Abstract
This investigation was conducted to study the relationship between intracellular Ca2+ and activation of large conductance Ca2+-activated K+ (BK) currents by unoprostone, the first synthetic docosanoid. We used HEK293 cells stably transfected with two BK channel splice variants, one sensitive to unoprostone and the other insensitive. We examined the effects of unoprostone on channel activity in excised inside-out patches and cell-attached patches. The half-maximal stimulation of the sensitive BK channels by Ca2+ was shifted from 3.4 ± 0.017 nM to 0.81 ± .0058 nM in the presence of 10 nM unoprostone. There was no effect on insensitive channels even at unoprostone concentrations as high as 1000 nM. There was no effect of unoprostone on the voltage dependence of the BK channels. Changes in open probability and effects of Ca2+ and unoprostone were best described by a synergistic binding model. These data would suggest that Ca2+ and unoprostone were binding to sites close to one another on the channel protein and that unoprostone binding causes the affinity of the calcium binding site to increase. This idea is consistent with three dimensional models of the Ca2+ binding site and a putative unoprostone binding domain. Our results have important implications for the clinical use of unoprostone to activate BK channels. Channel activation will be limited if intracellular Ca2+ is not elevated.
Keywords: BK channels, KCNMA1, Unoprostone, Rescula®, single channels, Ca2+-dependence
Graphical Abstract

Introduction
The goal of this study was to determine the calcium and voltage dependence of large conductance potassium channels (also known as BK, maxi-K, slo1, KCa1.1, or KCNMA1) in the presence of unoprostone. Unoprostone isopropyl is a synthetic docosanoid. It resembles the naturally occurring docosanoid metabolites of docosahexaenoic acid (DHA). The latter is an ω-3, polyunsaturated fatty acid abundantly located in the neural tissues of the retina and brain [19,32]. These studies are relevant to the use of unoprostone (trade name Rescula®,) in glaucoma therapy in protecting against vision loss. Primary open-angle glaucoma is a primary cause of blindness and ocular hypertensive medication delays or prevents the onset of this type of glaucoma [7]. Unoprostone causes a reduction in intraocular pressure [19]. It has been shown that unoprostone has direct effects on the trabecular network thereby reducing intraocular pressure that would otherwise be increased by humoral agents [32]. These effects were due to activation of large conductance Ca2+ activated (BK) channels that prevented contraction of the trabecular meshwork [32]. Unoprostone has also been suggested to protect cone photoreceptor cells from oxidative stress and light-irradiation–induced damage by activation of BK channels [34]. The latter finding has triggered clinical trials in retinal diseases characterized by photoreceptor degeneration [36].
BK channels are potassium channels characterized by their large conductance to potassium ions (K+). As with most other voltage-gated potassium channels, BK channels have a tetrameric structure. Each monomer of the channel-forming alpha subunit is the product of the KCNMA1 gene. These channels are activated (opened) by changes in membrane electrical potential and by increases in the concentration of intracellular calcium ion (Ca2+) [20,37]. BK channels are relatively ubiquitous potassium channels with at least six distinct functional types of BK-channels in the CNS alone [23]. Like many other potassium channels, BK channels occur in different cells as many different splice variants [13,16,28,33]. The cell-surface occurrence of different splice variants is important because different splice variants have different baseline activity and different sensitivity to calcium and other regulators of the channels. Altering the splice variant composition of BK channels can alter their activity and apparent sensitivity to calcium and other regulators of activity [28,33]. For example, one BK splice variant in a pituitary cell line, GH3, is sensitive to arachidonic acid (AA), but an alternative BK splice variant in a sub-clone of GH3 cells, GH4-C1, is not [9–12]. GH3 and GH4 cells contain two different BK splice variants that differ by the presence or absence of a 27 amino acid domain near the C-terminus of the BK-α subunit and only the splice variant containing the 27 amino acid domain is sensitive to arachidonic acid. In the present work, we used two engineered rat BK-α subunits that differ only in the presence or absence of the 27 amino acid domain near the C-terminus (rSlo(27) and rSlo(0) respectively, obtained from C. S. Park [13] to test whether the splice variants are sensitive to unoprostone, the first synthetic docosanoid. rSlo(27) exhibits an extremely high homology with the major BK channel α-subunit from rat brain (accession number: Nucleotide AF135265; Protein AAD34786) while rSlo(0) exhibits an extremely high homology to a mouse brain splice variants (muBKα, accession number: Nucleotide L16912; Protein AAA39746).
Unoprostone is a member of a larger class of drugs, the prostones. It has the interesting property of potentiating the sensitivity of BK channels. As mentioned above, unoprostone is used for treating high intraocular pressure. Whether, unoprostone can be used for other indications involving calcium-induced cytotoxicity will depend upon whether unoprostone can activate BK channels reliably in the target organ. As part of determining whether this was likely, we first needed to have specific information about the conditions under which unoprostone activates BK channels.
Methods
Drugs and chemicals
Unoprostone was obtained from Sucampo Pharmaceuticals as 1mM solution in dimethyl sulfoxide (DMSO) and diluted in saline to appropriate concentrations. Final DMSO concentrations were less than 0.01% and produced no effect on channel activity by itself. To ensure that there was no vehicle effect, we matched the DMSO concentration in the unoprostone-free salines with unoprostone-containing salines in the excised patch experiments. In addition, any effect of vehicle could be observed as effect of the DMSO on the activity of the BK channels in the unoprostone insensitive splice variant, rSlo(0). For all electrophysiological experiments, drug exposure was accomplished using a gravity perfusion/suction removal technique with a perfusion rate of 2.0 ml/minute and a dead volume of 1.0 ml. Previous experiments showed that exchange was 90 ± 7 % complete after 0.5 minutes. Cell-attached recordings were used immediately. For excised patches, after obtaining a high resistance ( >25 GΩ) seal, patches were excised into a solution with a known concentration of Ca2+ and depolarized to +40 mV. Control recordings were obtained in K2EGTA buffered solutions containing the desired Ca2+ concentration. After control recordings (typically 2–5 minutes), the patch was perfused with a second solution containing the desired concentration of unoprostone and recordings continued for an additional ten minutes. For experiments involving voltage dependence measurements, 2–3 minute control recordings were made at +10, +20, +30, +40, +50, and +60 mV. The patch was then depolarized to +40 mV and the unoprostone solution was introduced. After allowing the effect of the prostone to stabilize (5–10 min) the voltage paradigm described above was repeated.
Plasmids and constructs
All constructs were confirmed by DNA sequencing. The rSlo constructs were a generous gift of C.S. Park [13]. The two constructs are identical except for the 27 amino acid insertion or deletion near the calcium binding site. A schematic diagram of the two BK constructs is shown in Fig. 1.
Figure 1. A schematic diagram of the two BK channel constructs, rSlo(27) and rSlo(0).
A construct for muBKa1 (mouse) was truncated at position 953. Then the c terminus of rBKa1 was added to the truncated form to produce rSlo(0). In the second construct, bases corresponding to 27 amino acids were added before once again adding the c terminus of rBKa1 to produce rSlo(27). In this figure the sequence between the N-terminus and 9th alpha helical domain are identical, but the 27 amino acid region immediately prior to the calcium “bowl” are present in one construct and not the other. The sequence of the calcium “bowl” and the subsequent sequence to the C-terminus are identical.
Cell culture and transfection
HEK293 Maxi K α-subunit stable cell lines with either rSlo(27) or rSlo(0) were generated in our laboratory as previously described [17] and maintained in DMEM supplemented with 10% FCS, l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and geneticin (1 mg/ml). All other media and components were purchased from Invitrogen (Carlsbad, CA).
Single-channel recording
Experiments in this study were carried out using either the cell-attached or excised patch configuration of the patch-clamp technique in the HEK Maxi K α-subunit stable cell lines. Electrodes were fabricated from Corning 7052 glass (Garner Glass, Fullerton, CA) in two steps on a Narishige PP-83 electrode puller (Narishige, Tokyo, Japan). Electrodes were fire polished to a final tip resistance between 3 and 5MΩ. The bath and pipette solutions used in the cell-attached mode contained (in mM) 140 NaCl, 1 CaCl2, 5 KCl, and 10 HEPES, pH adjusted to 7.4 with 2 N NaOH. The bath and pipette solutions used in the excised patch mode contained (in mM) 150 KCl, 2 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.30 for the pipette and 140 KCl, 15 HEPES, 5 K2EGTA, and Ca2+ adjusted to produce various calcium activities; pH 7.4 for the bath. Recordings were performed at room temperature. After formation of a high-resistance (5 GΩ) seal, the channel currents were filtered at 1 kHz, recorded with an Axopatch 1-D amplifier (Molecular Devices), and sampled at 5 kHz with or without ionomycin (1 μM) added to the bath solution. Channel activity (NPo) was calculated from pClampfit 10.3 data-analysis software (Molecular Devices). Channel number (N) was determined from the maximal number of transitions during 10–20 min of recording, and channel open probability (Po) was calculated as the ratio of NPo to N.
Whole-cell recording
BK channel current was recorded using a whole-cell patch configuration. Briefly, cells on a coverslip were transferred to a cell chamber (0.5 ml) mounted on the stage of an inverted microscope (Diaphot, Nikon, Japan). Borosilicate glass electrodes (1.2-mm OD) were pulled with a PP-1 puller (Narashige, Inc. East Meadow, NY) and had tip resistances of 2–3 MV when filled with the pipette solution. A silver/silver choride electrode was used as the reference electrode. The tip potential was zeroed before patch pipette contact with the cell. After a giga-Ohm seal was obtained by applying a negative pressure, the cell membrane was ruptured by applying a gentle negative pressure to establish whole cell configuration. Series resistance was 3–6 MV and was compensated by 80% to minimize voltage errors. The liquid junction potentials were not corrected. Current signals were low-pass filtered at 5 kHz and digitized as for single channel records. All electrophysiological recordings were conducted at room temperature (22–23 degrees C). Extracellular solution contained (mM) NaCl 140, KCl 5.4, MgCl2 1.0, CaCl2 1.8, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10.0 (pH adjusted to 7.4 with NaOH). The pipette solution contained (mM) KCl 20, K-aspartate 110, MgCl2 1.0, HEPES 10, ethyleneglycoltetraacetic acid (EGTA) 5, and various free Ca2+ activities as calculated using the MaxChelator software [2].
Q-PCR of trabecular meshwork RNA and examination of alternative splicing
Total RNA from Human Trabecular Meshwork Cell was obtained from ScienCell Research Laboratories (San Diego, California, http://sciencellonline.com/human-trabecular-meshwork-cell-total-rna.html?_SID=U) and cDNA was synthesized using QuantiTect Reverse Transcription kit (QIAGEN). Two sets of PCR primers specific for Human potassium Large Conductance Calcium activated channel were designed based on published sequences to correspond to regions that were completely conserved in all published human sequences and are also present in rSlo(0) and rSlo(27). The first set of primers amplified a region from just N-terminal to the eighth alpha helical domain (see Fig. 1) to a region just before the ninth alpha helical region in the extended intracellular domain. The second set of primers amplified a region that included the “calcium bowl”, the tenth alpha helical region, and the splice site at which 27 amino acids were inserted in rSlo(27).
First primer set:
| Sense: CTACTTGGAAGGAGTCTCAAATG | antisense: GCTGAGCTGACGTCGCCA VVCIFG |
| Amino acids: YLEGVSNE |
Second primer set:
| Sense: CATGTGCGTTATCCTGTCAGCC | antisense: GTATCAGGGTGAGGATATTGTCA DNILTLI |
| Amino acids: MCVILSA |
We amplified (28 cycles) partial clones using Platinum Blue PCR SuperMix (Invitrogen). The PCR products were run on a 0.8%gel. Two bands were obtained from the first set of primers and a single band was obtained from the second set.. DNA from each of the bands was purified using QIAquick Gel Extraction Kit (QIAGEN). The products were cloned into PCR2.1-TOPO (Invitrogen). 13 clones from the first set of primers and 5 clones from the second set of primers were sent for sequencing.
Statistical analysis
The data are presented as means ± SE. Statistical significance was determined using either a Student’s t-test when two groups were compared or by a one-way ANOVA, followed by Holm-Sidak’s post hoc tests when multiple groups were compared. We assigned significance at p < 0.05.
Results
The response of rSlo(0) and rSlo(27) splice variants to calcium is the same
We first showed that the response of the two splice variants to intracellular calcium was indistinguishable in HEK293 cells which stably express rSlo(27) or rSlo(0) (Fig. 2). There is no statistically significant difference in channel open probability at any concentration of calcium.
Figure 2. The response to calcium of rSlo(0) and rSlo(27) splice variants is the same.
The response of the two splice variants to intracellular calcium was indistinguishable in HEK293 cells which stably express rSlo(27) or rSlo(0). In excised patches depolarized to +40 mV, increasing calcium produced a statistically significant increase in open probability for all calcium concentrations (p<0.5), but there is no statistically significant difference in channel open probability between rSlo(27) and rSlo(0) at any concentration of calcium (each bar is the mean ± s.d.of 4 separate experiments).
rSlo(27) responds to unoprostone; rSlo(0) does not
We examined the effects of unoprostone on HEK293 cells which stably express rSlo(27) or rSlo(0). All the cells were spherical and of uniform size (13 ± 0.2μm) as others have determined {Kaushik, 2014 21069 /id}{2013 21068 /id}{Dittami 21070 /id}{Gentet, 2000 21067 /id}. We first examined whole cell currents from HEK cells transfected with rSlo(27) internally perfused with 1μM Ca2+ (Fig. 3B) and the same cells with 10 nM unoprostone in the bath (Fig. 3C). I–V plots from cells with or without unoprostone treatment or untreated cells are shown for rSlo(27) (Fig. 3D) and rSlo(0) (Fig. 3E). Unoprostone increased BK channels currents. We then examined the properties of single BK channels. In excised, inside-out patches with symmetrical 140mM KCl in the bath and the pipette and 10 nM Ca2+ on the cytosolic surface of the patch, unoprostone in the bath strongly activates rSlo(27) with a half activating concentration of 4.7 ± 0.83 nM (at 10 nM Ca2+) (Fig. 4A, 4C) but does not activate rSlo(0) (Fig. 4B). The activity of the unoprostone-insensitive splice variant, rSlo(0), shows that addition of DMSO vehicle alone at the concentrations used in our experiments has little if any effect on BK channel activity. Neither splice variant is activated by unoprostone in the patch pipette.
Figure 3. Response of whole cell BK currents to unoprostone.
Whole cell currents in response to the voltage steps shown in panel A. Currents from HEK cells transfected with rSlo(27) internally perfused with 1μM Ca2+ (panel B) and the same cells with 10 nM unoprostone in the bath (panel C). I-V plots from the cells that are unoprostone-treated or untreated cells are shown for rSlo(27) (panel D) and rSlo(0) (panel E). Each point in the I-V relationships represents the mean ± s.e.of 3 separate experiments.
Figure 4. Response of BK channel variants to unoprostone.
In excised patches, unoprostone increases the open probability of rSlo(27) (panel A), but not rSlo(0) (panel B). Panel C summarizes the effect of unoprostone on open probability of the two splice variants (from means ± s.e.of 3 separate analyses of 1 minute continuous recording). With the patch depolarized to +40 mV and 50 nM Ca2+ in the bath, increasing concentrations of unoprostone increases open probability of rSlo(27) channels, but has little if any effect on rSlo(0). This is not because rSlo(0) is inactive (see Fig. 2) since when bath Ca2+ is increased to 500 nM (bottom trace), rSlo(0) activity is strongly increased.
Unoprostone does not alter the voltage dependence of BK channels
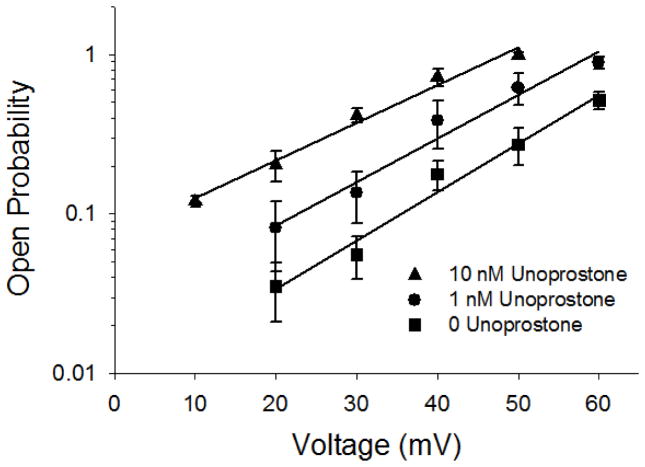
We have previously shown that the response of BK channels to lipid modulators appears to be dependent upon both intracellular calcium and voltage [10]; therefore, we examined the voltage dependence of BK channels in the presence or absence of unoprostone. We examined a range of voltages from +10 to +60 mV and 0, 1, and 10 nM unoprostone (Fig. 5). As expected unoprostone did increase the open probability at all potentials; however, the slopes of the open probability vs voltage relationships were not significantly different from one another implying that the voltage sensitivity (change in open probability per mV) did not change. This is not surprising since contemporary understanding of the channel suggests that the calcium sensor and the voltage sensor are distinct and different parts of the channel [37].
Figure 5. Unoprostone does not alter the voltage dependence of BK channels.
Although the open probability of BK channels is uniformly increased by unoprostone the slope of the Po vs voltage relationship is not significantly different in the presence of unoprostone (44 ± 4.8, 48 ± 5.2, and 46 ± 4.6 mV per 10 fold change in Po at 10, 1, and 0 nM unoprostone, respectively). Points are the mean ± s.e.of 3 separate experiments.
Unoprostone shifts the calcium-dependent activation of BK channels to lower calcium concentrations
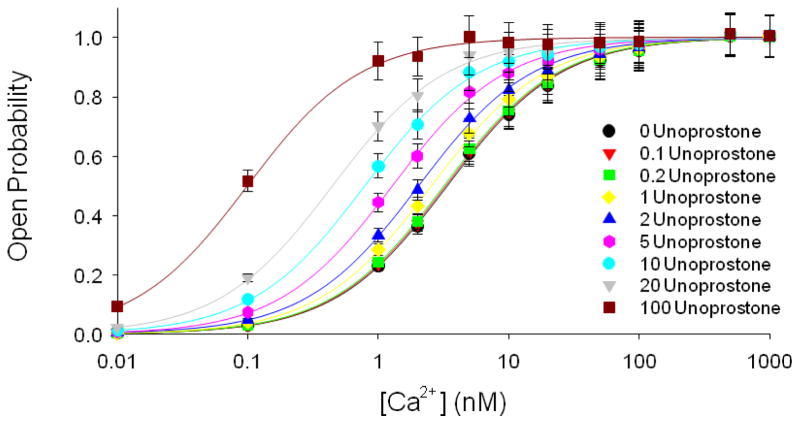
If unoprostone does not affect the voltage dependence of channel, then it must be altering the calcium dependence. To examine this, we examined BK channel activity in excised patches at +60 mV with final concentrations of calcium of 0.01, 0.1, 1, 2, 5, 10, 20, 50, 100, 500, or 1000 nM. To each of these patches we applied 0, 0.1, 0.2, 1, 2, 5, 10, 20, or 100 nM unoprostone. Open probability was used as a measure of channel activity. Figure 6 shows that the principal effect of unoprostone is to shift the calcium-concentration response curve to the left; i.e., unoprostone makes the channel more sensitive to calcium (Fig. 6).
Figure 6. Open probability of BK channels vs calcium concentration in the presence of different unoprostone concentrations.
We examined BK channel activity in excised patches at +60 mV with final concentrations of calcium of 0.01, 0.1, 1, 2, 5, 10, 20, 50, 100, 500, or 1000 nM. To each of these patches we sequentially applied 0, 0.1, 0.2, 1, 2, 5, 10, 20, or 100 nM unoprostone. The principal effect of unoprostone is to shift the calcium-concentration response curve to the left; i.e., unoprostone makes the channel more sensitive to calcium. The half-maximal stimulation of the sensitive BK channels by Ca2+ was shifted from 3.4 ± 0.017 nM to 0.81 ± .0058 nM in the presence of 10 nM unoprostone. Each point represents the mean ± s.e.of 3 separate experiments.
Unoprostone does not replace calcium
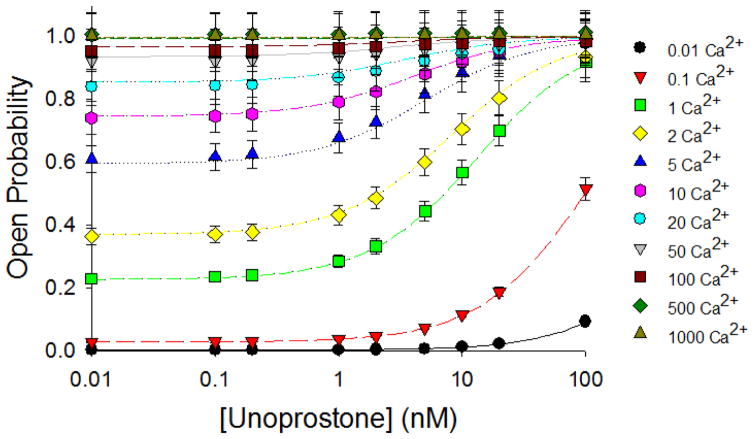
Adding unoprostone activates BK channels in the presence of calcium, but can it activate channels in the absence of calcium? The answer is “no”; in the absence of calcium or at low calcium, unoprostone is ineffective. At high calcium, unoprostone produces little additional effect on channel activity (Fig. 7). The relationship between unoprostone, calcium and BK channel activity can best be appreciated by examining a 3-D representation of the values. Figure 8 shows such a representation.
Figure 7. Unoprostone does not activate BK channels in the absence of calcium.
We examined BK channel activity in excised patches at +60 mV with final concentrations of 0, 0.1, 0.2, 1, 2, 5, 10, 20, or 100 nM unoprostone. To each of these patches we sequentially applied calcium of 0.01, 0.1, 1, 2, 5, 10, 20, 50, 100, 500, or 1000 nM. At low calcium concentrations, there was little activation of BK channels by unoprostone imply in that the principal effect of unoprostone was to increase calcium binding affinity. Each point represents the mean ± s.e.of 3 separate experiments.
Figure 8. A 3-D representation of the relationship between unoprostone, calcium, and open probability.

This figure represents all the data of figures 6 and 7 collected together and fit with to the model given in the discussion.
Unoprostone applied to the external surface of cells activates rSlo(27) (but not rSlo(0))
To approximate the situation during clinical application of unoprostone, we examined the effects of unoprostone applied in the bath outside the patch pipette in cell-attached patches; unoprostone activates rSlo(27) channels, but not rSlo(0) (Fig. 9). Interestingly, unoprostone in the patch pipette does not activate either splice variant. In these cell-attached patches that probably come closer to representing the situation during clinical administration of the drug, the half activating concentration of unoprostone on BK channels with a pipette potential of −40 mV is 13 ± 2.7 nM (Fig. 9). The question of why the half activating dosages should be different in cell attached patches and excised patches is important to understand and has clear implications for drug dosing and drug efficacy.
Figure 9. Unoprostone activates BK channels in cell-attached patches.
The single channel record at the top is a long continuous record from a cell-attached recording from a HEK cell stably transfected with rSlo(27) to which sequentially larger concentrations of unoprostone are added. The patches were depolarized to +60 mV. Sections of the record at A, B, C, and D are expanded in the traces below to show individual channel events. The single channel record at the bottom is a long continuous record from a cell-attached recording from a HEK cell stably transfected with rSlo(0) to which sequentially larger concentrations of unoprostone are added. Sections of the record at A, B, C, and D are expanded in the traces below to show individual channel events. This experiments show that under resting conditions with an intact cell, unoprostone applied extracellulary can activate rSlo(27) BK channels, but not rSlo(0) channels. To the right is the summary data showing the relationship of unoprostone concentration to BK channel open probability (Each point represents the mean ± s.e. of 3 separate experiments). When applied to the extracellular surface about 10 times as much unoprostone is necessary to activate channels than in excised patches.
RNA from human trabecular meshwork cells contains a BK channel splice variant that corresponds to rSlo(27)
We obtained total RNA from Human Trabecular Meshwork Cells from a commerscial supplier of human RNA (ScienCell Research Laboratories, San Diego, California) and prepared cDNA by reverse transcription. Two sets of PCR primers specific for human BK channels were designed based on published sequences (NCBI) to correspond to regions that were completely conserved in all published human sequences and are also present in rSlo(0) and rSlo(27). The first set of primers amplified a region from just N-terminal to the eighth alpha helical domain (see Fig. 1) to a region just before the ninth alpha helical region in the extended intracellular domain. The second set of primers amplified a region that included the “calcium bowl”, the tenth alpha helical region, and the splice site at which 27 amino acids were inserted in rSlo(27). We amplified partial clones with these primer pairs and the PCR products were run on a 0.8% gel. Two bands of similar molecular weight were obtained from the first set of primers and a single band was obtained from the second set (Fig. 10A).. After cutting out the bands and purifying the DNA, the DNA was cloned into PCR2.1-TOPO (Invitrogen). 13 clones from the first set of primers and 5 clones from the second set of primers were sent for sequencing.
Figure 10. RNA from human trabecular meshwork cells contains a BK channel splice variant that corresponds to rSlo(27).
Total RNA from Human Trabecular Meshwork Cells was used to prepare cDNA by reverse transcription. Two sets of PCR primers specific for human BK channels were designed based on published BK channel sequences (NCBI) to correspond to regions that were completely conserved in all published human sequences and are also present in rSlo(0) and rSlo(27). The first set of primers amplified a region from just N-terminal to the eighth alpha helical domain (see Fig. 1) to a region just before the ninth alpha helical region in the extended intracellular domain. The second set of primers amplified a region that included the “calcium bowl”, the tenth alpha helical region, and the splice site at which 27 amino acids were inserted in rSlo(27). We amplified partial clones with these primer pairs and the PCR products were run on a 0.8% gel. Two bands of similar molecular weight were obtained from the first set of primers and a single band was obtained from the second set (Fig. 10A).. The DNA was cloned into PCR2.1-TOPO (Invitrogen). 13 clones from the first set of amplimers and 5 clones from the second set were sequenced. All five clones from the second set of primers had the same sequence (Fig. 10C) containing a region completely homologous to rSlo(27) implying that BK channels in the cells from which this RNA was derived should be sensitive to unoprostone. Interestingly, 1 out of 13 clones amplified by the first set of primers was a splice variant containing a 29 amino acid insert (Fig. 10B).
All five clones from the second set of primers had the same sequence (Fig. 10C) containing a region completely homologous to rSlo(27) implying that the cells from which this RNA was derived should be sensitive to unoprostone. Interestingly, 1 out of 13 clones amplified by the first set of primers was a splice variant containing a 29 amino acid insert (Fig. 10B). We are unaware of a functional significance for this splice variant.
Discussion
We have provided an extensive characterization of the responses of BK channels to unoprostone at different membrane potentials and calcium concentrations. Unoprostone does not change the voltage sensitivity of the channels. It does however alter the apparent binding affinity for calcium without activating the channel directly. In this sense it behaves similarly to some other lipid modulators [8,10]. When taken all together, the data is best fit by a synergistic model in which the KM for calcium binding depends upon the unoprostone concentration [3,18,29,35]. The model for this effect is given by the following equation:
Where Po is BK channel open probability, [Ca2+] is intracellular calcium concentration, KM is the half-maximal activation concentration for calcium, [Uno] is the unoprostone concentration, and Ku is the concentration of unoprostone that reduces the half-activating calcium concentration by half. A least squares regression fit of all the data values in figures 6 and 7 (lines in the figures) gives a Km for calcium of 3.4 ± 0.017 nM similar to that described by others. The unoprostone synergism constant, Ku, is 3.0 ± 0.24 nM. These data might suggest that Ca2+ and unoprostone were competing for the same site on the channel protein, but this is inconsistent with unoprostone increasing the affinity for the channel. In addition, examination of open and closed interval data from patches containing only one channel show that unoprostone primarily produces an increase in the frequency of long-lived open events while Ca2+ reduces mean closed time suggesting that the effect of unoprostone on BK channels may not be by a direct effect on the Ca2+ binding site. This idea is consistent with three dimensional models of the Ca2+ binding site [37] and a putative unoprostone binding domain consisting of the 27 amino acids inserted into rSlo(27) spatially very close to the Ca2+ binding site.
Our results also imply that unoprostone is acting directly on the channel since it works in excised single channel patches; moreover, unoprostone only acts from the cytosolic surface of the excised patches consistent with the 27 amino acid insertion being close to the “calcium bowl” on the cytosolic surface of the channel [21,24].
Unoprostone has been suggested to act as a FP receptor agonist similar to known agonists such as PGF2α and latanoprost [15,26,27]. These agents bind to the FP receptor and cause [Ca2+]i mobilization and stimulate phosphoinositide hydrolysis [15,25]. However, the actions of unoprostone in excised patches could not be mediated by a G protein-linked receptor cascade. Also, even in cell-attached patches the concentration of unoprostone necessary to strongly stimulate BK channels was well below the reported EC50 values for unoprostone binding to FP receptors (5.9 μM) [19]. The highest concentration achieved in clinical use of unoprostone, approximately 100 nM [14], is consistent with the direct activation of BK channels we observe. It did require more unoprostone applied to the extracellular surface to activate BK channels in cell-attached patches. There are several potential explanations for this difference from the activation in excised patches. Obviously, if the intracellular calcium is particularly low, activation will be reduced; however, most cells even in a resting state have calcium in the 10 to 100 nM range which should be adequate to allow BK channel activation. Since we depolarized the cell-attached patches, a reduced membrane potentisal cannot completely explain the reduced activity in the cell-attached patches. However, unoprostone is relatively negatively charged. This implies that, if the cell interior is negative, that unoprostone will partition across the membrane with less inside the cell than outside. If the membrane were as negative as −60 mV, the amount inside the cell would be 10 fold less than outside. Interestingly, any activation of BK channels by unoprostone will tend to hyperpolarize cells and thereby limit the extent of activation. We feel that this hyperpolarization and unoprostone partitioning is sufficient to explain the reduced activation by unoprostone in cell-attached patches.
We tested to see if the human trabecular meshwork (TBM) actually contained a BK splice variant with the region that corresponded to the region in our constructs that produced unoprostone sensitivity (the rSlo(27) insert). We amplified TBM DNA and picked several clones from the DNA products. All of the clones contained a completely homologous insert to rSlo(27). This is actually not surprising since most patients respond to unoprostone. There are 15 human splice variants of BK channels in the NCBI database. Four of them have the rSlo(27) insert. When we amplified an adjacent region of DNA from the TBM, we did find at least one splice variant and 12 identical clones. Interestingly, this relatively rare splice variant (corresponding to NCBI accession #XP011538085) is homologous to rSlo(0) (i.e., it does not contain the insert that renders BK channels sensitive to unoprostone). There has been some suggestion that some individuals do not respond to unoprostone [4,31] and that at least some of the nonresponsiveness is genetically based [1]. Obviously, the majority of the DNA in our TBM sample encodes unoprostone-sensitive BK channels. The origin of the RNA is somewhat obscure, but probably represents RNA from primary cultures of a single patient as previously described [5,6,30]. If the rate of finding the one different clone reflects the rate of nonresponders, then approximately 8.3 ± 7.9% of patients will be non responders and we would have to examine RNA from at least 35 separate eyes to identify with 5% confidence a TBM without the responsive insert. That only 4 out of 15 published sequences have the responsive sequence suggest that using unoprostone in tissues other than TBM my require testing the genetic variability in BK channels to assess likely efficacy.
Our results have important implications for the use of unoprostone to activate BK channels. Channel activation will be limited if intracellular Ca2+ is not elevated. For example, the more strongly a trabecular cell is depolarized allowing calcium entry, the stronger the effect of unoprostone. This implies that unoprostone will “select” only those cells that can be most affected and that these cells are the cells that are preventing fluid loss and inducing high intraocular pressures. It also implies that if cells are not depolarized (with high intracellular calcium) that there will be only modest effects of unoprostone. This could lead to misinterpretation of results from clinical trials.
More fascinating is the possibility of the results suggesting other uses for unoprostone (or other related prostones). Ordinarily, depolarization of nerve cells accompanied by increased calcium entry is prevented from positive feedback by the activation BK channels with subsequent hyperpolarization. However, in several CNS disorders that are characterized by having inappropriate depolarization accompanied by increased calcium entry (epilepsy, ischemic stroke, Parkinson’s disease), normal activity of BK channels is not sufficient to prevent excitotoxicity. A BK channel activator seems like a logical therapeutic choice as one mode of treatment (particularly given the prostones anti-inflammatory action [22]). However, it would be critical to know the BK splice variant present in the target neurons since both sensitive and insensitive variants are present in the CNS. However, given the data we present in this report (especially figure 2 and 3), the calcium concentrations in ischemic neurons should be sufficient to allow a strong potentiation of BK channel activity in CNS neurons and hyperpolarization of the ischemic neurons, reducing excitatory amino acid release, and reducing ischemic calcium entry.
Highlights.
Two BK channel splice variants differing by a 27 amino acid insert were examined.
The response of the two splice variants to calcium is the same.
One splice variant responds to unoprostone; the other does not.
Unoprostone shifts the calcium-dependent activation of BK channels to lower calcium concentrations.
Unoprostone applied to the external surface of cells can activate BK channels.
Acknowledgments
This work was supported by a grant from Sucampo Pharmaceuticals, LLC, and NIH DK R37DK037963 to DCE.
Unoprostone is a product of Sucampo.
Footnotes
Abbreviations: BK – large conductance calcium-activated potassium channels or KCNMA1 channels
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abelson MB, McLaughlin J. A Look at Typical Non-Responders to Drug Therapy and the Factors That Have Made Them That Way. 2011 [Google Scholar]
- 2.Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca(2+) buffers. Methods Cell Biol. 2010;99:1–26. doi: 10.1016/B978-0-12-374841-6.00001-3. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld A, Gennings C, Cady R. Pharmacological synergy: the next frontier on therapeutic advancement for migraine. Headache. 2012;52:636–647. doi: 10.1111/j.1526-4610.2011.02058.x. [DOI] [PubMed] [Google Scholar]
- 4.Boland MV, Ervin AM, Friedman D, Jampel H, Hawkins B, Volenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA. 2012 [PubMed] [Google Scholar]
- 5.Clark AF, Lane D, Wilson K, Miggans ST, McCartney MD. Inhibition of dexamethasone-induced cytoskeletal changes in cultured human trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci. 1996;37:805–813. [PubMed] [Google Scholar]
- 6.Clark AF, Miggans ST, Wilson K, Browder S, McCartney MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- 7.Cuppoletti J, Malinowska DH, Tewari KP, Chakrabarti J, Ueno R. Cellular and molecular effects of unoprostone as a BK channel activator. Biochim Biophys Acta. 2007;1768:1083–1092. doi: 10.1016/j.bbamem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Denson DD, Li J, Wang X, Eaton DC. Activation of BK channels in GH3 cells by a c-PLA2-dependent G-protein signaling pathway. J Neurophysiol. 2005;93:3146–3156. doi: 10.1152/jn.00865.2004. [DOI] [PubMed] [Google Scholar]
- 9.Denson DD, Wang X, Worrell RT, AlKhalili O, Eaton DC. Cytosolic phospholipase A2 is required for optimal ATP activation of BK channels in GH(3) cells. J Biol Chem. 2001;276:7136–7142. doi: 10.1074/jbc.M009566200. [DOI] [PubMed] [Google Scholar]
- 10.Denson DD, Wang X, Worrell RT, Eaton DC. Effects of fatty acids on BK channels in GH(3) cells. Am J Physiol Cell Physiol. 2000;279:C1211–C1219. doi: 10.1152/ajpcell.2000.279.4.C1211. [DOI] [PubMed] [Google Scholar]
- 11.Denson DD, Worrell RT, Middleton P, Eaton DC. Ca2+ sensitivity of BK channels in GH3 cells involves cytosolic phospholipase A2. Am J Physiol. 1999;276:C201–C209. doi: 10.1152/ajpcell.1999.276.1.C201. [DOI] [PubMed] [Google Scholar]
- 12.Duerson K, White RE, Jiang F, Schonbrunn A, Armstrong DL. Somatostatin stimulates BKCa channels in rat pituitary tumor cells through lipoxygenase metabolites of arachidonic acid. Neuropharmacology. 1996;35:949–961. doi: 10.1016/0028-3908(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 13.Ha TS, Jeong SY, Cho SW, Jeon H, Roh GS, Choi WS, Park CS. Functional characteristics of two BKCa channel variants differentially expressed in rat brain tissues. Eur J Biochem. 2000;267:910–918. doi: 10.1046/j.1432-1327.2000.01076.x. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi K, Iizuka Y, Tsukahara S. Metabolites of isopropyl unoprostone as potential ophthalmic solutions to reduce intraocular pressure in pigmented rabbits. Jpn J Pharmacol. 1999;81:56–62. doi: 10.1254/jjp.81.56. [DOI] [PubMed] [Google Scholar]
- 15.Kelly CR, Williams GW, Sharif NA. Real-time intracellular Ca2+ mobilization by travoprost acid, bimatoprost, unoprostone, and other analogs via endogenous mouse, rat, and cloned human FP prostaglandin receptors. J Pharmacol Exp Ther. 2003;304:238–245. doi: 10.1124/jpet.102.042556. [DOI] [PubMed] [Google Scholar]
- 16.Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Al-Khalili O, Ramosevac S, Eaton DC, Denson DD. Protein-protein interaction between cPLA2 and splice variants of alpha-subunit of BK channels. Am J Physiol Cell Physiol. 2010;298:C251–C262. doi: 10.1152/ajpcell.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLean MR, Morecroft I. Increased contractile response to 5-hydroxytryptamine1-receptor stimulation in pulmonary arteries from chronic hypoxic rats: role of pharmacological synergy. Br J Pharmacol. 2001;134:614–620. doi: 10.1038/sj.bjp.0704273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melamed S. Neuroprotective properties of a synthetic docosanoid, unoprostone isopropyl: clinical benefits in the treatment of glaucoma. Drugs Exp Clin Res. 2002;28:63–73. [PubMed] [Google Scholar]
- 20.Miller C. An overview of the potassium channel family. Genome Biol. 2000;1:REVIEWS0004. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moczydlowski EG. BK channel news: full coverage on the calcium bowl. J Gen Physiol. 2004;123:471–473. doi: 10.1085/jgp.200409069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nighot M, Moeser A, Ueno R, Blikslager A. Gastro protective properties of the novel prostone SPI-8811 against acid-injured porcine mucosa. World J Gastroenterol. 2012;18:4684–4692. doi: 10.3748/wjg.v18.i34.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhart PH, Chung S, Levitan IB. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989;2:1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharif NA, Crider JY, Husain S, Kaddour-Djebbar I, Ansari HR, Abdel-Latif AA. Human ciliary muscle cell responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther. 2003;19:437–455. doi: 10.1089/108076803322473006. [DOI] [PubMed] [Google Scholar]
- 26.Sharif NA, Kelly CR, Crider JY. Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci. 2003;44:715–721. doi: 10.1167/iovs.02-0323. [DOI] [PubMed] [Google Scholar]
- 27.Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003;19:501–515. doi: 10.1089/108076803322660422. [DOI] [PubMed] [Google Scholar]
- 28.Shipston MJ. Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol. 2001;11:353–358. doi: 10.1016/s0962-8924(01)02068-2. [DOI] [PubMed] [Google Scholar]
- 29.Spinella M. The importance of pharmacological synergy in psychoactive herbal medicines. Altern Med Rev. 2002;7:130–137. [PubMed] [Google Scholar]
- 30.Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- 31.Susanna R, Jr, Medeiros FA, Vessani RM, Giampani J, Jr, Borges AS, Jordao ML. Intraocular pressure fluctuations in response to the water-drinking provocative test in patients using latanoprost versus unoprostone. J Ocul Pharmacol Ther. 2004;20:401–410. doi: 10.1089/jop.2004.20.401. [DOI] [PubMed] [Google Scholar]
- 32.Thieme H, Stumpff F, Ottlecz A, Percicot CL, Lambrou GN, Wiederholt M. Mechanisms of action of unoprostone on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2001;42:3193–3201. [PubMed] [Google Scholar]
- 33.Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol. 2001;537:57–68. doi: 10.1111/j.1469-7793.2001.0057k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuruma K, Tanaka Y, Shimazawa M, Mashima Y, Hara H. Unoprostone reduces oxidative stress- and light-induced retinal cell death, and phagocytotic dysfunction, by activating BK channels. Mol Vis. 2011;17:3556–3565. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Xu X, Tao W, Li Y, Wang Y, Yang L. A systems biology approach to uncovering pharmacological synergy in herbal medicines with applications to cardiovascular disease. Evid Based Complement Alternat Med. 2012;2012:519031. doi: 10.1155/2012/519031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto S, Sugawara T, Murakami A, Nakazawa M, Nao I, Machida S, Wada Y, Mashima Y, Myake Y. Topical isopropyl unoprostone for retinitis pigmentosa: microperimetric results of the phase 2 clinical study. Ophthalmol Ther. 2012;1:5. doi: 10.1007/s40123-012-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]