Abstract
High-dose calcium channel blocker (CCB) shows strong blood pressure (BP) lowering effect. Currently available of controlled-release (CR) nifedipine 80 mg per day clinical data are limited to monotherapy and short-term or long-term retrospective studies. We report the safety and efficacy results of a 52-week, prospective open-label study, in which Japanese patients with essential hypertension were treated with CR nifedipine [80 mg per day; 40 mg bis in die (BID; twice daily)] in combination with other antihypertensive drugs. The patients with inadequate BP control despite treatment with CR nifedipine (40 mg once daily) in combination with other antihypertensive drugs were enrolled. The primary objective of this study was to assess the long-term safety of CR nifedipine (80 mg per day). Efficacy variables included changes in the mean sitting BP, the target BP achievement rate and the BP response rate. CR nifedipine (80 mg per day) was generally well tolerated, with the most common drug-related treatment-emergent adverse event being tachycardia (6.9% of patients). Serious treatment-emergent adverse events were reported in three (4.2%) patients. By week 52, the mean reductions in sitting systolic and diastolic BP were 19.4 and 13.6 mm Hg, respectively. The target BP achievement and BP response rates after 52 weeks of treatment were 32.4 and 63.4%, respectively. Based on these findings, long-term treatment with CR nifedipine at 40 mg BID in combination with antihypertensive drugs was well tolerated and effective in Japanese patients with essential hypertension.
Keywords: combination therapy, controlled-release nifedipine, essential hypertension
Introduction
Hypertension is an important risk factor in cardiovascular disease and is associated with high mortality and morbidity rates.1 The disease affects ~1 billion people worldwide.2 In Asian countries, the prevalence of hypertension ranges from 20 to 35%.3, 4 This emphasizes the importance of effectively lowering blood pressure (BP) in patients with hypertension.
The primary goal of antihypertensive therapy is to improve BP control and reduce the long-term risk of cardiovascular morbidity and mortality.2, 5, 6 In Japan, the 2009 Japanese Society of Hypertension (JSH) guidelines recommend that systolic BP (SBP) and diastolic BP (DBP) are lowered to <130/85 mm Hg in nonelderly patients (age <65 years), to <140/90 mm Hg in elderly patients (⩾65 years) and to <130/80 mm Hg in patients with diabetes mellitus (DM), chronic kidney disease (CKD) or prior myocardial infarction (pMI).6 This may be achieved by using several antihypertensive drug classes, including calcium channel blockers (CCBs), angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, diuretics and β-blockers.6 Despite the availability of effective antihypertensive therapies and the importance of achieving a good BP control, many Japanese patients with hypertension do not achieve the JSH targets. In J-HOME study,7 ~60% of patients did not achieve the targets for office-measured (<140/90 mm Hg) and home-measured (<135/85 mm Hg) BP. Similar results were also reported in an observational study of target BP achievement rates in patients from Fukushima Prefecture, Japan.8
Nifedipine is a dihydropyridine CCB with a predominant vasodilatory activity that was originally marketed as an immediate-release capsule formulation. Modified-release formulations that prolong the release of nifedipine have since been developed for clinical use.9 In addition, a controlled-release (CR) tablet formulation of nifedipine (Adalat; BAY a 1040 CR tablet; Bayer Yakuhin, Osaka, Japan) was developed in Japan. This formulation of nifedipine retains the antihypertensive effects, reduces side effects and improves compliance with once daily (quaque die; QD) administration.10, 11 At the time we designed this study, the maximum approved dose of CR nifedipine for hypertensive patients was 40 mg per day in Japan, which has since been increased to 80 mg per day for patients with hypertension or 60 mg per day in patients with angina. The efficacy of the increased dose of CR nifedipine was demonstrated in a recent study, in which BP control was improved by CR nifedipine monotherapy at 80 mg per day [40 mg bis in die (BID; twice daily)] in patients with uncontrolled essential hypertension.12 More recently, a phase III trial demonstrated superior antihypertensive efficacy of nifedipine 80 mg per day (40 mg BID) vs nifedipine 40 mg per day in Japanese patients with essential hypertension as monotherapy.13 Furthermore, administration of 20–40 mg of CR nifedipine in combination with other antihypertensive drugs is effective for the treatment of essential hypertension.14, 15 Indeed, treatment guidelines suggest that combination therapy with ⩾2 antihypertensive drugs is often required to achieve BP control, with CCBs being recommended in most of the preferred combinations.5, 6
In the past, Furberg et al.16 suggested that high dosage administration of nifedipine increased mortality in patients with coronary heart disease. The large clinical trials with long-acting CCB17 or CR CCB18 in standard dosage did not show the increase of cardiovascular event rate or mortality. Thus the long-term safety and efficacy of high-dose CR nifedipine (80 mg per day) in prospective design and in combination with other hypertensive drugs are particularly important.
Therefore, we conducted a long-term (52-week), open-label study of Japanese patients with poorly controlled essential hypertension despite treatment with nifedipine (40 mg per day) in combination with other antihypertensive drugs. Our aim was to investigate the safety and efficacy of increasing the dose of CR nifedipine to 80 mg per day (40 mg BID).
Methods
Study design
This study was a phase III, 52-week, open-label study conducted across four centers in Japan. At visit 1 (week −2), eligible patients started treatment with CR nifedipine (40 mg QD) in the morning together with their prior antihypertensive drug for 2 weeks. At the end of this period (that is, week 0/visit 2), the patients started taking CR nifedipine (40 mg BID) in the morning and evening every day for 52 weeks. The use of other antihypertensive drugs and their dosages were to be kept as stable as possible, although changes were permitted if needed. In the event of excessive hypotension or other adverse events (AEs), the doses of the other antihypertensive drugs could be reduced, or the drugs discontinued. If hypotension or AEs persisted despite discontinuation of the other antihypertensive drugs, the nifedipine dose was reduced to 40 mg QD. Visits were scheduled at weeks −2, 0, 2, 4, 6 and 8, and then every 4 weeks thereafter.
This study was registered at www.ClinicalTrials.gov (identification number: NCT01294215). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice. The protocol for the study was reviewed and approved by each study site's Independent Ethics Committee or Institutional Review Board before starting the study. All patients provided a written informed consent before the enrolment.
Patients
Patients were eligible if they satisfied the following criteria: age ⩾20 years; essential hypertension; treatment with nifedipine (40 mg QD) and one or more antihypertensive drugs other than a CCB for ⩾4 weeks before week −2; mean sitting DBP (msDBP) at weeks −2 and 0 of ⩾90 mm Hg in elderly patients, ⩾85 mm Hg in nonelderly patients, or ⩾80 mm Hg in patients with DM, (CKD) or pMI; and an absolute difference in msDBP of <10 mm Hg between weeks −2 and 0. Patients with severe hypertension (msDBP ⩾110 mm Hg or mean sitting SBP (msSBP) ⩾180 mm Hg), secondary hypertension, hypertensive emergency or a history of cardiovascular or cerebrovascular ischemic events within 6 months, intracranial or subarachnoid hemorrhage within 6 months, hematopoietic dysfunction, malignant tumor, cardiogenic shock, congestive heart failure, aortic stenosis, mitral stenosis, pulmonary hypertension, alcoholism or drug abuse, having dialysis, liver dysfunction, renal dysfunction or human immunodeficiency virus infection were excluded from the study. Pregnant or nursing women were also excluded.
Safety assessments
Safety assessments consisted of the monitoring and recording of all AEs, electrocardiography measures and laboratory tests. AEs that occurred after the patient gave the informed consent until the end of the treatment period were recorded in clinical report forms, and their severity and relationship to the study drug were determined by the investigator. AEs included abnormal laboratory or physical findings, symptoms or diseases that occurred after providing the informed consent. Deteriorations in pre-existing medical conditions were also considered AEs. Pre-existing conditions that did not deteriorate during the treatment were recorded in the patient's medical history. Surgery conducted during treatment was not considered an AE if the procedure was planned before the patient enrolled in the study. Treatment-emergent AEs (TEAEs) were defined as AEs that occurred after starting the treatment with the study drug and within 30 days after the last dose of the study drug. AEs and drug-related AEs were classified using the Medical Dictionary for Regulatory Activities (MedDRA) Version 14.1.
Other safety assessments included a 12-lead electrocardiography, which was conducted at weeks 0, 24 and 52. Laboratory tests (hematology, clinical chemistry and urinalysis) were conducted at weeks −2, 4, 12, 24, 36 and 52.
Efficacy assessments
Efficacy variables included the changes from baseline in trough sitting DBP/SBP (that is, DBP/SBP before morning drug administration), the proportions of patients achieving the BP targets recommended in the JSH 2009 guidelines,6 responder rates (reduction in DBP of >10 mm Hg from baseline), or achievement of the DBP and SBP targets recommended in the JSH 2009 guidelines,6 and the changes from baseline in trough pulse rate (that is, pulse rate before morning drug administration). On the basis of the target BP level recommended by JSH 2014 guidelines,19 achievement rate for elderly patients aged 75 or over (<150/90 mm Hg) and nonelderly patients without DM or CKD (<140/90 mm Hg) were also evaluated.
Statistical analysis
Continuous variables are expressed as the mean±s.d. at each visit and for changes over time. Frequency tables were generated for categorical or qualitative variables and data are presented as the n (%). The changes in DBP, SBP and pulse rate from week 0 to the last available visit (LAV) were evaluated by using paired t-tests, and P<0.05 was considered statistically significant. The BP achievement/response rates were calculated for all patients, and in subgroups of patients divided by age (<65 vs ⩾65 years) and the presence of concomitant disease (DM, CKD or pMI). The analyses of changes in DBP, SBP and pulse rate were conducted post hoc. All statistical analyses were performed by using SAS Release 9.1 (SAS Institute, Cary, NC, USA).
Results
Patient demographics and baseline characteristics
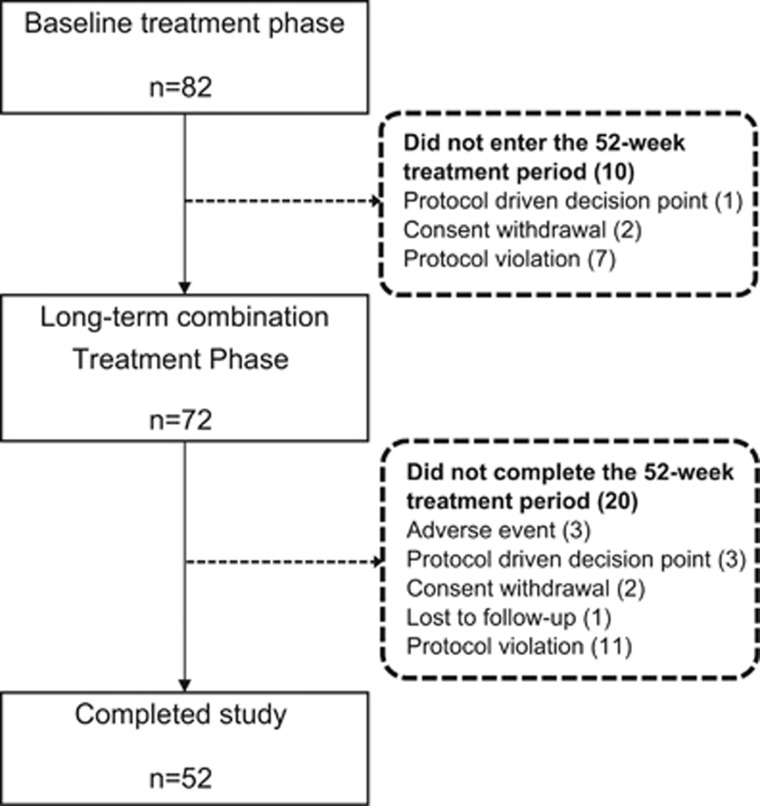
A total of 82 patients were enrolled and 10 patients did not complete the baseline treatment phase (Figure 1). Of 72 patients who completed the baseline treatment period and entered the long-term combination treatment period (52 weeks), 52 (72.2%) completed the study. The main reasons for discontinuation were protocol violation (n=11), discontinuations because of AEs (n=3), protocol-driven decisions (that is, patients who discontinued study drug administration because of a failure to achieve the target BP set out in the study protocol; n=3), withdrawal of consent (n=2) and loss to follow-up (n=1) (Figure 1). The baseline characteristics of the patients are presented in Table 1. The mean age was 58.7 years, the mean duration of hypertension was 11.4 years and the baseline msSBP/msDBP was 150.5/93.5 mm Hg. Overall, 73.6% of patients were nonelderly (aged <65 years), 63.9% of patients were past or current smokers, 34.7% had DM and 2.8% had pMI.
Figure 1.
Patient disposition.
Table 1. Baseline characteristics of patients.
| Variable | Value |
|---|---|
| N | 72 |
| Age, years | 58.7±9.5 |
| <65 years, n (%) | 53 (73.6) |
| ⩾65 years, n (%) | 19 (26.4) |
| Sex, n (%) | |
| Male | 53 (73.6) |
| Female | 19 (26.4) |
| BMI (kg m−2) | 27.8±5.6 |
| msDBP (mm Hg) | 93.5±6.9 |
| msSBP (mm Hg) | 150.5±16.9 |
| Duration of hypertension, years | 11.4±11.1 |
| Smoking history, n (%) | |
| Non-smoker | 24 (33.3) |
| Past or current smoker | 46 (63.9) |
| BP classification | |
| Grade 1 | 38 |
| Grade 2 | 25 |
| Diabetes mellitus, n (%) | 25 (34.7) |
| Chronic kidney disease, n (%) | 0 (0.0) |
| Prior myocardial infarction, n (%) | 2 (2.8) |
Abbreviations: BMI, body mass index; msDBP, mean sitting diastolic blood pressure; msSBP, mean sitting systolic blood pressure.
BP classification: SBP 140–159 mm Hg and/or DBP 90–99 mm Hg as Grade 1 and SBP 160–179 mm Hg and/or DBP 100–109 mm Hg as Grade 2.
Data are presented as the mean±s.d. or n (%).
Concomitant antihypertensive drugs that were being used at the baseline included ARBs (73.6%), diuretics (22.2%), β-blockers (20.8%), angiotensin-converting enzyme inhibitors (8.3%), renin inhibitors (6.9%) and α-blockers (2.8%) (Table 2). The proportion of patients who received ⩾2 concomitant antihypertensive drugs was 23.6%.
Table 2. Concomitant antihypertensive agents used at the baseline.
| Antihypertensive agent | n (%) |
|---|---|
| ARB | 53 (73.6) |
| ACEI | 6 (8.3) |
| Diuretics | 16 (22.2) |
| β-blockersa | 15 (20.8) |
| α-blockers | 2 (2.8) |
| Renin inhibitors | 5 (6.9) |
| Antiadrenergic agents | 0 (0.0) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers.
Includes dual α/β-blockers.
Safety
Drug-related TEAEs occurred in 29.2% of patients (Table 3). Tachycardia (6.9%) and gingival hypertrophy (4.2%) were the most common drug-related TEAEs. One case of orthostatic hypotension was also reported as a drug-related TEAE. Serious AEs were reported in three (4.2%) patients (acute myocardial infarction, cerebral hemorrhage and gallbladder stone, all of them were also serious TEAEs). One acute myocardial infarction case was drug-related serious AE. The majority of drug-related TEAEs were mild or moderate in intensity, with the exception of one event that was considered severe (acute myocardial infarction). Overall, three patients (4.2%) discontinued the study drug because of AEs, of whom two did so because of drug-related AEs (acute myocardial infarction and gingival hypertrophy). There were no deaths.
Table 3. Adverse events.
| Description | n (%) |
|---|---|
| n | 72 |
| Any AE, n (%) | 64 (88.9) |
| Any TEAEs, n (%) | 56 (77.8) |
| Any SAEs, n (%) | 3 (4.2) |
| Any drug-related AEs, n (%) | 21 (29.2) |
| Drug-related SAEs, n (%) | 1 (1.4) |
| Discontinuation of study drug because of AEs, n (%) | 3 (4.2) |
| Drug-related TEAEs, n (%) | 21 (29.2) |
| Drug-related TEAEs by MeDRA system organ class, n (%) | |
| Cardiac disorders | 7 (9.7) |
| Tachycardia | 5 (6.9) |
| Acute myocardial infarction | 1 (1.4) |
| Ventricular extrasystoles | 1 (1.4) |
| Gastrointestinal disorders | 6 (8.3) |
| Gingival hypertrophy | 3 (4.2) |
| Constipation | 2 (2.8) |
| Diarrhea | 1 (1.4) |
| General disorders and administration site conditions | 3 (4.2) |
| Feeling abnormal | 1 (1.4) |
| Edema | 1 (1.4) |
| Peripheral edema | 1 (1.4) |
| Investigations | 2 (2.8) |
| Elevated gamma-glutamyltransferase | 1 (1.4) |
| Glucose present in urine | 1 (1.4) |
| Metabolism and nutrition disorders | 2 (2.8) |
| Diabetes mellitus | 2 (2.8) |
| Nervous system disorders | 1 (1.4) |
| Dizziness | 1 (1.4) |
| Vascular disorders | 2 (2.8) |
| Orthostatic hypotension | 1 (1.4) |
| Flushing | 1 (1.4) |
Abbreviations: AE, adverse event; MeDRA, Medical Dictionary for Regulatory Activities; SAEs, serious adverse events; TEAEs, treatment-emergent adverse events.
There were no clinically relevant differences in the incidences of TEAEs or drug-related TEAEs among subgroups of patients divided by sex, age (< and ⩾65 years of age, among patients without DM, chronic renal disorder or pMI), body weight and medical status (patients with DM, chronic renal disorder or pMI).
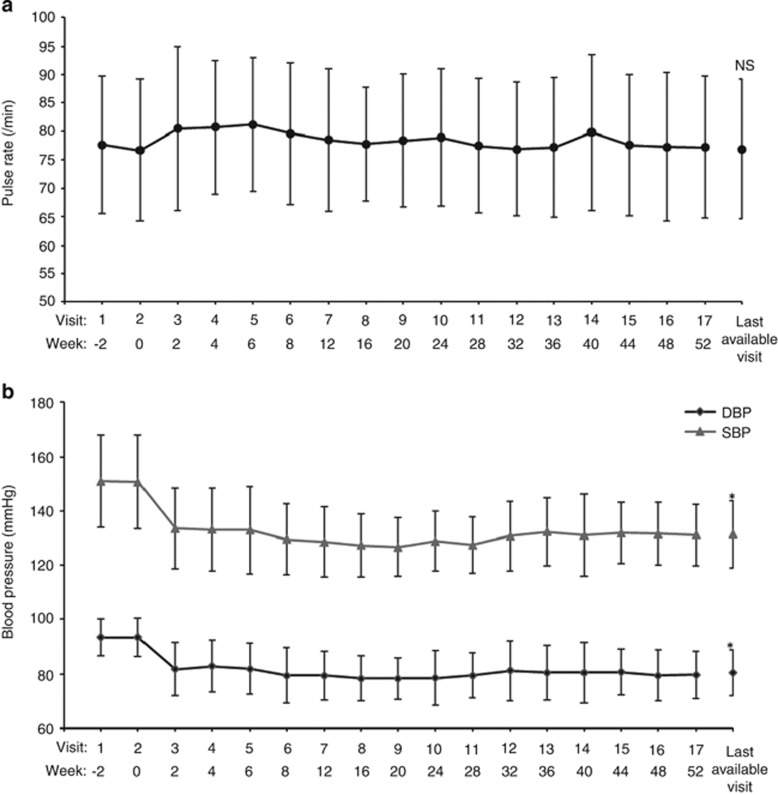
The pulse rate increased by 3.8 beats per min from baseline to week 6, but then returned to and stayed at the baseline level for the remainder of the long-term treatment period (Figure 2a). The mean pulse rate was 76.7±12.5 beats per min at week 0 and 76.9±12.3 beats per min at the LAV (P=0.8923, paired t-test).
Figure 2.
Changes in pulse rate (a) and mean sitting blood pressures (b) over time. *P<0.0001 at the last available visit vs week 0 (one-sample t-test; post hoc analysis).
Efficacy
One patient was excluded from this efficacy analysis owing to inadequate efficacy data. The msDBP and msSBP decreased to 81.8±9.8 mm Hg and 133.5±14.8 mm Hg, respectively, by week 2 of treatment with CR nifedipine at 80 mg per day, and these levels were maintained throughout the 52-week treatment period (Figure 2b). The reductions in msDBP (−11.6±8.3 mm Hg from baseline) and msSBP (−16.8±14.2 mm Hg from baseline) were observed from week 2. msDBP decreased significantly from 93.5±7.0 mm Hg at the baseline to 80.4±8.4 mm Hg at the LAV (P<0.0001, paired t-test). msSBP also decreased significantly from 150.6±17.1 mm Hg at baseline to 131.4±12.4 mm Hg at the LAV (P<0.0001, paired t-test). Among patients with data at week 52, the mean reductions in msDBP and msSBP from baseline were −13.6±8.1 and −19.4±13.1 mm Hg, respectively.
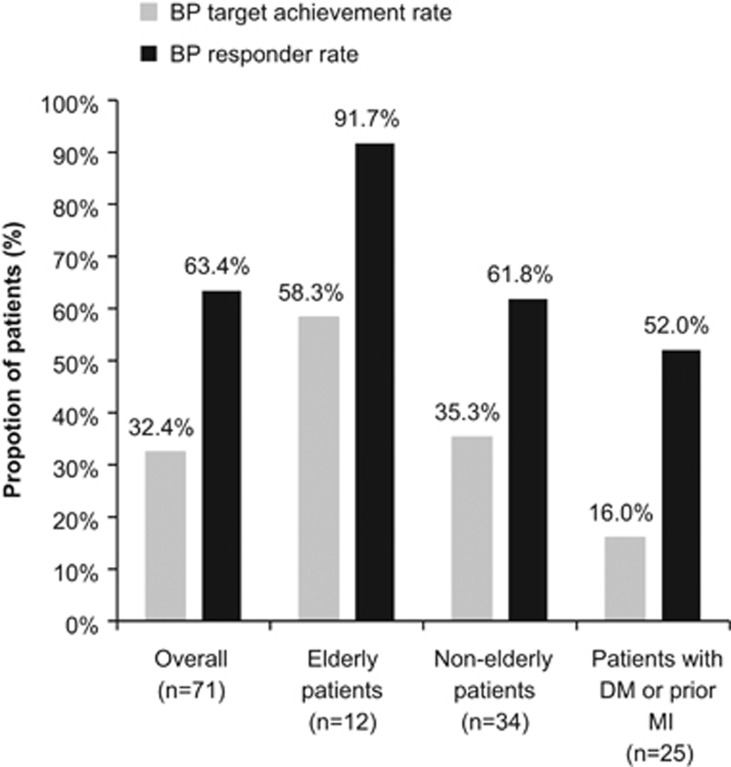
Among patients with data at week 52, the target BP achievement rate at the end of the long-term treatment period was 32.4% (23/71 patients) (Figure 3). Among patients without concomitant diseases (that is, DM or pMI), the target BP achievement rates were 58.3% (7/12) in elderly patients and 35.3% (12/34) in nonelderly patients. Overall, 16.0% (4/25) of patients with any concomitant disease (DM or pMI) achieved the target BP (Figure 3). According to the JSH 2014 guidelines, 100% (4/4) of elderly aged 75 or over and 77.3% (34/44) of subjects under the age of 75 without DM were achieved the target BP.
Figure 3.
Target BP achievement and BP responder rates after 52 weeks of treatment with CR nifedipine (80 mg per day) in all patients, elderly patients (aged ⩾65 years; without concomitant diseases), nonelderly patients (<65 years; without concomitant diseases) and in patients with concomitant diseases (diabetes mellitus or prior myocardial infarction).
The overall BP response rate at the end of the long-term treatment period was 63.4% (45/71 patients) (Figure 3). Furthermore, BP responder rates in elderly patients, nonelderly patients and patients with concomitant diseases (DM or pMI) were 91.7% (11/12 patients), 61.8% (21/34) and 52.0% (13/25), respectively (Figure 3).
Discussion
In Japan, CCBs such as nifedipine are among the most frequently prescribed antihypertensive agents because of their established BP-lowering efficacy and their ability to maintain BP control.
The results of a long-term (52-week) safety study of CR nifedipine at a dose of 40 mg per day have been reported.20 Clinical trials have also demonstrated that CR nifedipine is well tolerated when administered in combination with candesartan or valsartan,14, 15 for 16 weeks long at the dose of 20–40 mg per day. More recently, a small-scale retrospective study of patients with a poorly controlled essential hypertension despite antihypertensive therapy showed that administration of CR nifedipine at 80 mg per day monotherapy was well tolerated over 24 months of follow-up.12 A phase III trial of CR nifedipine 80 mg per day monotherapy13 also demonstrated the short-term efficacy and safety.
In the past, a meta-analysis, published by Furberg et al.,16 suggested that high dosage administration of short-acting nifedipine increased mortality in patients with coronary heart disease. Two large clinical trials with standard dose of long-acting CCB17 or CR CCB18 did not show such increased mortality, indicating the importance of pharmacokinetics of CCB.
Thus, all together, it is of value to show the long-term safety and effectiveness of 80 mg per day CR nifedipine in combination with other hypertensive drugs in prospective cohort.
In the present study, we found that this dose of CR nifedipine was well tolerated and lowered BP quickly and in a sustained manner for 52 weeks. The majority of drug-related TEAEs were mild or moderate in severity. Concomitant administration of other antihypertensive agents did not appear to affect the long-term tolerability of CR nifedipine administered at 80 mg per day.
Long-term administration of CR nifedipine (80 mg per day) in combination with other antihypertensive agents effectively reduced BP, with effects that were sustained for 52 weeks. These results confirm those of a recent retrospective study that reported similar reductions in BP over 6 months of treatment (−15.9/−6.7 mm Hg) with CR nifedipine at 80 mg per day in patients with uncontrolled essential hypertension.12
In a nationwide study, 30–50% of patients receiving antihypertensive therapy achieved the target BP level; however, the hypertensive patients with DM showed a significantly lower achievement rate (target BP 140/90 mm Hg; non DM 36.9%, DM 32.5%, P<0.001 and Target BP 130/80 mm Hg; non DM 13.1%, DM 11.3%, P<0.05)21 In other reports, the achievement rate was 32.2% in nonelderly patients, and just 25.0% in high-risk patients, even though they required a strict BP control and had low BP targets (for example, <130/80 mm Hg for patients with DM or CKD).22, 23, 24 However, the low proportion of responders among nonelderly patients is perhaps unsurprising because in JSH 2009 guidelines, the target BP is lower in these patients (<130/85 mm Hg) than in older patients with hypertension (<140/90 mm Hg).7 In fact, the achievement rate was 77.3% based on the target BP level of JSH 2014 guidelines (<140/90 mm Hg). In our study, the BP responder rates were over 50% in each of the subgroups of patients, although the achievement rate was somewhat lower in the high-risk group of patients with concomitant diseases (DM or pMI) than in lower-risk groups of elderly or nonelderly patients without these diseases.
And the treatment guidelines for hypertension suggest that the majority of patients with hypertension will require treatment with two or more antihypertensive drugs for an adequate BP control.5, 6
Therefore, a comprehensive BP control, together with lifestyle interventions and the management of concomitant diseases or other risk factors is essential in high-risk patients, and it is important to select the most appropriate treatment, including drug selection, for the individual patient.
Because the primary objective of this study was assessing the long-term safety of CR nifedipine at 80 mg per day in combination with other antihypertensive drugs, the sample size was not estimated for detecting the efficacy of CR nifedipine in combination with antihypertensive drugs. Nevertheless, we observed nominally significant changes in DBP and SBP from week 0 to the LAV.
The relatively small sample size of this study did not allow us to reach clear conclusions on the safety and efficacy of nifedipine in the subgroups of patients (that is, elderly patients and patients with concomitant diseases), or to comprehensively assess the significance of changes in efficacy variables. The primary objective of this study was the long-term safety of CR nifedipine (80 mg per day) in essential hypertension treated with CR nifedipine (40 mg per day) in combination with other antihypertensive drugs. Thus this study had no control group such as patients continuing 40 mg per day CR nifedipine or another antihypertensive agent. This would be another limitation of this study. Accordingly, it is not possible to confirm that the improvements in BP were solely because of the administration of the study drug, or whether other study-related factors contributed to these improvements. Considering these limitations, there is a need for larger, well-controlled clinical trials to confirm the findings of this report, especially in ‘real-world' clinical settings. Studies are also needed to assess the effects of CR nifedipine on home-measured BP and ambulatory BP monitoring, as well as provide direct comparisons with other high-dose antihypertensive agents.
In conclusion, long-term administration of CR nifedipine at a dose of 80 mg per day was well tolerated when used in combination with other antihypertensive agents in these Japanese patients with essential hypertension. We observed marked reductions in BP within 2 weeks of treatment. Notably, these improvements were sustained for 52 weeks. Taken together, these results indicate that a high daily dose of CR nifedipine (80 mg per day) is a useful option for treating essential hypertension.
Acknowledgments
This study was funded by Bayer Yakuhin (Osaka, Japan). We are indebted to Prof. Kenjiro Kimura, Prof. Hiromi Rakugi and Prof Yusuke Ohya for the scientific advice. We thank Prof. Naoyuki Hasebe, Prof. Sadayoshi Ito, Prof. Kazuomi Kario, Prof. Yasuaki Dohi, Dr Yuhei Kawano, Prof. Masatsugu Horiuchi and Dr Tsutomu Imaizumi for helpful comments. We thank Rod McNab and Maxwell Chang of inScience Communications, Springer Healthcare and Nicholas D. Smith, PhD, for providing the medical writing assistance, which was funded by Bayer Yakuhin (Osaka, Japan). List of study participants: T Sugiura (Sugiura iin), T Iwai (Sagamino central hospital), H Oda (Oda clinic) and H Yamamoto (Yamamoto clinic).
Footnotes
KS reports no conflicts of interest with Bayer Yakuhin. MK, YM, KA and MK report employment by Bayer Yakuhin.
References
- 1Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360. [DOI] [PubMed] [Google Scholar]
- 2Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 3Hypertension in the Asia Pacific region - the problem and the solution (2007). http://www.apsh.org./webCast.html Accessed 12 November, 2012.
- 4Chiang CE, Chen CH. Hypertension in the Asia-Pacific region. J Hum Hypertens 2008; 22: 441–443. [DOI] [PubMed] [Google Scholar]
- 5Mancia G, De Backer G, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187. [DOI] [PubMed] [Google Scholar]
- 6Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H; Japanese Society of Hypertension Committee. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 2009; 32: 3–107. [PubMed] [Google Scholar]
- 7Ohkubo T, Obara T, Funahashi J, Kikuya M, Asayama K, Metoki H, Oikawa T, Takahashi H, Hashimoto J, Totsune K, Imai Y; J-HOME Study Group. Control of blood pressure as measured at home and office, and comparison with physicians' assessment of control among treated hypertensive patients in Japan: First Report of the Japan Home versus Office Blood Pressure Measurement Evaluation (J-HOME) study. Hypertens Res 2004; 27: 755–763. [DOI] [PubMed] [Google Scholar]
- 8Yokokawa H, Goto A, Sanada H, Watanabe T, Yasumura S. Gaps between hypertension treatment guidelines and clinical practice in Japan: baseline survey results from Fukushima Research of Hypertension (FRESH). J Clin Hypertens (Greenwich) 2009; 11: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Bayer. The history of Bayer: Adalat® http://www.bayer.co.jp Accessed 12 November 2012.
- 10Nakamichi N, Yangida T, Hikima Y. A phase I clinical trial of nifedipine controlled-release formulation (BAY a 1040-OD tablet): a single-dose study. Jpn Pharmacol Ther 1995; 23: s241–s255. [Google Scholar]
- 11Nakamichi N, Yangida T, Hikima Y. A phase I clinical trial of nifedipine controlled-release formulation (BAY a 1040-OD tablet): a repeated-dose study. Jpn Pharmacol Ther 1995; 23: s257–s269. [Google Scholar]
- 12Kobayashi N, Ishimitsu T. Assessment on antihypertensive effect and safety of nifedipine controlled-release tablet administered at 80 mg/day in practical clinic. Clin Exp Hypertens 2012; 34: 191–200. [DOI] [PubMed] [Google Scholar]
- 13Shimamoto K, Hasebe N, Ito S, Kario K, Kimura K, Dohi Y, Kawano Y, Rakugi H, Horiuchi M, Imaizumi T, Ohya Y. Nifedipine controlled-release 40 mg b.i.d. in Japanese patients with essential hypertension who responded insufficiently to nifedipine controlled-release 40 mg q.d.: a phase III, randomized, double-blind and parallel-group study. Hypertens Res 2014; 37: 69–75. [DOI] [PubMed] [Google Scholar]
- 14Hasebe N, Kikuchi K. Controlled-release nifedipine and candesartan low-dose combination therapy in patients with essential hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J Hypertens 2005; 23: 445–453. [DOI] [PubMed] [Google Scholar]
- 15Saito I, Saruta T. Controlled release nifedipine and valsartan combination therapy in patients with essential hypertension: the adalat CR and valsartan cost-effectiveness combination (ADVANCE-combi) study. Hypertens Res 2006; 29: 789–796. [DOI] [PubMed] [Google Scholar]
- 16Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation 1995; 92: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 17ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The antihypertensive and lipid-lowering treatment to prevent heart attack trial, major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002; 288: 2981–2997. [DOI] [PubMed] [Google Scholar]
- 18Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener G, Danchin N, Just H, Fox KA, Pocock SJ, Clayton TC, Motro M, Parker JD, Bourassa MG, Dart AM, Hildebrandt P, Hjalmarson A, Kragten JA, Molhoek GP, Otterstad JE, Seabra-Gomes R, Soler-Soler J, Weber S. Coronary disease Trial Investigating Outcome with Nifedipine gastrointestinal therapeutic system investigators. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849–857. [DOI] [PubMed] [Google Scholar]
- 19Shimamoto K, Ando K, Fujita T, Hasebe N, HIGAKI J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Ito H, Iwao H, Kai H, Kario K, Kashihara N, Kawasno Y, Mitsuyama-Kim S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saito S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; on behalf of The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–392.24705419 [Google Scholar]
- 20Ishii M, Matsuoka M, Iimura O, Yoshinaga K, Yagi S. Long-term administration study of BAY a 1040-OD tablets (nifedipine sustained-release formulation) in patients with essential hypertension: investigation on monotherapy and combined therapy. Jpn Pharmacol Ther 1995; 23: S335–S358. [Google Scholar]
- 21Mori H, Ukai H, Yamamoto H, Saitou S, Hirao K, Yamauchi M, Umemura S. Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res 2006; 29: 143–151. [DOI] [PubMed] [Google Scholar]
- 22Fujita T, Teramoto T. Japan Guideline Assessment Panel. Prog Med 2006; 22: 2297–2306. [Google Scholar]
- 23Teramoto T, Fujita T. Japan Guideline Assessment Panel 2. Prog Med 2010; 30: 1437–1449. [Google Scholar]
- 24Miura K, Nagai M, Ohkubo T. Epidemiology of hypertension in Japan: where are we now? Circ J 2013; 77: 2226–2231. [DOI] [PubMed] [Google Scholar]