Abstract
Background
Subjective Cognitive Decline (SCD) is increasingly considered promising to detect preclinical Alzheimer’s disease. How SCD is ascertained is critical for determining its potential utility in identifying at-risk individuals, yet SCD measures differ along several dimensions.
Objective
We aimed to examine the extent to which reports of SCD in healthy elders (HE) may be influenced by the characteristics of the SCD measures. We investigated variations in rates of SCD endorsement across different measures, including an open-ended question. We also examined the association of responses across measures, and the degree to which specific SCD items were associated with objective memory performance.
Methods
99 HE completed a series of questionnaires from which 10 items examining SCD for memory and other aspects of cognition were drawn. We applied Cochran’s Q tests to assess differences in rates of SCD, correlation analyses to examine association of SCD responses and regression models to determine the association between SCD items and delayed verbal memory.
Results
Rates of SCD varied as a function of the assessment format, ranging from 1 to 7 % for memory and 5 to 20% for concentration. SCD was lower for memory versus non-memory domains. SCD items were associated both within and across domains. The most accurate predictor of memory was memory-related SCD in comparison to others the same age.
Conclusion
Characteristics of SCD items influence rates of endorsement. Querying SCD using an “age-anchored” question may provide the most accurate reflection of actual cognitive performance.
Keywords: subjective cognitive decline, subjective memory complaints, cognitive complaints, early detection, metacognition, aging
INTRODUCTION
Subjective Cognitive Decline (SCD) refers to a self-experienced worsening of cognitive abilities. Although individuals with varying levels of cognitive ability can experience cognitive decline, the term SCD is generally used to refer to the experience of cognitive decline in the context of objective cognitive performance that is within normal limits. In the effort to detect preclinical Alzheimer’s disease (AD), SCD is thought to be promising because in some individuals it may represent an early symptom of neurodegeneration and thus a potential sign of the disease [1,2]. SCD has been related to amyloid burden [3,4] and patterns of subtle brain changes such as gray matter atrophy [5–7], cerebral hypometabolism [7] and altered default mode network connectivity [8]. The growing attention to SCD is evident in the conceptual framework for research recently published by the SCD-Initiative (SCD-I) Working Group, a collaborative effort to establish a consensus on terminology and approaches to studying SCD. While SCD has potential diagnostic utility, the extent to which it offers a window into an individual’s “true” level of cognitive functioning or likelihood for subsequent cognitive decline is subject to debate [9–15]. Moreover, it’s well established that factors such as depression, anxiety, personality traits (e.g., neuroticism), and metacognitive abilities contribute to the experience of cognitive decline [16–19].
It is perhaps not surprising then, that the estimated prevalence of SCD in cognitively normal adults, varies across studies (4.5% [20], 10.4% [21] and 17% [22]). One critical factor certain to contribute to variable rates of SCD is variability in the methods used to assess SCD. Measures of SCD commonly used in the literature differ along a number of dimensions, including but not limited to the number of questions, response options, standard of comparison against which respondents are asked to judge themselves, cognitive domains evaluated, and the time frame across which individuals evaluate themselves. There is evidence to suggest that variability across these dimensions influences individuals’ responses, however, this has not directly been examined in the context of SCD. In social and health sciences, it is well established that features of instruments shape the answers provided by the respondent [23–25]. For instance, shorter and simpler phrasing helps the respondent to select an answer and limits the “don’t know” responses [23]. In addition, ambiguous terms in a question can lead to different interpretations of the question’s meaning and therefore elicit responses not necessarily expected by the researcher [23,25,26].
Another important dimension is whether the question is open or closed-ended. The former prompts the respondent to answer what is on his or her mind without cues, and offers the possibility of obtaining more detailed information than can be collected in other self-report formats. It also reduces the social desirability bias in the absence of cues [27]. Yet the open-ended format may also entail a certain form of ambiguity and the subject could deliberately fail to state “things that go without saying” [27]. Finally, closed-ended questions have been shown to result in a higher rate of endorsement of the behavior of interest than open ended questions [24].
In addition to the characteristics of the question, the type of response scaling is also an important consideration. Dichotomous scales have both the advantage and disadvantage of forcing the participant to take a position (e.g., presence or absence of SCD) rather than establishing the severity of an outcome as would be done using an ordinal scale [24]. The specific response options are also important to consider. For example, in a study asking participants how frequently they felt really irritated, Schwartz et al. [28] showed that specific response options induced distinct interpretations of the question. When the rating scale ranged from “less than once a year” to “more than once a month”, participants felt they had to consider major and remarkable irritations, whereas when the scale contained high-frequency points such as “several times a day”, irritations were understood to be minor ones.
In summary, features of the questions influence the nature of the responses. We therefore aimed to examine the extent to which reports of SCD in cognitively healthy elders (HE) may be influenced by the manner in which SCD is queried. To this end, we directly compared within a cohort the rates of SCD item endorsement drawn from multiple instruments evaluating depression, quality of life, and general self-ratings, including an open-ended question that may provide information not gathered in a forced choice format [29,30].
The overall aim of this paper is to examine the extent to which the format of questions influence SCD endorsement. We address the following three primary questions in this regard: 1) Do rates of SCD endorsement differ as a function of question format; 2) Are individuals’ responses to SCD items associated across different question formats; and 3) In which format are reports of SCD most closely related to objective cognition? We examined these questions for SCD related to both memory and non-memory domains. For the first question, we hypothesized that SCD rates would be lowest when participants are asked to compare themselves to others their same age (i.e., age-anchored format) as opposed to another reference group or no reference group. This hypothesis was based on the idea that older adults, when not given a reference point, may perceive questions about cognition in reference to their baseline level of cognition from which they are likely to have experienced some level of decline. However, such decline would not necessarily be described as worse than the decline experienced by their peers. Therefore, the proportion of individuals reporting a complaint would be reduced when the question involves an estimation of one’s own abilities in comparison to a group of the same age. For the second question, we hypothesized that responses to the different formats would be associated with one another, because although each question was shaped in a distinct way, they all aimed to ascertain the same construct. We further hypothesized that the strength of the association would be higher between more similar formats, that is, those sharing specific characteristics (i.e., type of scaling and comparison group). And finally for the third question, we hypothesized that endorsement of memory difficulties in comparison to others of a similar age would be most closely related to objective memory functioning, consistent with the recent finding linking amyloid burden most tightly to age-anchored SCD specifically [4].
Finally, as a secondary set of analyses, we examined rates of and associations between SCD items across cognitive domains, hypothesizing that participants would more frequently endorse complaints about memory functioning than other aspects of cognition as observed in previous studies [31,32]; and that SCD items would be correlated across cognitive domains as also observed elsewhere [3].
MATERIALS AND METHODS
Participants
150 cognitively healthy elders (HE) at 2 centers were enrolled in a study of memory awareness. At Columbia University Medical Center (CUMC), 51 HE were selected from a database of controls, previously recruited from local senior centers and market mailing procedures, and who agreed to be contacted for research studies. At the University of Pennsylvania PENN Memory Center (n = 99), participants were recruited from a database of cognitively normal individuals who previously agreed to be contacted for research studies. Participants were eligible for the study if they were aged 55 or above, and scored at least 27 on the Mini Mental State Examination (MMSE) [33]. Participants were screened to exclude individuals with neurologic, psychiatric, or severe medical disorders.
The study was designed in compliance with guidelines on experimentation with human subjects, and the protocol was approved by the Institutional Review Boards of both medical centers. All individuals provided informed consent prior to participation.
Procedure
Participants underwent a two-hour test session during which they completed measures of objective and subjective cognition, mood, and quality of life. All questions regarding subjective cognition were administered prior to cognitive testing to ensure that the participants’ reports regarding their cognition were not influenced by experience with cognitive testing.
Measures
Subjective Cognitive Decline
10 SCD items were collected including a single open-ended question and 9 questions drawn from the different scales outlined below. Memory and concentration were the only domains that were covered across more than one scale and were therefore the only domains examined as part of Question 2. Characteristics of each SCD item are summarized in Table 1.
Table 1.
Characteristics of all SCD Items
| Domain | Instrument | Specific Query | Comparison group |
Response Scale |
Endorsement % (n) |
SCD Rate % |
|---|---|---|---|---|---|---|
| Memory | GDS | Do you feel you have more problems with memory than most? |
Most others | No | 93.3 (140) | 6.7 |
| Yes | 6.7(10) | |||||
| BAS | Please rate your ability to remember as compared to other people your age |
Others of same age |
Excellent | 12.0 (18) | 3.4 | |
| Above average | 32.7 (49) | |||||
| Average | 52.0 (78) | |||||
| Below average | 2.7 (4) | |||||
| Very impaired | 0.7 (1) | |||||
| QOL | How about your memory? How has it been lately? |
None | Excellent | 14.0 (21) | 0.7 | |
| Good | 60.7 (91) | |||||
| Fair | 24.7 (37) | |||||
| Poor | 0.7 (1) | |||||
| OEa | I’d like you to tell me what you think about your memory abilities |
Others of same ageb |
No complaint | 32.9 (47) | 4.9 | |
| Age-related complaints |
62.2 (89) | |||||
|
Significant complaints |
4.9 (7) | |||||
| Concentration | GDS | Do you have trouble concentrating? | None | No | 80.0 (120) | 20.0 |
| Yes | 20.0 (30) | |||||
| BAS | Please rate your ability to concentrate and attend as compared to other people your age |
Others of same, age |
Excellent | 16.0 (24) | 5.3 | |
| Above average | 42.7 (64) | |||||
| Average | 36.0 (54) | |||||
| Below average | 4.6 (7) | |||||
| Very impaired | 0.7 (1) | |||||
|
Decision- making |
GDS | Is it easy for you to make decisions? | None | Yes | 76.0 (114) | 24.0 |
| No | 24.0 (36) | |||||
|
Clarity of Mind |
GDS | Is your mind as clear as it used to be? | Previous Self | Yes | 52.7 (79) | 47.3 |
| No | 47.3 (71) | |||||
| Speech | BAS | Please rate your ability to speak clearly as compared to other people your age |
Others of same age |
Excellent | 48.0 (72) | 0.7 |
| Above average | 32.0 (48) | |||||
| Average | 19.3(29) | |||||
| Below average | 0.7 (1) | |||||
| Very impaired | 0 (0) | |||||
| Word-Finding | BAS | Please rate your ability to say the word you are thinking of as compared to other people your age |
Others of same age |
Excellent | 15.3 (23) | 13.3 |
| Above average | 22.0 (33) | |||||
| Average | 49.3 (74) | |||||
| Below average | 13.3 (20) | |||||
| Very impaired | 0 (0) |
Rates of SCD refer to frequency of responses in bold. aN = 143 for the OE. bAlthough not explicit in the question, answers were scored with reference to expectations based on age. GDS = Geriatric Depression Scale; BAS = Brief Anosognosia Scale; QOL = Quality of Life-AD; OE = Open-Ended question.
Open-Ended (OE) Question
Examiners stated: “I would like you to tell me what you think about your memory abilities.” Responses were audio-recorded. In order to score the responses, a 3-point ordinal rating scale was developed to characterize the degree of the participants’ complaints about memory functioning. The development of the ordinal scale was done retrospectively, after listening to the content of the responses to determine what distinctions could be drawn between different responses. An effort was made to develop as finely tuned scale as possible to distinguish between different levels of complaints. Responses were scored as: no complaint, shallow/age-related complaint (“My memory has gotten worse since I’ve gotten older but it’s not too bad”), and significant complaints (“I’m worried about my memory; it seems to be getting worse”). Responses were scored separately by two raters blind to each other’s ratings. Responses which received discrepant ratings were scored by a third rater and the final rating assigned was that which was endorsed by two of the three raters. Table 2 includes examples of participants’ OE responses.
Table 2.
Responses to the Open-Ended Format
| Complaint Level | Verbatim Examples |
|---|---|
|
None (n = 47) |
I think it’s pretty good. I haven’t noticed any problems or changes. |
| I think it’s been pretty good. As a young person I had a very good memory or an excellent memory. | |
| I have a good memory, normal memory. I am still working. I remember lots of things. | |
| I think it is good, pretty good with faces and names and with certain areas of my interest. | |
| It is excellent, the work I do, dealing with big companies. Really important things to remember… I don’t have any problem with it. | |
|
Age related (n = 89) |
I find I don’t come up with names as rapidly as it used to, but I’ll come up with it later. |
| I think it’s generally good, but I have trouble especially calling up names and also words that I know on crossword puzzles and until I get a hint I can’t remember them. I think that is much more than it used to be. But I notice I’m not alone in that, and otherwise I think my memory is good. | |
| I guess my memory is average for my age. There are times when I have some memory loss but so do my other friends of the same age. | |
| I am considered a very bright person but I start a sentence and suddenly I forget where I was. Every people of my age is feeling the same thing… I don’t remember where I put my glasses, again people much younger than me do the same thing, But I don’t like it. | |
| I have my good days and my bad days actually. My memory is not too good, I can’t remember your name. I guess I am about average. They used to be really good, right now for my age, I am not doing bad. | |
|
Significant (n = 7) |
It’s starting to worry me because I find that I forget a lot of things like people’s names. It seems to be getting worse. |
| I worry a lot about it because my mother had AD and I know that I have APOE 3 and 4 gene. I do forget and I get very distracted. I get ADD when I’m doing things…. | |
| It’s dwindling. Hereditary….My mother at my age started to forget things and that really bothers me, forgetting. Because I know that I sound paranoid and I’m always accusing people of something. | |
| They’re declining. Short time memory is going rapidly away. I never was too much account for names but it’s gone beyond that now. Places, time, appointment book. I don’t trust my brain. It’s awful I do this because I always earned my living being cognitive instead of physical and my cognitive functions are beginning to go. | |
| I forget things. I have to ask things a second or third time. More than before. | |
| I think as I age my memory gets worse and worse and it’s frightening for me because my father had AD and it seems to run in my family. | |
| Not good, my memory is deteriorating; I don’t know why. Age, lack of exercise, I drink wine. I forget things, sort of kind of diagnosis AD, I diagnosed myself not by a doctor, I can’t remember. I can talk to you, I can read something and then my mind goes somewhere else. |
Forced Choice SCD Formats
Geriatric Depression Scale (GDS) [34]
This thirty-item Yes/No self-report is used to identify depression in older adults. Participants were prompted to endorse those items they have experienced in the past week. Questions querying SCD were: “Do you feel you have more problems with memory than most?”, “Do you have trouble concentrating?”, “Is it easy for you to make decisions?” and “Is your mind as clear as it used to be?” Presence of SCD was defined as an endorsement of “yes” for the first two questions and of “no” for the last two.
Brief Anosognosia Scale (BAS) [35]
Previously used to examine awareness of symptoms in cognitively impaired elders [17], the BAS was used as a measure of subjective cognition in this study. Participants were asked to judge themselves with regard to eight specific abilities in comparison to others their age (i.e., age-anchored format) on a scale ranging from “Excellent” to “Very impaired.” The cognitive abilities assessed were: remembering, concentrating and attending, speaking clearly, and saying the word you are thinking of. Ratings of “Below Average” or “Very Impaired” were classified as SCD.
Quality of Life-AD (QOL) [36]
This thirteen-item scale ranging from “Excellent” to “Poor” assesses an individual’s perception of his or her quality of life across a number of specific domains. The specific question regarding memory was: “How about your memory? How has it been lately?” Endorsement of the ratings “Fair” or “Poor” led to classification as SCD.
Learning and Memory
Philadelphia Repeatable Verbal Learning Test (PrVLT)[37]
The PrVLT is a list-learning task modeled after the 9-word California Verbal Learning Test in which participants are required to learn 9 words (comprising three different semantic categories: fruit, tools, and furniture) over the course of five trials. This is followed by an interference trial, a short free and cued recall, and a delayed free and cued recall, respectively. The primary dependent variable in the current study was free delayed recall as it is has been shown to be sensitive to early memory changes associated with AD [38].
Statistical Analysis
Analyses were performed using SPSS Version 21. In an effort to quantify rates of SCD endorsement (i.e., presence or absence of SCD), ordinal response choices for all measures were coded dichotomously. The dichotomous variables were defined according to the coding in Table 1, with the bolded responses considered an endorsement of SCD. The decision of where to divide the ordinal categories was driven by the specific response options in each of the scales, with the goal of categorizing any negatively laden or below average rating as endorsement of a complaint. Certain response options (i.e., “Fair” on the QOL scale) were somewhat ambiguous, however, and the implications of the selected cut-points are addressed in the discussion. Analyses were conducted using both ordinal and dichotomous responses for all measures except for the GDS in which only dichotomous data were available.
To examine whether participants differed with regard to subjective or objective cognition as a function of recruitment site, we used chi-square tests of independence and independent t-tests.
To compare rates of SCD across scales and domains, Cochran’s Q test, a procedure for determining whether the proportions of 3 or more dichotomous variables are equal, were performed. Post-hoc pairwise comparisons were conducted using the McNemar test adjusted with Bonferroni correction. To determine the association of responses across scales and across domains, correlation analyses were computed with Spearman’s coefficient for ordinal variables and the Phi coefficient for dichotomously-coded variables.
Finally, in order to determine the extent to which specific SCD items are associated with objective memory, a series of linear regression models were conducted. First, only demographic variables (age, education, and gender), depression and global cognitive status were entered as predictors of objective memory (PrVLT delayed memory). Variables that were significantly associated with objective memory were included as covariates in the subsequent regression model to determine the extent to which SCD items were associated with objective memory above and beyond such factors. Individual SCD items were then entered as predictors of objective memory in each of 10 regression models. SCD items were coded in either their dichotomous or ordinal form, depending on which form was most strongly associated with objective memory. In a final step, all individual SCD items found to be significantly related to objective cognition in individual models were then entered simultaneously into a final model to determine whether any individual SCD item was most clearly related to objective memory. Regression results were not corrected for multiple comparisons.
RESULTS
Descriptive statistics
The mean age and educational level of the sample was 75.19 (range: 56–97, SD = 9.01) and 16.02 (range: 8–21, SD = 2.66) respectively. The proportion of women was 68.7%. 99.7% of participants indicated Non-Hispanic as their ethnicity, with race reported as 85% Caucasian, 13% African American and 1% Asian. Mean score was 29.28 (SD = 0.96) on the MMSE and 7.36 (SD = 1.98) out of 9 for the PrVLT long delay. Scores of depressive symptomatology on the GDS were low, with a mean score of 4.01 out of 30 (SD = 3.71). Participants didn’t differ with respect to the frequency of SCD or objective cognition across enrollment sites.
Rates of SCD endorsement
Within Cognitive Domains
Table 1 shows the frequency of responses on all scales as well as the operationally defined rates of SCD using a dichotomous coding. None of the SCD items was related to age (p > 0.05). For memory, there was a significant difference in SCD rates across the four scales (Q (3) = 12.51, p = 0.006). More people endorsed having “more memory problems than most” (GDS; 6.7%) than having “poor memory” without reference to any group (QOL; 0.7%) (χ2 (1) = 7.11, p = 0.004). Other pairwise comparisons were not significant. For concentration, more participants endorsed having “trouble concentrating” when there was no reference group specified (GDS; 20.0 %) (χ2 (1) = 16.11, p < 0.001) than having below average or very impaired concentration in relation to others the same age (BAS; 5.3%).
Across Cognitive Domains
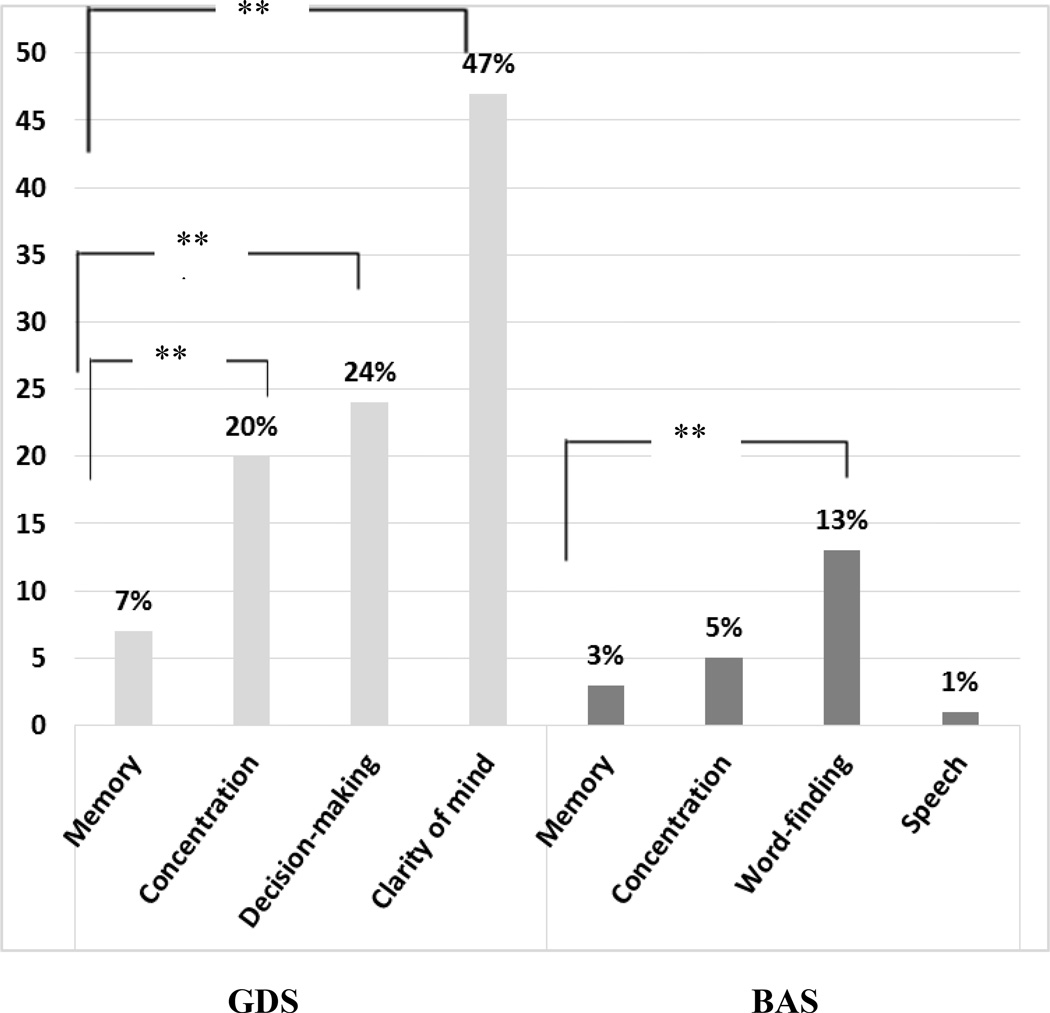
In the context of scales which queried memory as well as other aspects of cognition a difference was found in rates of endorsement across the cognitive domains queried on the GDS (Q(3) =80.34, p < 0.001). Complaints about memory were less frequently endorsed than those regarding clarity of mind (χ2 (1) = 55.38, p < 0.001), decision-making (χ2 (1) =18. 38; p < 0.001) and concentration (χ2 (1) =10. 62, p < 0.001). On the BAS, using dichotomous coding, a difference was also found across the items (Q (3) = 27.41, p < 0.001). Complaints about word-finding were more frequent than complaints about memory (χ2 (1) = 9.33, p < 0.001) and speech (χ2 (1) =15.43, p < 0.001). See Figure 1.
Figure 1.
Rates of Domain-Specific SCD on the GDS and the BAS
GDS = Geriatric Depression Scale; BAS = Brief Anosognosia Scale.
**p < .01.
Association of Responses to SCD Items
Within Cognitive Domains
Bivariate correlations revealed that each memory item correlated with every other. The strongest association was between the 2 age-anchored items, OE and BAS, when coded dichotomously (φ = 0.66, p < 0.001). See shaded cells in Table 3. With regard to concentration, the 2 items (BAS and GDS) were correlated to each other either when the BAS was in its ordinal (ρ = 0.21, p < 0.001) or dichotomous form (φ = 0.33, p < 0.001).
Table 3.
Correlations among SCD Items Within and Across Cognitive Domains
| Cognitive Domain | Memory | Concentration | |||
|---|---|---|---|---|---|
| Reference Group | Same age (BAS) | Most others (GDS) |
None (QOL) |
Same age (BAS) |
|
| Memory | Most others (GDS) | 0.26**(0.55 **) | |||
| None (QOL) | 0.56**(0.44 **) | 0.36**(0.31**) | |||
| Same age (OE) | 0.44**(0.66 **) | 0.27**(0.32 **) | 0.45**(0.37 **) | ||
| Concentration | None (GDS) | 0.07 | 0.21**(0.33**) | ||
| Same age (BAS) | 0.67**(0.29**) | ||||
| Word-Finding | Same age (BAS) | 0.48**(0.15) | |||
| Speech | Same age (BAS) | 0.41**(0.01 **) | |||
| Decision-Making | None (GDS) | 0.23** | |||
| Clarity of Mind | Previous Self (GDS) | 0.18* | |||
Shaded results are within cognitive domains and across scales. Non-shaded results are across domains and within scales. Correlations are reported for the variables coded originally with correlations between the variables dichotomously coded in parentheses. GDS = Geriatric Depression Scale; BAS = Brief Anosognosia Scale; QOL = Quality of Life-AD; OE = Open-Ended question.
None = No reference group.
p < 0.05;
p < 0.01.
Across Cognitive Domains
Correlation analyses among the GDS items indicated that the memory item was associated with decision-making (φ = 0.23, p < 0.05) and clarity of mind (φ = 0.18, p < 0.05) but not with concentration (non-shaded cells in Table 3). Among the dichotomized BAS items, memory was correlated with concentration but not word-finding or speech. Correlations between memory and other items emerged and were higher when coded in their original ordinal format (concentration: ρ = 0.67, p < 0.001; word-finding: ρ = 0.48, p < 0.001; speech: ρ = 0.41, p < 0.001).
Association of SCD with Objective Memory Performance
We first performed a linear regression including global cognition, demographic variables (age, education, and gender), and depression as predictors, with objective memory as the outcome. PrVLT long delay was log transformed to meet the assumption of normality for the regression. The overall model was significant (F(5, 144) = 3.66, p = 0.004, R2= 0.113) with MMSE (p = 0.021) and gender (p = 0.002) emerging as independent predictors of memory, with females scoring higher than males. Each SCD item was then entered as a single predictor into 10 subsequent models controlling for gender and MMSE, the two variables that were significantly predictive of memory according to the results of the first model (See Table 4). SCD items were entered as dichotomous or ordinal according to the format that was most predictive of objective memory.
Table 4.
Individual Models Examining SCD Items as Predictors of Delayed Memory Scores
| Domain | Predictors | Reference Group | B(SE) | R2 | p |
|---|---|---|---|---|---|
|
Memory |
BAS | Same age | 0.40 (.12) | 0.17 | 0.001 |
| OE | Same age | 0.31 (.10) | 0.17 | 0.003 | |
| GDS | Most others | 0.17 (.09) | 0.13 | 0.06 | |
| QOL | None | 0.10 (.03) | 0.16 | 0.01 | |
| Concentration | GDS | None | 0.07 (.06) | 0.12 | 0.21 |
| BAS | Same age | 0.23 (.10) | 0.38 | 0.02 | |
| Decision Making | GDS | None | 0.13 (.05) | 0.15 | 0.01 |
| Clarity of Mind | GDS | Previous self | −0.03 (.05) | 0.11 | 0.51 |
| Word-Finding | BAS | Same | −0.02 (.03) | 0.12 | 0.41 |
| Speech | BAS | Same | −0.24 (.27) | 0.12 | 0.39 |
R2 reflects variance accounted for by SCD item, gender, and MMSE.
BAS = Brief Anosognosia Scale; OE = Open-Ended question; GDS = Geriatric Depression Scale; QOL = Quality of Life-AD. Variables were coded in the format that was most highly related to the delayed memory score, this included dichotomous coding for GDS items, the OE and BAS memory, concentration, and speech and ordinal coding for the QOL, and BAS word finding.
Each of the memory-specific SCD predictors except for the GDS memory item was significant when entered individually into the model, although to varying degrees. Other significant non-memory SCD predictors included age-anchored concentration on the BAS, and decision-making on the GDS. Table 4 displays results with variables coded in the format that was most highly related to objective cognition; this included dichotomous coding for GDS items, the OE item, and BAS memory, concentration, and speech. QOL and BAS word finding were coded ordinally.
All individual SCD items found to be significantly related to objective cognition in the previous models were then simultaneously entered (dichotomously or ordinally coded according to which form was most predictive of memory) into a final model with gender and MMSE (Table 5). One exception was the exclusion of the OE item (that was scored in an age-anchored fashion similar to BAS) to avoid high multi-collinearity with the BAS item. Gender and MMSE retained their significance, and the age-anchored BAS item emerged as the only significant SCD predictor (B = 0.276, p = 0.032). A second model was run replacing the BAS item with the OE item. Results were similar except that the OE item did not reach statistical significance (p = 0.055). An independent samples t-test confirmed differences in objective memory as a function of the BAS memory item, with individuals endorsing SCD achieving delayed memory scores of 3.60 (3.51) versus 7.49 (1.79), (t(1, 149) = 4.62, p < 0.001).
Table 5.
Predictors of Objective Memory Performance in a Single Model
| B(SE) | R2 | F | p-value | ||
|---|---|---|---|---|---|
| Block 1 | (Gender, MMSE) | 0.110 | 9.10 | < 0.001 | |
| Block 2 | 0.216 | 6.58 | < 0.001 | ||
| Gender | −0.154 (.05) | 0.001 | |||
| MMSE | −0.052 (.02) | 0.021 | |||
| Memory | |||||
| BAS | 0.276 (.13) | 0.032 | |||
| QOL | 0.044 (.04) | 0.230 | |||
| Concentration | |||||
| BAS | 0.112 (.10) | 0.263 | |||
| Decision-making | |||||
| GDS | 0.086 (.05) | 0.099 | |||
BAS = Brief Anosognosia Scale; GDS = Geriatric Depression Scale; QOL = Quality of Life-AD.
Variables were coded in the format that was most highly related to the delayed memory score, this included dichotomous coding for the BAS and the GDS items and ordinal coding for the QOL.
DISCUSSION
The overall purpose of this study was to understand the extent to which reports of SCD in cognitively normal older adults may differ when assessed in different formats. The SCD items examined in this study varied along several dimensions, including whether they were open-ended versus closed-ended, the reference group to which responders were asked to compare themselves (others of same age, previous self, most others, none), the type of scaling (dichotomous versus ordinal) as well as the specific response options.
Summary of Findings
Rates of SCD endorsement
First, we investigated rates of SCD across different question formats. As expected, rates of SCD varied as a function of the different formats in which it was assessed, ranging from 1 to 7% for memory, and 5 to 20% for concentration. Our initial hypothesis was that participants would be least likely to report a cognitive complaint when comparing themselves to others the same age (age-anchored format) because rather than implying an intra-individual decline with age, which we assume most people may experience, this format instead emphasizes a comparison to a group of peers that may be experiencing varying degrees of age-related decline. Therefore, the proportion of individuals reporting a complaint would be reduced when the question involves an estimation of his/her own abilities in comparison to a group of the same age. This was true for the two concentration items, with 15% fewer complaints endorsed in the age-anchored format (BAS) than when no reference group was provided (GDS).
This was also true for the memory domain when considering the answers provided to the OE question. When people were asked to talk openly about their memory abilities (without a reference group), more than half of participants (63%) reported a memory difficulty that they believed was age-appropriate. In contrast, only 4.9% of participants voiced a significant complaint (i.e., one that was not typical for their age); for example, “It’s starting to worry me because I find that I forget a lot of things.” This was similar to the rate of SCD on the age-anchored BAS item (3.4%), suggesting that forced choice questions at least map broadly onto information gathered in an OE format.
In line with these findings, the lack of any comparison group on the QOL item was expected to result in high rates of memory complaints. Contrary to expectations, however, rates of memory-specific SCD were actually lowest on this scale (0.7%). This surprising finding almost certainly reflects the decision to define SCD as a rating of “poor” only and to collapse ratings of “fair” with “good” and “excellent” into a single non-SCD category. The ambiguous meaning of the word “fair” presented a challenge for coding SCD as present or absent. Indeed, although we did not consider “fair” to be indicative of a complaint for the dichotomous grouping, one could argue that anything below “good” could be considered a report of SCD. Collapsing fair and poor would have resulted in a very high prevalence of SCD at 25%, potentially leading to a lack of specificity. These results illustrate the fact that response options are as relevant for determining rates of SCD as the questions themselves.
The highest SCD rate (7%) was endorsed when participants were asked to compare themselves to “most” others. The expression “than most” thus appears to be considered by respondents as including others of the same age as well as younger people. Given the participants’ mean age of 75, it is reasonable for them to have experienced, and thus reported, more problems with memory “than most” given the well-known changes that occur even in the context of aging [39–41].
As part of the examination of SCD rates, we also explored differences across cognitive domains. In these analyses, we expected that participants would report more complaints regarding memory than other cognitive domains [31,32]. However, the results indicated that the most frequently endorsed complaints were those regarding clarity of mind on the GDS, and word-finding on the BAS, with memory complaints reported far less often. Indeed, almost half of the participants answered “No” to the question: “Is your mind as clear as it is used to be?” (47%), in contrast to the 7% reporting more memory problems than most. It is possible that older adults experience disturbance in their clarity of mind more frequently than memory problems in particular, which is consistent with the fact that participants more frequently endorsed difficulty concentrating than remembering in general. It is also very likely that the framing of the question regarding clarity of mind, which directly asks individuals to compare themselves to their previous level of functioning, accounts for the very high endorsement rate on this particular item. Word-finding difficulty was also reported more frequently (13%) on the BAS than memory difficulty. This is consistent with reports that word-finding difficulty is among the most common and distressing cognitive complaints reported in older adulthood [42–44].
Association of Responses to SCD Items
With respect to the association of SCD items, all SCD items within a domain were associated. Within the memory domain, the association between responses was the strongest when the comparison group was similar across formats (comparison to people of the same age), underscoring the important role of the comparison group. However, memory related SCD items were also correlated with decision-making, clarity of mind, and concentration, suggesting that the experience of cognitive decline in one area is often accompanied by the experience of decline in another.
The degree of interrelation of SCD items across cognitive domains was also clearly influenced by the manner in which SCD was queried, as evidenced by the high correlations among items within the BAS scale, all of which were obtained in an age-anchored format as opposed to the GDS items which are each phrased differently. However, the relatively strong associations among BAS items are seen only when the item is coded in its original ordinal format, suggesting that the nature of the associations can be driven largely by the manner in which SCD is coded.
Association of SCD with Objective Memory Performance
Finally, in order to determine whether any particular SCD item mapped most closely onto objective cognition we investigated the specific association between each SCD item and performance on an objective memory test with the expectation that complaints of memory difficulty as compared to others of the same age would be most highly related to objectively measured memory ability. Interestingly, endorsement of memory-specific SCD in nearly all formats (as well as non-memory items including decision making and concentration) added predictive utility for objective memory over and above that of global cognition and gender, suggesting that participants tend to have an accurate perception of their cognitive abilities. However, SCD for memory in comparison to others of the same age accounted for the most variance in memory in the individual models, and was the only SCD predictor to retain its predictive utility when all significant SCD items were entered into a final model. This finding highlights the importance of the reference group, and suggests that there is some degree of specificity to the content of SCD as non-memory SCD items were less predictive of objective memory.
Study implications
Overall, these results suggest that the specific manner in which SCD questions and response options are phrased plays an important role in determining rates of SCD. Moreover, the current findings suggest that age-anchored memory complaints may have the greatest accuracy for detecting potentially meaningful cognitive decline in older adults whose performance remains within normal limits on cognitive testing. This type of complaint (i.e. to feel worse than people of the same age), has been shown to be associated with amyloid-beta deposition, a neuropathological hallmark of AD [4]. Jessen’s framework of SCD [1] suggests that this kind of subjective complaint should be considered a “criterion plus” of SCD, that is to say one which increases the likelihood of the presence of preclinical AD. Our findings support that proposal.
Limitations
This study had several limitations. First, the vast majority of subjects didn’t endorse memory related SCD (7% at its maximum). The low incidence of SCD was a limitation for statistical analyses and prevented us from performing some types of analyses. Nevertheless all analyses examining the association of SCD items and the relationship between SCD and objective memory incorporated the full range of the SCD scales (using ordinal measurements when possible), and were thus not bound by the relatively low rate of SCD when coded dichotomously.
Another potential limitation was the order of task administration. We administered subjective cognition evaluation systematically prior to objective cognition to prevent participants from being influenced by their performance on the cognitive assessment. However, one could argue that having previously been asked to consider and discuss one’s subjective perception of cognitive functioning might influence subsequent performance on memory testing. Therefore, future studies should include a counterbalanced design in order to control for this possible effect. It may also be considered problematic to have drawn single items from scales, including scales which were not originally designed to measure SCD. The use of single items raises questions about their psychometric properties, however, this procedure was undertaken in order to directly observe the effects of question format on SCD endorsement, an endeavor that would have been complicated by using total scores from SCD questionnaires. Lastly, the various scales used to measure SCD in the current study did not differ in a single dimension. Rather, it was often the case that the phrasing of the question, the response options, and the cognitive domain all differed, rendering interpretation of the factors that led to differences in SCD rates challenging. Moreover, SCD items differed with regard to the context in which they were acquired, the emotional valence of particular words included in the question, and the time frame under consideration. The extent to which these different variables influence SCD endorsement is unknown and should be further investigated while holding other aspects of the SCD format constant.
Future Directions
This study provides preliminary insight into the effect of SCD ascertainment on rates of endorsement, and suggests that moving forward, researchers and clinicians alike may gather particularly useful information regarding subtle cognitive change by asking individuals to consider cognitive functioning specifically in reference to similarly aged peers.
ACKNOWLEDGMENTS
This study was supported by the Marian S. Ware Alzheimer Program, NIA P30-01024, the CDC funded Penn Healthy Brain Research Center, and Dr. Cosentino’s Paul B. Beeson Career Development Award in Aging (K23 AG032899) funded jointly by the National Institute on Aging (NIA) and the American Federation of Aging Research. This study was also supported by T32 AG000026.
Footnotes
There is no conflict of interest.
REFERENCES
- 1.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, Monteiro I, Torossian C, Vedvyas A, Ashraf N, Jamil IA, de Leon MJ. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4:S98–S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M, Wagner M, Koppara A, Klöppel S, Jessen F for the Alzheimer’s Disease Neuroimaging Initiative. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2013;10:99–108. doi: 10.1016/j.jalz.2013.05.1764. [DOI] [PubMed] [Google Scholar]
- 6.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, Popp J, Daamen M, Gorris D, Heneka MT, Boecker H, Biersack HJ, Maier W, Schild HH, Wagner M, Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR, Gao S, O’Neill DP, Saykin AJ. Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis. 2013;35:751–760. doi: 10.3233/JAD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, van den Bussche H, Scherer M, Maier W, Wagner M German Study on Aging, Cognition and Dementia in Primary Care Patients. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10:76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 11.Luck T, Luppa M, Matschinger H, Jessen F, Angermeyer MC, Riedel-Heller SG. Incident subjective memory complaints and the risk of subsequent dementia. Acta Psychiatr Scand. 2014 doi: 10.1111/acps.12328. in press. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;6:1–13. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 13.Van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MMB. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Reid LM, Maclullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 15.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127:344–350. doi: 10.1111/ane.12038. [DOI] [PubMed] [Google Scholar]
- 17.Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43:1004–1019. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merema MR, Speelman CP, Foster JK, Kaczmarek EA. Neuroticism (not depressive symptoms) predicts memory complaints in some community-dwelling older adults. Am J Geriatr Psychiatry. 2013;21:729–736. doi: 10.1016/j.jagp.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Pearman A, Storandt M. Predictors of subjective memory in older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59:P4–P6. doi: 10.1093/geronb/59.1.p4. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 21.Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191–1202. doi: 10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz N. Self-reports: How the questions shape the answers. Am Psycho. 1999;54:93–105. [Google Scholar]
- 24.Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to Their Development and Use. Oxford: Oxford University Press; 2014. [Google Scholar]
- 25.Schaeffer NC, Dykema J. Questions for Surveys: Current trends and future directions. Public Opin Q. 2011;75:909–961. doi: 10.1093/poq/nfr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudman S, Bradburn NM. Asking questions. San Francisco: Jossey-Bass; 1982. [Google Scholar]
- 27.Schwarz N, Oyserman D. Asking questions about behavior: cognition, communication, and questionnaire construction. Am J Eval. 2001;22:127–160. [Google Scholar]
- 28.Schwarz N, Strack F, Müller G, Chassein B. The Range of response alternatives may determine the meaning of the question: Further Evidence on informative functions of response alternatives. Soc Cogn. 1988;6:107–117. [Google Scholar]
- 29.Mattos P, Lino V, Rizo L, Alfano Â, Araújo C, Raggio R. Memory complaints and test performance in healthy elderly persons. Arq Neuropsiquiatr. 2003;61:920–924. doi: 10.1590/s0004-282x2003000600006. [DOI] [PubMed] [Google Scholar]
- 30.Snitz BE, Yu L, Crane PK, Chang C-CH, Hughes TF, Ganguli M. Subjective cognitive complaints of older adults at the population level: An item response theory analysis. Alzheimer Dis Assoc Disord. 2012;26:344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh-Manoux A, Dugravot A, Ankri J, Nabi H, Berr C, Goldberg M, Zins M, Kivimaki M, Elbaz A. Subjective cognitive complaints and mortality: Does the type of complaint matter? J Psychiatr Res. 2014;48:73–78. doi: 10.1016/j.jpsychires.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS) Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 35.Deckel AW, Morrison D. Evidence of a neurologically based “denial of illness” in patients with Huntington’s disease. Arch Clin Neuropsychol. 1996;11:295–302. [PubMed] [Google Scholar]
- 36.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. J Ment Health Aging. 1999;5:21–32. [Google Scholar]
- 37.Price CC, Garrett KD, Jefferson AL, Cosentino S, Tanner JJ, Penney DL, Swenson R, Giovannetti T, Bettcher BM, Libon DJ. Leukoaraiosis severity and list-learning in dementia. Clin Neuropsychol. 2009;23:944–961. doi: 10.1080/13854040802681664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libon DJ, Bondi MW, Price CC, Lamar M, Eppig J, Wambach DM, Nieves C, Delano-Wood L, Giovannetti T, Lippa C, Kabasakalian A, Cosentino S, Swenson R, Penney DL. Verbal serial list learning in mild cognitive impairment: a profile analysis of interference, forgetting, and errors. J Int Neuropsychol Soc. 2011;17:905–914. doi: 10.1017/S1355617711000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craik FI, McDowd JM. Age differences in recall and recognition. J Exp Psychol Learn Mem Cogn. 1987;13:474–479. [Google Scholar]
- 40.Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- 41.Balota DA, Dolan PO, Duchek JM. Memory in healthy older adults. In: Tulving E, editor. The Oxford Handbook of Memory. Oxford University Press; 2000. pp. 411–425. [Google Scholar]
- 42.Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008;14:1004. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derouesné C, Dealberto MJ, Boyer P, Lubin S, Sauron B, Piette F, Kohler F, Alpérovitch A. Empirical evaluation of the “Cognitive Difficulties Scale” for assessment of memory complaints in general practice: A study of 1628 cognitively normal subjects aged 45–75 years. Int J Geriatr Psychiatry. 1993;8:599–607. [Google Scholar]
- 44.Lovelace E, Twohig P. Healthy older adults perceptions of their memory functioning and use of mnemonics. Bull Psychon Soc. 1990;28:115–118. [Google Scholar]