Abstract
Background and aims
To decrease infectious disease transmission, China is expanding methadone maintenance treatment (MMT). This study evaluated the prevalence of hepatitis C virus (HCV) infection at MMT entry, seroconversion rates after admission and potential risk factors for HCV seroconversion during MMT in Wuhan, China.
Design
Cross-sectional survey of all patients entering MMT and prospective follow-up of patients HCV seronegative at admission.
Setting
All MMT clinics in Wuhan, China.
Participants
A total of 12 755 opiate-dependent individuals entering MMT between May 2006 and June 2011; 1200 participants HCV seronegative at admission were followed.
Measurements
Serological tests for HCV and self-report data on risk behaviors at MMT admission; urine toxicology results and repeated assessments of serological status and risk behaviors during treatment on patients HCV seronegative at admission.
Findings
HCV seroprevalence at admission was 72.1% [95% confidence interval (CI) = 71.3–72.9%] and 555/1200 (46.3%, 95% CI = 43.5–49.1%) patients seroconverted to HCV during MMT. The mean time to HCV seroconversion was 3 (95% CI = 2.84–3.07) years with a cumulative seroconversion rate of 34.5 (95% CI = 31.5–36.9) per 100 person-years. Significant predictors of HCV conversion included injection drug use in the past 30 days [relative hazard (RH) 2.0, 95% CI: 1.6 – 2.4, P=0.002] and the rate of opiate-positive urine tests during MMT (RH 2.0, 95% CI = 1.3–3.1, P<0.001).
Conclusions
Methadone maintenance treatment patients in Wuhan, China show a high prevalence of hepatitis C virus at admission (72.1%) and a high rate of seroconversion during treatment (46.3%). Seroconversion is associated with continuing injection drug use.
Keywords: Behavioral risks, hepatitis C virus, injection drug use, methadone maintenance treatment, opiate dependence, seroconversion
INTRODUCTION
An estimated 130–170 million people world-wide are chronically infected with hepatitis C virus (HCV), an agent that can cause considerable morbidity and mortality [1]. Estimates of HCV prevalence rates among people who inject drugs (PWIDs) vary widely, with rates up to 85–95% infected in some regions, including Asia [2–5]. HCV infection incidence rates often exceed human immunodeficiency virus (HIV) incidence rates in PWIDs [6–9] by a substantial margin and illustrate the hyperendemic nature of HCV infection in this population. In China, the number of registered individuals who use drugs exceeded 2 million in 2013 [10], of whom 75–85% use heroin and 50–70% inject the drug. Injection drug use is now the predominant mode of both HCVand HIV transmission, with HCV prevalence ranging from 11 to 91% and HIV prevalence ranging from 12.6 to 52.5% among PWIDs in different regions of China [11]. As the number of people who use and who inject drugs continues to increase, HCV is quickly becoming a major public health problem in China [12].
Methadone maintenance treatment (MMT) was introduced in China in 2004 [13] as a strategy to reduce drug use and transmission of HIV [14–17]. Since then, MMT has been expanded into a nation-wide program consisting of more than 763 clinics, covering all provinces, with a cumulative total of more than 412 000 patients enrolled by the end of 2013 [18]. MMT in China has been associated with many positive outcomes, including reductions in heroin use, risky injection practices and criminal behaviors among patients [17]. Although MMT has been reported to reduce the risk of HIV seroconversion [19] and reductions in HIV risk behaviors, including reduced injection frequency and related risk behaviors [20,21], previous studies have failed to demonstrate significant reductions in HCV seroconversion among MMT patients [22–24].
Very few studies have addressed HCV, drug use and related risk factors or the effects of MMT on HCV transmission in China. Consequently, the aim of the current study was to evaluate the prevalence of HCV at MMT admission, HCV seroconversion rates during MMT and potential risk factors for HCV seroconversion among opiate-dependent patients entering MMT in Wuhan China.
METHODS
Settings and participants
We reviewed the clinical records of all patients enrolled in all the 20 MMT clinics in Wuhan, China. Between May 2006 and June 2011, there were 16 085 entrants; 12 755 (79.3%) received HCV test at MMT entry and 3330 refused the test or were not tested for other reasons. Of the remaining 3558 MMT patients who tested HCV-negative at MMT entry, 47.8% (1702 of 3558) agreed to participate in the prospective follow-up. To ensure that HCV antibody seroconversions investigated in the current study were the result of transmission during the MMT program, only participants who had HCV-negative blood specimens collected twice, at MMT entry and at least 3 months later, and who completed at least one follow-up interview were included in the final study sample; 502 of 1702 (29.5%) eligible patients were excluded from the study sample because either they did not return for follow-up interviews or the duration of follow-up was less than 6 months. Consequently, 1200 participants were included in the prospective follow-up sample, where they received HCV tests at least every 6 months for the entire study duration or until they tested HCV-positive. Patients testing HCV-positive at any time during MMT were not tested again during subsequent follow-up interviews.
The study was approved by the Institutional Review Boards (IRB) of the Wuhan Center for Disease Control and Prevention (CDC). Participants were not provided with incentives. The self-report and urine and serological testing collected in the study did not differ substantially from the data collected during the routine treatment and follow-up for patients enrolled in MMT in Wuhan. Study participation was voluntary, and data on reasons for refusing to participate were not collected. Participation in standard evaluation components offered during MMT is also voluntary, and patients in MMT in Wuhan can refuse follow-up blood tests and follow-up evaluation.
MMT programs in Wuhan, and similarly elsewhere in China, offer fairly limited services, including daily dispensing of methadone at the clinics and occasional group counseling, typically offered once per month and attended by relatively small groups of volunteering patients. MMT patients are required to pay a daily fee of approximately US$1.50 (10 RMB) every time they come to receive their methadone dose. This fee is fixed, regardless of the medication dose or services received on any given day (e.g. patients can request a visit with a doctor or a nurse to discuss issues related to their MMT treatment or other concerns). In general, patients are prescribed maintenance doses within a therapeutic range, between 60 and 120 mg per day. However, a substantial proportion of MMT patients do not attend clinics every day. During MMT, patients are required to submit a urine sample for toxicology testing (morphine metabolites only) approximately once per month; however, compliance with this requirement is fairly low despite that, in general, there are no treatment-related consequences for continued drug use or testing positive during MMT.
HCV antibody determinations
Participants were tested for HCV and HIV at treatment entry; those who tested negative for HCV at treatment entry were retested 3 months later; those who tested negative on both occasions were classified as HCV seronegative at admission and retested approximately every 6 months thereafter. Specimens of 10 ml venous blood were collected in K3-ethylenediamine tetraacetic acid (EDTA) tubes, kept at +4°C, and transferred to the laboratory of the Wuhan CDC. Plasma specimens were separated on the same day and stored at −20°C frozen in aliquots until analysis. HCV antibody testing was performed using the third-generation enzyme-linked immunoassay (EIA-3) system (Kehua Biotechnology Inc., Shanghai, China). Specimens found to be initially reactive by EIA-3 were repeated in duplicate. Repeat reactive specimens were assumed to be seropositive.
Risk factors
To identify potential risk factors for HCV, we used de-identified data extracted from the computerized database maintained by the Chinese CDC. MMT clinic staff (doctors and nurses) collect data on all patients at MMT treatment entry and at approximately 6-month follow-ups in all MMT clinics in China using a questionnaire developed by the Chinese CDC. Information collected and stored in the computerized database maintained by the Chinese CDC included sociodemographics data (e.g. employment, living status), self-report data (life-time and the past 30 days) on illicit drug use history, needle/equipment sharing history, drug rehabilitation and treatment history, limited data on sexual risk behaviors and criminal history and results of urine toxicology screens tested for opiate (morphine) metabolites. While the database aims to include a broad range of relevant information, many self-report variables have missing data, limiting an ability to conduct comprehensive multivariate analyses of all inter-related and potentially important factors and risk behaviors that may affect the HCV transmission in this population. Consequently, this study focused on evaluation of factors related to ongoing drug use and injection drug use during MMT based both on self-report and urine toxicology tests results.
Statistical analyses
Descriptive statistics were used to characterize the study sample. The χ2 tests were used to evaluate the statistical significance of differences on categorical variables among seroconverters and non-converters, as well as among the study cohorts (participants versus non-participants). Kaplan–Meier survival analyses were conducted to calculate the HCV seroconversion rates and to compare seroconversion rates between the groups of participants who reported past-month injection drug use (IDU) and those who did not. The time characteristics of seroconversion for HCV in the study sample were calculated using the person-years method [25], where the date of seroconversion was taken as the mid-point of the follow-up period. Univariate analyses of the statistical significance of factors associated with HCV seroconversion were performed using hazard ratios (HR) and 95% confidence intervals (CI). The Cox model for multivariate analyses was used to evaluate the relationship between IDU in the past 30 days and the rates of opiate-positive urine toxicology tests on seroconversion rates. An analysis of variance was used to compare the rates of opiate-positive urine tests between seroconverters and non-converters. Comparisons were based on all available data collected between the MMT entry and the first HCV-positive test for seroconverters and during the entire follow-up for non-converters. Additionally, a proportion of study participants who seroconverted during the first year of the follow-up was calculated.
RESULTS
Participant characteristics and HCV prevalence at MMT admission
The median age and duration of drug use at enrollment for all 16 085 patients were 39 years [interquartile range (IQR) = 33–44 years] and 11 years (IQR = 7–14 years), respectively. The study sample was predominantly male (73%), with 99% of Han ethnicity, 73% with junior high school or lower education, 43% married or with a steady partner and 87% unemployed. More than 78% reported injection or injection and inhalation as the main drug use route, and more than 58% reported more than 10 years’ duration of drug use. The prevalence of HIV among all patients enrolled in MMT in Wuhan was less than 0.5%, and data collected by Wuhan CDC also showed that the prevalence of HIVamong PWID in Wuhan who were not in treatment was less than 1%. At MMT entry, 9197 of 12 755 (72.1%, 95% CI = 71.3–72.9%) patients were HCV-positive. In the followed sample, two of 1200 (0.2%) participants were infected with HIVand 18 of 1200 (1.5%) with syphilis. The differences in baseline characteristics between those who refused to participate and those who agreed to participate were not statistically significant. See Table 1 for a complete set of baseline characteristics of the study participants.
Table 1.
Baseline characteristics of study cohorts.
| Characteristic | All MMT patients n=16 085 | With baseline HCV test n=12 755 | Refused HCV test at baseline n=3330 | Did not agree to be followed n=1856 | Followed n=1200 |
|---|---|---|---|---|---|
|
| |||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Gender | |||||
| Male | 11 769 (73.2) | 9319 (73.1) | 2450 (73.6) | 1383 (74.5) | 908 (76.0) |
| Female | 4316 (26.8) | 3436 (26.9) | 880 (26.4) | 473 (25.5) | 292 (24.0) |
| Age (years) | |||||
| ≤ 30 | 2398 (14.9) | 1968 (15.4) | 430 (12.9) | 240 (12.9) | 101 (8.4) |
| 31–39 | 6402 (39.8) | 5162 (40.5) | 1240 (37.3) | 698 (37.6) | 272 (22.6) |
| ≥ 40 | 7285 (45.3) | 5625 (44.1) | 1660 (49.8) | 918 (49.5) | 827 (69.0) |
| Education | |||||
| Elementary or lower | 1127 (7.0) | 858 (6.7) | 269 (8.1) | 129 (7.0) | 67 (5.6) |
| Junior high school | 10 720 (66.6) | 8440 (66.2) | 2280 (68.4) | 1262 (68.0) | 724 (60.3) |
| Senior high school | 3891 (24.2) | 3166 (24.8) | 725 (21.8) | 419 (22.5) | 371 (30.9) |
| Junior college or higher | 347 (2.2) | 291 (2.3) | 56 (1.7) | 46 (2.5) | 38 (3.2) |
| Marital status | |||||
| Married or have a regular partner | 6985 (43.4) | 5524 (43.3) | 1461 (43.9) | 883 (47.6) | 590 (49.2) |
| Divorced/widowed | 2833 (17.6) | 2264 (17.8) | 569 (17.1) | 288 (15.5) | 214 (17.8) |
| Single | 6267 (39.0) | 4967 (38.9) | 1300 (39.0) | 685 (36.9) | 396 (33.0) |
| Employment | |||||
| Employed | 2179 (13.5) | 1682 (13.2) | 497 (14.9) | 234 (12.6) | 141 (11.8) |
| Unemployed | 13 906 (86.5) | 11073 (86.8) | 2833 (85.1) | 1622 (87.4) | 1059 (88.2) |
| Living status | |||||
| With family | 12 040 (74.9) | 9538 (74.8) | 2502 (75.2) | 1417 (76.3) | 911 (75.9) |
| With friends | 1724 (10.7) | 1426 (11.2) | 298 (8.9) | 209 (11.3) | 90 (7.5) |
| Alone | 2321 (14.4) | 1791 (14.0) | 530 (15.9) | 230 (12.4) | 199 (16.6) |
| Drug administration method | |||||
| Inhaled | 3490 (21.7) | 2799 (21.9) | 691 (20.8) | 657 (35.4) | 478 (39.8) |
| Injected | 11 174 (69.5) | 8892 (69.7) | 2282 (68.5) | 1095 (59.0) | 628 (52.3) |
| Mixed (injected and inhaled) | 1421 (8.8) | 1064 (8.2) | 357 (10.7) | 104 (5.6) | 94 (7.9) |
| Duration of drug use (years) | |||||
| ≤ 5 | 3062 (22.2) | 2821 (22.1) | 748 (22.5) | 525 (28.3) | 80 (6.7) |
| 6–9 | 3098 (19.3) | 2516 (19.7) | 582 (17.5) | 342 (18.4) | 274 (22.8) |
| ≥10 | 9418 (58.5) | 7418 (58.2) | 2000 (60.0) | 989 (53.3) | 846 (70.5) |
| Drug use in the past 30 days | |||||
| Yes | 15 440 (96.0) | 12242 (96.0) | 3198 (96.0) | 1791 (96.5) | 1158 (96.5) |
| No | 645 (4.0) | 513 (4.0) | 132 (4.0) | 65 (3.5) | 42 (3.5) |
| Injection drug use in the past 30 days | |||||
| Yes | 10 428 (64.8) | 8446 (66.2) | 1982 (59.5) | 916 (51.1) | 566 (48.9) |
| No | 5657 (35.2) | 4309 (33.8) | 1348 (40.5) | 875 (48.9) | 592 (51.1) |
MMT = methadone maintenance treatment; HCV = hepatitis C virus.
HCV seroconversion rates and time to seroconversion
The median duration of follow-up for the 1200 initially HCV-negative study participants was 1.8 years (IQR = 1.1–2.7 years), and the mean number of follow-up interviews was 4.0 ± 2.0. As of December 2011, 555 of 1200 (46.3%, 95% CI = 43.5–49.1%) of the initially HCV seronegative participants had become HCV-infected, yielding a cumulative seroconversion rate of 34.5 per 100 person-years (95% CI = 31.5–36.9). During the first year of follow-up, 193 of 1200 (16.1%, 95% CI = 14.1–18.3%) study participants seroconverted to HCV.
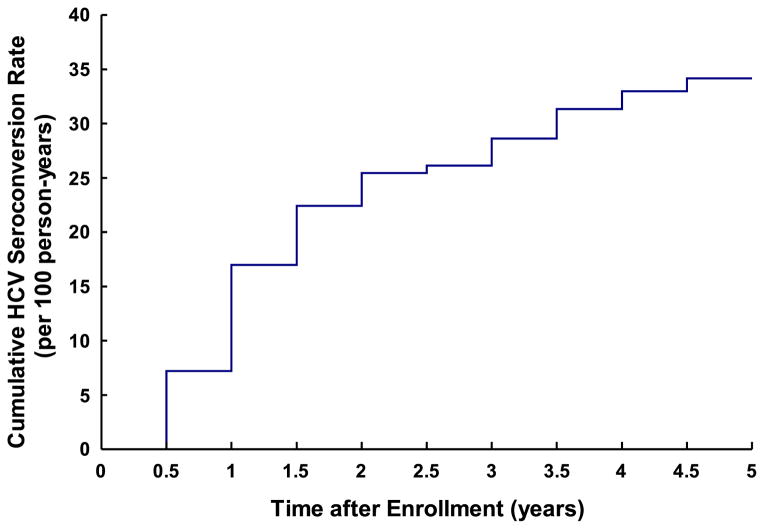
The total HCV surveillance time during the study period was 2419.3 person-years. The mean time to HCV seroconversion was 2.96 years (95% CI = 2.84–3.07) and a substantial proportion of the HCV seroconversions occurred during the first 3 years of follow-up (Fig. 1).
Figure 1.
Cumulative rate of hepatitis C virus (HCV) seroconversion among methadone maintenance treatment (MMT) patients in Wuhan between May 2006 and December 2011
Factors associated with HCV seroconversion
In a multivariate Cox model, time to HCV seroconversion was associated significantly with injection drug use in the past 30 days [relative hazard (RH) = 2.0, 95% CI = 1.6–2.4, P=0.002] and the rate of opiate-positive urine tests during MMT (RH = 2.0, 95% CI = 1.3–3.1, P<0.001). Additionally, 79.5% of study participants had at least one opiate-positive urine toxicology test during MMT, and HCV seroconverters had a significantly higher rate of opiate-positive tests than non-converters (19.4 versus 16.0%, t = 3.12, P=0.002). These two variables (IDU in the past 30 days and the rate of opiate-positive urine test) correlated significantly (r=0.208, P<0.001).
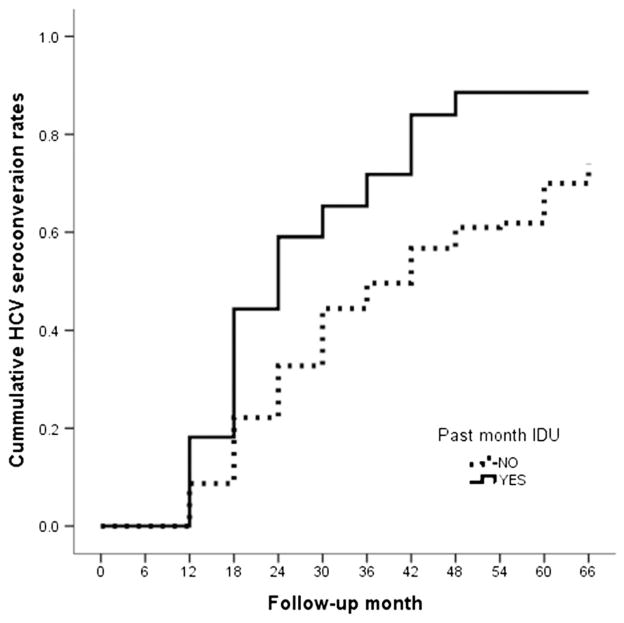
The Kaplan–Meier survival analyses also showed significant differences in time to seroconversion between those who reported drug injection in the past 30 days and those who reported no injection in the past 30 days. The median time to HCV seroconversion for these two groups of patients was 2.04 versus 3.48 years (log-rank χ2=61.39, P<0.01), respectively (also see Fig. 2).
Figure 2.
Hepatitis C virus (HCV) seroconversion rates among study participants who reported past 30-day injection drug use (IDU) and those who did not
Factors related to MMT
The CDC database collected self-reported data at each follow-up on the average daily methadone dose in the past 3 months. At the most recent follow-up, this information was reported by 1143 participants. The reported doses ranged between 8 and 170 mg daily, with 53% of participants reporting the average doses between 60 and 120 mg. While data based on objective measures of MMT adherence for the entire time between treatment enrollment and follow-up assessments was not available, at the most recent follow-ups 1143 of 1200 participants provided self-reported number of days in the past 3 months when they came to clinic and took their methadone dose. The range was from 1 to 90 days, with approximately 22% reporting up to 30 days, 37% reporting between 30 and 60 days and the remaining 41% reporting between 60 and 90 days out of 90 possible days of clinic attendance. These two variables did not significantly predict seroconversion rates or time to seroconversion independently or in multivariate analyses.
DISCUSSION
In this study of patients enrolled in all 20 MMT clinics operating in Wuhan, China, we found an alarmingly high HCV prevalence among patients entering MMT (72%) and a very high rate of HCV seroconversion (46%), with a cumulative seroconversion rate of 34.5 per 100 person-years during MMT. The high HCV prevalence among study participants indicates a potentially very high HCV prevalence among all drug users in Wuhan and their engagement in high-risk behaviors prior to entering MMT. Consistent with other studies of MMT in China [26], a considerable proportion of the patients entering MMT in Wuhan continued during MMT to use illicit opioids and potentially to engage in other behaviors exposing them to risk of HCV infection, sexually transmitted diseases and other infectious or communicable diseases. The findings of the present study, showing that predictors of HCV seroconversion included injection drug use and that a substantial proportion of HCV seroconversions occurred during the first 3 years of MMT, add urgency to the importance of improving MMT treatment services to obtain maximum benefit from this approach.
Continuing illicit opioid use during MMT adversely affects MMT adherence [27] and MMT treatment outcomes. Findings from this study indicate that ongoing illicit drug use, and in particular injection drug use during MMT, are also associated with higher rates of HCV seroconversion during treatment. While objective data on treatment adherence and dosing characteristics were not available in this study, self-reported data support the substantial variability of these factors in the studied cohort.
Optimally effective scale-up of MMT to prevent HCV transmission may require a close monitoring of patients for continued injection drug use and provision of additional interventions to reduce drug use during treatment, in particular injection drug use, with a goal to reduce transmission risk significantly. In some settings, more intensive and multi-component interventionism, including antiretroviral treatment and availability of needle and syringe exchange programs, along with MMT, have reduced HCV transmission [28–30]. However, China, and Wuhan specifically, have not undertaken a large scale-up of needle and syringe distribution programs. There was a very small needle and syringe exchange program on the outskirts of Wuhan, enrolling fewer than 100 individuals. Syringes and needles can be purchased without significant restrictions and without prescription in pharmacies throughout China, including Wuhan. They are inexpensive, and generally pharmacies have no shortages of supply. As supported by the findings of a recent systematic review, multi-component interventions combining drug treatment, behavioral counseling to reduce or eliminate injection drug use and encourage safer injection practices, and provision of sterile needles, syringes and other injection equipment, are likely to have the greatest impact in preventing HCV transmission [31]. Similar approaches may be effective in MMT programs in China.
Also of note, we found that the HCV seroconversion rate for those reporting past-month IDU was significantly faster than for those denying past month IDU, but there was nevertheless a substantial rate of HCV seroconversion among participants denying past-month IDU. One reason for this may have been the difference between the short (30-day) interval for self-reported injection drug use and the longer (6-month) potential exposure period. Other reasons could include under-reporting of injection drug use or, less likely, exposure through non-injection transmission routes.
Limitations
Approximately 20% of participants enrolling in MMT did not have an initial HCV test, and slightly fewer than half (47.8%) of participants who were HCV seronegative at MMT admission participated in the follow-up assessments. Consequently, the results may not reflect the overall population of patients entering MMT or of patients testing seronegative at MMT admission in Wuhan. Post-hoc comparisons of participating and non-participating MMT patients found no significant differences between the groups, although non-participants reported slightly higher IDU. The 46.3% seroconversion rate found in this study may underestimate the overall seroconversion rate, as patients who refused to participate or were lost to follow-up (e.g. because they left treatment early) may be more likely to continue drug use and high-risk behaviors.
The study findings are based mainly on self-reported data collected every 6 months, while the report covered the past 30 days. Although restricting recall to the past 30 days may miss some periods of drug or injection drug use, recall for more recent behavior generally has greater reliability and validity. Of note, we obtained a similar pattern of findings based on urine toxicology screens—participants who seroconverted had higher rates of urine tests positive for opiates during MMT—supporting the validity of the self-report data. Missing data in many of the variables included in the computerized clinical records database precluded fully comprehensive multivariate analyses of all potentially relevant characteristics of the studied cohort and their impact on HCV seroconversion. Additionally, data on HCV seroincidence among people who use drugs who are not engaged in MMT in Wuhan were not available, precluding discussion or interpretation of potential beneficial effects of MMT participation on HCV seroconversion rates. Finally, MMT patients in Wuhan may not be fully representative of drug using individuals in other regions of China, and the effectiveness of MMT for reducing risk behaviors or HCV conversion may also differ in other regions or depending on the availability of other harm reduction interventions [11,31].
CONCLUSIONS
The findings in the current study of a high prevalence of HCV at MMT entry and high rates of HCV seroconversion during MMT support the critical importance of developing comprehensive and more effective infectious disease prevention interventions for out-of-treatment PWIDs and for individuals receiving MMT [31]. The current study is a critical first step towards identifying factors potentially contributing to HCV transmission among MMT patients and developing specific interventions to reduce HCV seroconversion risk during MMT, with the goal of substantially reducing the rates of HCV seroconversion in this high-risk population.
Acknowledgments
This study was supported by China CDC, NIDA DA026797 and the Connecticut Mental Health Center, Department of Mental Health and Addiction Services. None of these institutions influenced the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of interests
None.
References
- 1.World Health Organization. [accessed 17 June 2014];Guidance on prevention of viral hepatitis B and C among people who inject drugs. Available at: http://apps.who.int/iris/bitstream/10665/75192/1/WHO_HIV_2012.18_eng.pdf. (Archived by WebCite® at http://www.webcitation.org/6QP7PVFIz) [PubMed]
- 2.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:362–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008;122:990–1003. doi: 10.1016/j.puhe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 6.Christensen PB, Krarup HB, Niesters HG, Norder H, Georgsen J. Prevalence and incidence of bloodborne viral infections among Danish prisoners. Eur J Epidemiol. 2000;16:1043–9. doi: 10.1023/a:1010833917242. [DOI] [PubMed] [Google Scholar]
- 7.Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Aust NZ J Public Health. 2007;31:30–5. doi: 10.1111/j.1753-6405.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Roy E, Alary M, Morissette C, Leclerc P, Boudreau JF, Parent R, et al. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007;18:23–7. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 9.Hahn JA, Page-Shafer K, Lum PJ, Bourgois P, Stein E, Evans JL, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–64. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 10.Narcotics Control Bureau of the Chinese Ministry of Public Security. Annual Report on Drug Control in China. 2013. [Google Scholar]
- 11.Bao YP, Liu ZM. Systematic review of HIV and HCV infection among drug users in China. Int J STD AIDS. 2009;20:399–405. doi: 10.1258/ijsa.2008.008362. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Health, People’s Republic of China; United Nations Programme on HIV/AIDS; World Health Organization. [accessed 17 June 2014];Update on the HIV/AIDS epidemic and response in China. 2005 Available at: http://data.unaids.org/publications/External-Documents/rp_2005chinaestimation_25jan06_en.pdf. (Archived by WebCite® at http://www.webcitation.org/6QP7qbU7r)
- 13.Pang L, Hao Y, Mi G, Wang C, Luo W, Rou K, et al. Effectiveness of first eight methadone maintenance treatment clinics in China. AIDS. 2007;21(Suppl 8):S103–7. doi: 10.1097/01.aids.0000304704.71917.64. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China’s response to HIV/AIDS. Lancet. 2007;369:679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan SG, Wu Z. Rapid scale up of harm reduction in China. Int J Drug Policy. 2007;18:118–28. doi: 10.1016/j.drugpo.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health of the People’s Republic of China. [accessed 17 June 2014];China AIDS Response Progress Report. 2012 Available at: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_CN_Narrative_Report%5B1%5D.pdf. (Archived by WebCite® at http://www.webcitation.org/6QP821iaO)
- 17.Yin W, Hao Y, Sun X, Gong X, Li F, Li J, et al. Scaling up the national methadone maintenance treatment program in China: achievements and challenges. Int J Epidemiol. 2010;39 (Suppl 2):29–37. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center of AIDS Prevention and Control (NCAIDS) Annual Report. 2013. Unpublished document. [Google Scholar]
- 19.Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in-and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–56. [PubMed] [Google Scholar]
- 20.Willner-Reid J, Belendiuk KA, Epstein DH, Schmittner J, Preston KL. Hepatitis C and human immunodeficiency virus risk behaviors in polydrug users on methadone maintenance. J Subst Abuse Treat. 2008;35:78–86. doi: 10.1016/j.jsat.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu EW, Liang T, Shen LM, Zhong H, Wang B, Wu ZY, et al. Impact of methadone maintenance treatment on HIV risk behaviors of heroin drug users. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:981–4. [PubMed] [Google Scholar]
- 22.Crofts N, Nigro L, Oman K, Stevenson E, Sherman J. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction. 1997;92:999–1005. [PubMed] [Google Scholar]
- 23.Selvey LA, Denton M, Plant AJ. Incidence and prevalence of hepatitis C among clients of a Brisbane methadone clinic: factors influencing hepatitis C serostatus. Aust NZ J Public Health. 1997;21:102–4. doi: 10.1111/j.1467-842x.1997.tb01664.x. [DOI] [PubMed] [Google Scholar]
- 24.Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. J Urban Health. 2000;77:331–45. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow N, Day N. Statistical Methods in Cancer Research. Vol. 2. The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer: IARC Scientific Publications; 1987. [PubMed] [Google Scholar]
- 26.Lin C, Wu Z, Rou K, Yin W, Wang C, Shoptaw S, et al. Structural-level factors affecting implementation of the methadone maintenance therapy program in China. J Subst Abuse Treat. 2010;38:119–27. doi: 10.1016/j.jsat.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffa JD, Grebely J, Tossonian H, Wong T, Viljoen M, Khara M, et al. The impact of ongoing illicit drug use on methadone adherence in illicit drug users receiving treatment for HIV in a directly observed therapy program. Drug Alcohol Depend. 2007;89:306–9. doi: 10.1016/j.drugalcdep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in HCV prevalence? Model projections for different epidemic settings. Addiction. 2012;107:1984–95. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–562. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-anlysis of interventions to prevent hepatitis C virus infections in people who inject drugs. J Infect Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]