were not significant in multivariate analyses.
TABLE 3.
Multivanate analysis of factors associated with receipt of CPM versus other types of surgery
| Among all women (n = 560) |
Among women where BCS was an option or was recommended (n = 367) |
|||
|---|---|---|---|---|
| CPM versus UM | CPM versus BCS | CPM versus UM | CPM versus BCS | |
| OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | |
| Age at diagnosis (year) | 0.97 (0.91–1.04) | 0.99 (0.93–1.06) | 0.99 (0.90–1.09) | 1.00 (0.93–1.08) |
| Married/living as married (ref = unmarried) | 0.96 (0.50–1.83) | 1.30 (0.71–2.36) | 0.87 (0.34–2.23) | 1.58 (0.76–3.28) |
| Parous (ref = nulliparous) | 1.39 (0.78–2.50) | 2.17 (1.23–3.83)* | 1.77 (0.76–4.14) | 2.16 (1.08–4.33)¥ |
| BMI (kg/m2) | 0.96 (0.92–1.01) | 0.93 (0.89–0.97)* | 0.92 (0.85–0.99)¥ | 0.91 (0.86–0.97)* |
| T2 tumor size or greater (ref = T1) | 0.88 (0.53–1.46) | 2.03 (1.22–3.38)* | 0.95 (0.46–1.96) | 2.37 (1.28–4.37)* |
| Any nodal involvement (ref = N0) | 0.71 (0.43–1.16) | 1.82 (1.08–3.06)¥ | 0.43 (0.21–0.89)¥ | 0.98 (0.51–1.89) |
| Her2 positivity (ref = Her2 negative) | 0.86 (0.53–1.41) | 1.82 (1.08–3.08)¥ | 0.61 (0.30–1.25) | 1.39 (0.73–2.65) |
| BRCA testing (ref = tested/BRCA negative) | ||||
| Tested/BRCA+ | 12.63 (3.59–44.48)* | 10.30 (3.77–28.18)* | 28.63 (3.32–246.48)* | 13.09 (4.32–39.61)* |
| Not tested/unknown | 0.38 (0.18–0.83)¥ | 0.47 (0.21–1.06) | 0.39 (0.11–1.36) | 0.49 (0.16–1.51) |
| First-degree relative w/breast or ovarian cancer (ref = negative first-degree family history) | 1.72 (0.85–3.45) | 1.68 (0.86–3.25) | 2.45 (0.80–7.49) | 1.69 (0.78–3.68) |
| Anxiety (ref = HADS <8) | ||||
| HADS 8–10 | 2.13 (1.14–3.98)¥ | 2.84 (1.51–5.31)* | 2.97 (1.19–7.39)¥ | 3.46 (1.63–7.35)* |
| HADS ≥11 | 2.04 (1.00–4.16)¥ | 2.23 (1.11–4.51)¥ | 4.88 (1.62–14.65)* | 3.34 (1.40–7.97)* |
| Depression (ref = HADS <8) | ||||
| HADS 8–10 | 1.33 (0.66–2.71) | 1.52 (0.74–3.11) | 0.76 (0.27–2.13) | 1.14 (0.47–2.75) |
| HADS ≥11 | 1.93 (0.68–5.49) | 1.74 (0.63–4.84) | 3.01 (0.43–21.04) | 1.30 (0.36–4.79) |
| Fear of recurrence (ref = moderate/low concern) | 0.56 (0.32–0.98)¥ | 0.49 (0.28–0.86)¥ | 0.28 (0.12–0.65)* | 0.35 (0.17–0.71)* |
| Decisional involvement (ref = shared) | ||||
| Mainly patient's decision | 3.23 (1.98–5.27)* | 2.78 (1.72–4.50)* | 1.89 (0.94–3.79) | 3.09 (1.75–5.46)* |
| Mainly doctor's decision | 0.09 (0.02–0.37)* | 0.08 (0.02–0.32)* | 0.32 (0.04–2.68) | 0.09 (0.01–0.56)* |
| Very/extremely high confidence with decision (ref = low/moderate confidence) | 2.60 (0.78–8.71) | 5.20 (1.72–15.73)* | 4.27 (0.92–19.75) | 3.89 (0.98–15.50) |
CPM contralateral prophylactic mastectomy, UM unilateral mastectomy, BCS breast-conserving surgery, HADS Hospital Anxiety and Depression Scale, ref reference category
p ≤ 0.01
p ≤ 0.05
DISCUSSION
Despite being candidates for BCS, many young women with unilateral breast cancer are having bilateral mastectomies. Many of these women reported making the final decision about surgery on their own rather than sharing it with their physician; furthermore, the association between receipt of CPM and certain emotional factors indicate that psychosocial characteristics potentially play an important role in the decision-making process.
CPM is conventionally considered for women at high risk for a second primary cancer, such as those who test positive for a BRCA mutation. In our cohort, in which most women did undergo genetic testing, mutation carriers were more likely to undergo CPM compared with noncarriers, which is consistent with other studies and with standard clinical recommendations.20–23 Whereas other studies have documented a relationship between a positive family history and CPM, having a first-degree relative with breast or ovarian cancer was not independently associated with receipt of CPM in our cohort.5–7,20,23,24
Women who had CPM were more likely to perceive the decision as patient-driven and less likely to report a physician-driven or shared decision compared with women who had UM or BCS. This has been shown in other studies, in which women who said the decision about surgery was made on their own were more likely to choose mastectomy.25,26 The role of emotions and how they impact perceptions about risk and potentially influence behavior, also must be considered.27 “Peace of mind” and concerns about recurrence often are cited as reasons for choosing CPM.28,29 Hawley et al. reported that women who were more worried about recurrence when they made their surgical decision were more likely to have CPM.24 In our study, where fear of recurrence was measured in most women during the postsurgical period, patients who had CPM reported less worry about the cancer coming back compared with those who had UM or BCS. Furthermore, women who had CPM expressed greater confidence with their decision, another indication of the possible “peace of mind” gained by choosing CPM. In contrast, higher generalized anxiety levels were associated with CPM; given that anxiety has been shown to affect breast cancer risk perceptions, efforts to better manage anxiety when women are making surgical decisions are warranted.30–32 For some women, a desire to avoid developing a CBC no matter how small the risk, as well as avoid additional treatment if a second cancer were to develop, might be of principal concern. However, women also should understand their risks of local, contralateral, and systemic recurrence and that removing the contralateral breast does not affect these risks equally.
Issues related to cosmesis and reconstruction often are important considerations when making surgical decisions. A recent qualitative study reported that 43.3% of women said a desire for symmetry was a factor in their decision to have CPM.33 A desire to have both breasts appear the same after surgery was cited as an extremely or very important reason for choosing CPM among 57% of women surveyed in our prior work.29 Although we did not have specific information about cosmesis in this analysis, more than 90% of women who had CPM did have reconstruction. Women with larger breasts can be more difficult to reconstruct and make symmetric; additional surgery to the contralateral breast, e.g., reduction mammoplasty or mastopexy, might be indicated, possibly making some women less likely to choose CPM. Higher BMI and obesity have been associated with an increased risk of complications from reconstruction.34,35 We found that lower BMI was associated with a greater likelihood of having CPM versus BCS, which might indicate that concerns about reconstruction and potential for complications have influenced surgical choice in our population, although further research is warranted.
Our findings should be considered in the context of some limitations. Our study population is predominantly white, insured, and most women were treated in the Northeast metropolitan area and generalizability might be limited. Certain factors that have been found previously to be associated with CPM that could not be assessed in this study, because this information was not available, including pre-operative MRI use and presence of multifocal or multicentric disease.5,7,20,21,24,36 Whereas all data was collected as part of an ongoing, prospective cohort study, this analysis was cross-sectional in design. Anxiety, depression, and fear of recurrence were measured at study baseline, which in most cases corresponded to the postsurgical period, therefore, the direction of any associations must be interpreted with caution. Additionally, because this data was collected in the first year following diagnosis, patients are on different trajectories in care, including timing of receipt of genetic results and receipt of chemotherapy treatment. It is possible that negative experiences following surgery (e.g., complications) might influence perceptions about past decisions surrounding surgery. As most prior studies of CPM have been limited to evaluating sociodemographic and clinical factors, despite the limitations of when psychological characteristics were measured, we believe they provide important information, are hypothesis-generating, and merit investigation in future studies. We also cannot exclude the possibility of some response bias: although 89% of eligible women were included in the final analytic sample (560/627), a smaller proportion (56%) of women invited to participate in the YWS were enrolled in the study at the time of this analysis (961/1705).
In aggregate, our findings suggest a need for improved communication surrounding surgical choices, together with better management of anxiety surrounding diagnosis and risk of recurrence. Interventions that enhance risk communication, reduce anxiety, and encourage shared patient-physician decision-making may be beneficial in this setting.
ACKNOWLEDGMENT
Susan G. Komen (A. Partridge); NIH 5 R25 CA057711 (S. Rosenberg). K. Sepucha receives salary and research support from the Foundation for Informed Medical Decision Making (Foundation), a not-for-profit (501 (c) 3) private foundation (http://www.informedmedicaldecisions.org). The Foundation develops content for patient education programs and has recently merged with Healthwise, another not-for-profit (501 (c) 3).
Footnotes
CONFLICT OF INTEREST None.
REFERENCES
- 1.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 3.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010;17:2554–62. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 4.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 5.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–64. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 6.Jones NB, Wilson J, Kotur L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol. 2009;16:2691–6. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 7.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 8.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16:935–41. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy JA, Milner TD, O'Donoghue JM. Contralateral risk-reducing mastectomy in sporadic breast cancer. Lancet Oncol. 2013;14:e262–9. doi: 10.1016/S1470-2045(13)70047-0. [DOI] [PubMed] [Google Scholar]
- 10.Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr., Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29:1564–9. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD002748.pub3. doi: 10.1002/14651858.CD002748. [DOI] [PubMed] [Google Scholar]
- 12.Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju160. doi: 10.1093/jnci/dju160. [DOI] [PubMed] [Google Scholar]
- 13.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20:4113–20. doi: 10.1245/s10434-013-3108-1. [DOI] [PubMed] [Google Scholar]
- 14.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol. 2013;20:3212–7. doi: 10.1245/s10434-013-3116-1. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe SM, Liederbach E, Czechura T, Pesce C, Winchester DJ, Yao K. Impact of bilateral versus unilateral mastectomy on short term outcomes and adjuvant therapy, 2003–2010: a report from the National Cancer Data Base. Ann Surg Oncol. 2014;21:2920–7. doi: 10.1245/s10434-014-3687-5. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psych Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Lasry JC, Margolese RG. Fear of recurrence, breast-conserving surgery, and the trade-off hypothesis. Cancer. 1992;69:2111–5. doi: 10.1002/1097-0142(19920415)69:8<2111::aid-cncr2820690817>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–92. [PubMed] [Google Scholar]
- 19.Sepucha KR, Belkora JK, Chang Y, et al. Measuring decision quality: psychometric evaluation of a new instrument for breast cancer surgery. BMC Med Inform Decis Mak. 2012;12:51. doi: 10.1186/1472-6947-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Ann Surg Oncol. 2012;19:2600–6. doi: 10.1245/s10434-012-2299-1. [DOI] [PubMed] [Google Scholar]
- 21.Stucky CC, Gray RJ, Wasif N, Dueck AC, Pockaj BA. Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):330–7. doi: 10.1245/s10434-010-1259-x. [DOI] [PubMed] [Google Scholar]
- 22.Howard-McNatt M, Schroll RW, Hurt GJ, Levine EA. Contralateral prophylactic mastectomy in breast cancer patients who test negative for BRCA mutations. Am J Surg. 2011;202:298–302. doi: 10.1016/j.amjsurg.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila) 2010;3:1026–34. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley ST, Jagsi R, Morrow M, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149(6):582–89. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–33. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 26.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101:1337–47. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Risky feelings: why a 6% risk of cancer does not always feel like 6% Patient Educ Couns. 2010;81(Suppl):S87–93. doi: 10.1016/j.pec.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz SJ, Morrow M. Contralateral prophylactic mastectomy for breast cancer: addressing peace of mind. JAMA. 2013;310:793–4. doi: 10.1001/jama.2013.101055. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159:373–81. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apicella C, Peacock SJ, Andrews L, Tucker K, Daly MB, Hopper JL. Measuring, and identifying predictors of women's perceptions of three types of breast cancer risk: population risk, absolute risk and comparative risk. Br J Cancer. 2009;100:583–9. doi: 10.1038/sj.bjc.6604910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–51. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Perez M, Schootman M, et al. A longitudinal study of factors associated with perceived risk of recurrence in women with ductal carcinoma in situ and early-stage invasive breast cancer. Breast Cancer Res Treat. 2010;124:835–44. doi: 10.1007/s10549-010-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beesley H, Holcombe C, Brown SL, Salmon P. Risk, worry and cosmesis in decision-making for contralateral risk-reducing mastectomy: analysis of 60 consecutive cases in a specialist breast unit. Breast. 2013;22:179–84. doi: 10.1016/j.breast.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–74. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Fischer JP, Nelson JA, Au A, Ct T, 3rd, Serletti JM, Wu LC. Complications and morbidity following breast reconstruction: a review of 16,063 cases from the 2005–2010 NSQIP datasets. J Plast Surg Hand Surg. 2013 doi: 10.3109/2000656X.2013.819003. doi: 10.3109/2000656X.2013.819003. [DOI] [PubMed] [Google Scholar]
- 36.Sorbero ME, Dick AW, Beckjord EB, Ahrendt G. Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol. 2009;16:1597–605. doi: 10.1245/s10434-009-0362-3. [DOI] [PubMed] [Google Scholar]