Abstract
Enrichment technique was employed for the isolation of the crude oil degrading bacteria. The isolated bacteria were screened for their degradative ability and the best degrading bacteria were selected based on their growth. Specific activities of Catechol-2,3-dioxygenase and effects of temperature and pH and their stabilities on the enzyme relative activities were observed. Bacteria isolated from the soil sample include; Bacillus cereus, B. amyloliquficiens, B. firmus, Acinetobacter calcoaceticus, Pseudomonas sp. P. fluorescens, P.putida, P.aeruginosa, Achromobacter xylosoxidans and Achromobacter sp. Screening of the degradative ability of the bacteria revealed P. aeruginosa, Bacillus cereus, Acinetobacter calcoaceticus and Achromobacter sp. to be the best degraders. The pH and temperature range with time for the enzyme activity were 6.0-8.0 and 30oC-50oC respectively. The enzyme exhibited activity that was slightly more tolerant to alkaline pH. Therefore, engineering of Catechol 2,3-dioxygenase may be employed for application on bioremediation of polluted sites.
Keywords: Bacteria; catechol 2,3-dioxygenase; crude oil; pH; soil and temperature.
INTRODUCTION
In Nigeria, the growth and activities of petroleum and petroleum associated industries has led to increased oil pollution in our environment, due to problems arising from petroleum exploration, exploitation, transportation and consumption coupled with poor maintenance of oil pipelines resulting in seepages and ruptures [1]. The rising in the incidence of damage and vandalization of pipelines by restive oil communities mostly in the Niger Delta area of the country has resulted into huge problem in these recent years. Ilaje community, located in the Southern part of Ondo State, closer to the Atlantic Ocean, is one of the oil producing communities in the Niger Delta region of Nigeria. Oil explorations in these communities at times result to contamination of lands and water bodies due to spillage [2]. Contamination of soils and aquifers by oil spills has been a continual general pollution problem that resulted into desolation of almost all compartments of the environment and thereby inflicting serious health repercussion and ecological instability [3]. Toxic effects of oil and their products on the soil environment include enhancing hydrophobicity of soils, disturbance of water availability to vegetation, and direct toxicity to plants and microorganisms. The technology commonly used for soil remediation includes mechanical, burying, evaporation, dispersion, and washing. However, these technologies are expensive and can lead to partial decomposition of contaminants. Bioremediation on the other hand, is an efficient and cost effective approach to reduce or completely remove the hazardous and toxic organic chemicals from the contaminated environment by microbes which are known for their capabilities of catabolic processes. Microbial remediation of a hydrocarbon–contaminated site is achieved by the help of a diverse group of microorganisms applying enzymes in their metabolism, especially the indigenous soil bacteria. Aerobic biodegradation of aromatic compounds involves their conversion into dihydroxylated intermediates (e.g., catechol or its alkyl- or chloro-substituted derivatives) which are then further metabolized by intradiol (catechol-1,2-dioxygenase) or extradiol (catechol-2,3-dioxygenase) dioxygenases [4]. Intradiol enzymes are known to incorporate two oxygen atoms between vicinal hydroxyl groups, while the extradiol dioxygenases cleave the aromatic ring of the substrate outside the two hydroxyl groups to produce muconic-semialdehyde [5]. Catechol 2,3-dioxygenases are homotetramers and they have been detected from many Gram-negative bacteria (Pseudomonas, Sphingomonas, Acinetobacter, Burkholderia,) and Gram-positive bacteria (Nocardia, Rhodococcus and Bacillus) strains [6]. The main aim of this study is to assay for the specific activity of Catechol 2,3-dioxygenase from bacterial with the highest degrading activities obtained from crude oil polluted site in Ayetoro, Ondo state. In addition, the study aims to evaluate the environmental conditions for the activity of Catechol 2,3-dioxygenase produced by these bacteria and to determine the optima conditions for pH and temperature of the enzyme activity.
MATERIALS AND METHODOLOGY
Sources and Collection of Samples Soil
Crude oil contaminated and the non-contaminated soils were collected aseptically into a labeled sterile plastic bag at Ayetoro, Ilaje Local Government Area of Ondo State. Bonny-light crude oil was obtained from Nigeria National Petroleum Commission (NNPC) Bayelsa State.
Isolation and Selection of Oil Degrading Bacteria
Soil samples (2g) were suspended in 98 ml of Bushnell Haas broth supplemented with 1% v/v crude oil and were incubated at 30oC for 7days. After which UV spectrophotometer was used to determine the optical density at 600nm at 24 hours interval. After one week, 1 ml of each suspension from the enrichment process was serially diluted and 1ml from10-4 aliquot was seeded into Bushnell Haas agar plates and was incubated at 30oC for 48hrs. Pure colonies of each isolates were obtained by streak plate method on nutrient agar plates and were incubated at 30oC overnight, and were stored on agar slant.
Characterization and Identification of the Isolates
The morphological characteristics (colony colour, size, elevation and shape) of the colonies were observed, Gram staining and biochemical tests (starch hydrolysis, casein hydrolysis, H2S production, catalase test, oxidase test, methyl red, Voges Proskauer (MR-VP), citrate utilization and sugar fermentation) were performed [7-9], and were identified [10].
Characterisation of the Degradation Potential
Bacterial inoculum (1ml) was transferred into 5ml Bushnell-Hass medium and was supplemented with 1ml of crude oil as the carbon source, incubated at 30°C at 170 rpm in shaker incubator for a period of 7days. The growth was monitored through culture densities, by taking the O.D readings daily at 600nm against Bushnell Haas medium as blank.
Medium and Culture Conditions
LB medium (5g/l yeast extract, 10g/l casein peptone, 10g/l Nacl,) pH 7.0 was used to grow the isolates. The bacteria inocula (10%) from LB culture were then transferred to 1000ml of freshly prepared mineral salts medium {2.75g/l of K2HPO4, 2.225g/l of KH2PO4, 1.0g/l of (NH4)2SO4, 0.2g/l of Mgcl2.6H20, 0.1g/l of Nacl, 0.02g/l of Fecl3.6H2O and 0.01g/l of Cacl2} pH 7.0, supplemented with 1% v/v crude oil. The medium were incubated at 30oc on a rotary shaker at 200rpm.
Preparation of Crude Cell Extract and Protein Determination
After centrifugation at 5000xg, 10min at 4oC, bacterial supernatant were suspended in the lysis buffer (50mM Tris phosphate pH 8.0, 1% glycerol for the stabilization of the protein and prevention of aggregation, and 0.1% Triton X-100 for the prevention of aggregation of hydrophobic and membrane proteins). Total protein concentrations in cell extracts were determined with BSA (bovine serum albumin) as a standard [11, 12].
Determination of Catechol-2,3-Dioxygenase Activity
Meta-Cleavage dioxygenase activity was assayed by monitoring the increase in absorbance at the corresponding wavelength of the meta-cleavage product with a DU800 spectrophotometer (Beckman Coulter, Inc., USA) equipped with a thermo-controlled cuvette holder. The pH of the buffer used in this study was adjusted at 30oC. The reaction mixtures contained 2ml of 50mMol Tris- HCL buffer (pH 7.5), 6ml distilled water, 0.2ml cell free extract. The contents were mixed by inversion and 0.2ml catechol was added. The increase in absorbance at 375nm caused by the formation of the reaction product 2-hydroxymuconic semialdehyde was monitored. One unit of the specific activity is defined as the amount of enzyme that converts 1.0µl of meta-cleavage product per minute at 30oC.
pH and Temperature Optima of Catechol 2,3-Dioxygenase
The effect of pH on catechol-2,3-dioxygenase activity was determined by measuring the activity at 30°C over the pH range of 3.0-12 using the following buffers: 50mM sodium acetate (pH 3.0 to 5.0), 50mM phosphate (pH 6.0 and 7.0), 50mM Tris- HCl (pH8.0 and 9.0) and 50mM carbonate (pH 10 and 12).
The optimum temperature was determined by assaying the enzyme activity at various temperatures (30-80°C) in 50 mM Tris/HCl buffer (pH 8.0). The enzyme and substrate solutions were pre-incubated, mixed and the enzymatic reaction was then carried out.
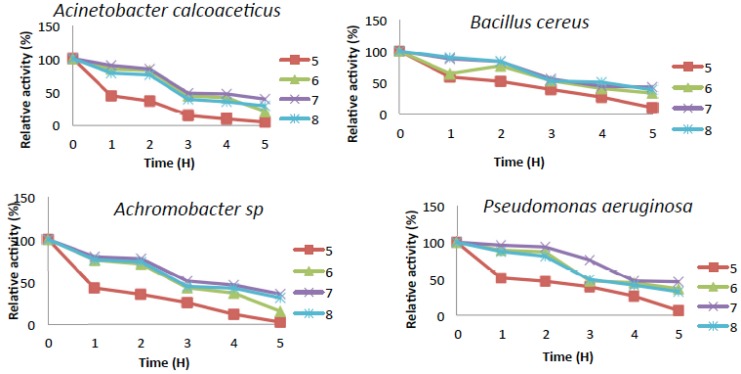
Determination of Thermal and pH Stability of Catechol-2,3–Dioxygenase
The thermal stability of the enzyme was determined by incubating the enzyme and enzymatic reaction mixtures within the temperature range of 40°C to 60°C for 60 min and measuring the activity at 5 minutes interval of incubation at the same temperatures.
pH stability of catechol-2,3-dioxygenase was determined by dissolving and mixing equal volume of enzymes with equal volume of different buffers and allowing the mixture to stand for the period of five minutes which was followed by the measurement of the enzyme’s activity at every one hour interval for the period of five hours.
Statistical Analysis
The statistical analysis was performed using Microsoft office Excel 2007 for calculating mean, standard deviation and standard error.
RESULTS
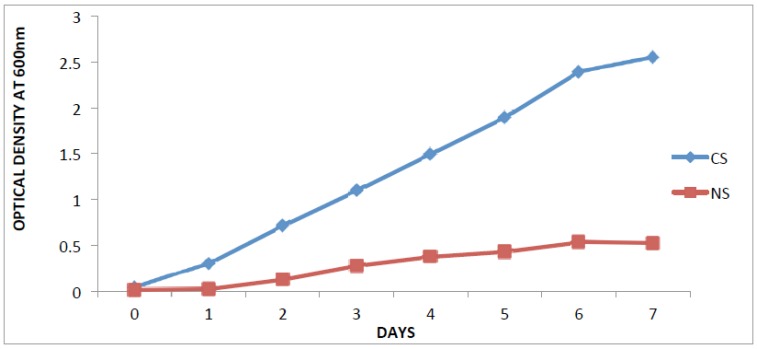
The optical density at (OD600nm) of the crude oil contaminated soil varied between 0.04 and 2.55 while that of the non-contaminated soil was between 0.01 and 0.52.
Characterization and Identification of the Isolates
The results of the morphological and biochemical analyses of bacterial isolates from the soil sample with bonny light crude oil, revealed ten bacterial strains namely; Bacillus cereus, Bacillus firmus, Bacillus amyloliqufaciens, Acinetobacter, Pseudomonas sp. Pseudomonas floresens, Pseudomonas putida, Pseudomonas aeruginosa, Achromobacter sp. and Achromobacter xylosoxidans.
Characterization of the Degradation Potential
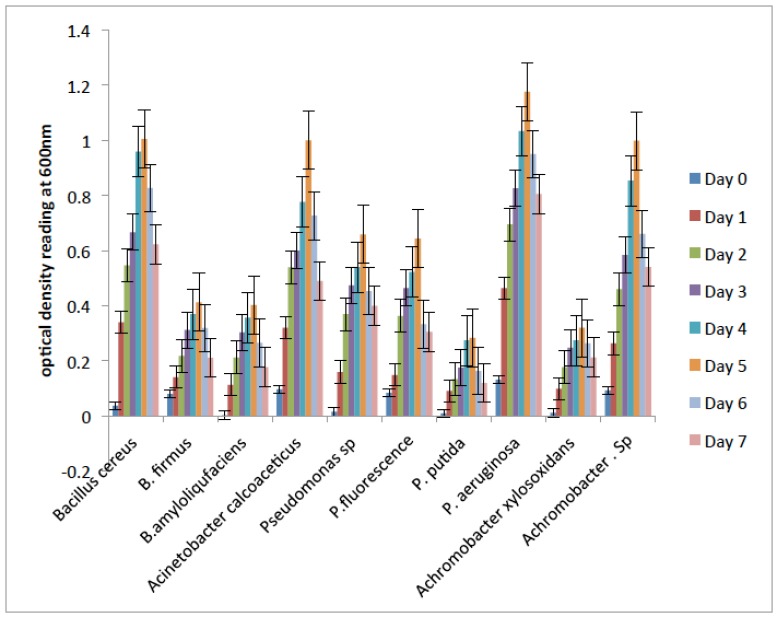
Fig. (2) shows the degradation abilities demonstrated by the bacterial isolates during their growth on crude oil as their sole carbon and energy source. The results revealed that Pseudomonas aeruginosa, Bacillus cereus, Acinetobacter calcoaceticus and Achromobacter sp. were characterized by rapid initial growth phase within 24 hours and their optimum OD values were observed on the 5th day. The result showed that the four bacterial isolates maximally utilized the hydrocarbon. On the other hand, the other bacterial isolates ultilized the hydrocarbon differently with Pseudomonas putida and Achromobacter xylosoxidans having lower growth rate. Hence, the four bacterial isolates with the best degradative abilities on crude oil based on visual observation for turbidity are the most efficient isolates, and were selected for further study.
Fig. (2).
Degradation potentials of the bacterial isolates on crude oil.
Catechol-2,3-Dioxygenase Activity
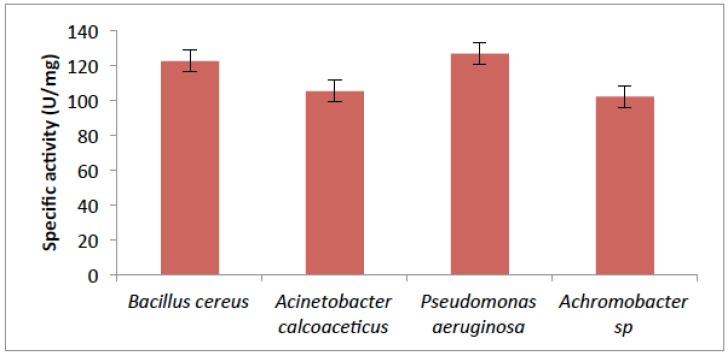
As shown in Fig. (3), the absorbance at 375nm which corresponds to the enzyme activity of the four isolates. B.cereus has the highest activity of 122.57 µ mg–1 protein; Acinetobacter calcoaceticus had activity of 105.32 µ mg–1 protein. While the assay of catechol 2,3-dioxygenase (C23O) from Pseudomonas aeruginosa, showed the utmost activity of (126.835 µ mg–1 protein) with Achromobacter sp. having the lowest activity of 102.10 µ mg–1 protein.
Fig. (3).
Activity of Catechol- 2,3-dioxygenase from some bacteria isolates.
Effect of Temperature and Thermal Stability on the Activity of Catechol 2,3-dioxygenase
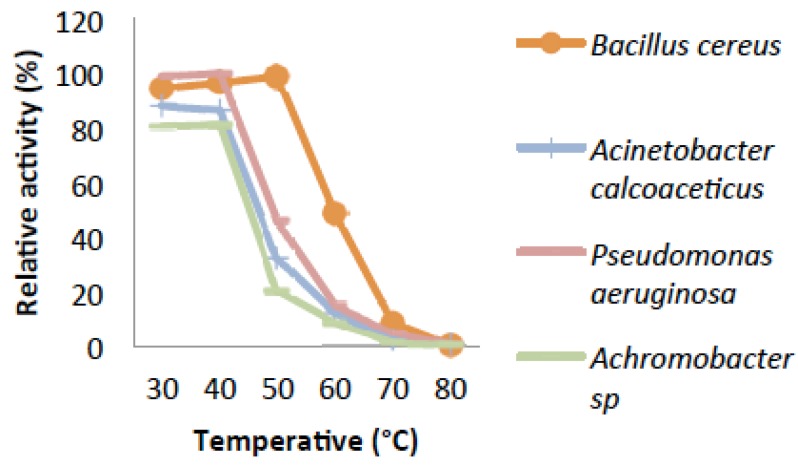
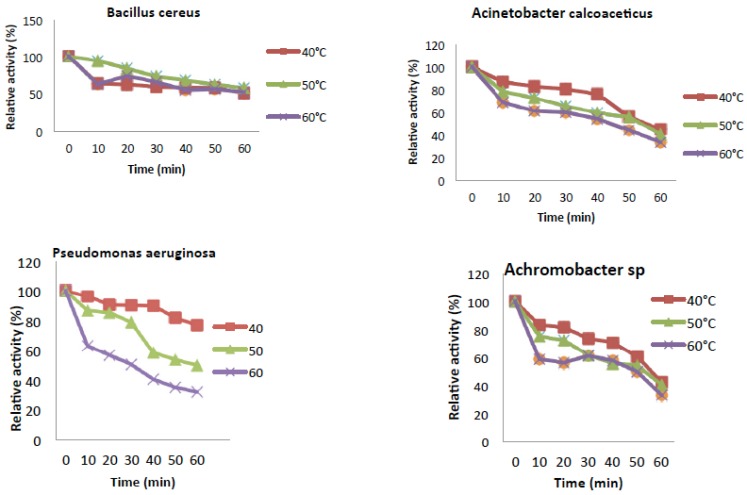
The temperature optimum of the reaction rate of catechol 2,3-dioxygenase (C23O) from Pseudomonas aeruginosa and Achromobacter sp. were estimated to be at 40°C (Fig. 4). Catechol 2,3-dioxygenase isolated from Acinetobacter calcoaceticus as well as Bacillus cereus showed the highest activity at 30°C. Fig. (5) revealed that the enzyme from all the bacteria were thermally stable at 40°C.While, Pseudomonas aeruginosa and Achromobacter sp. were also partially stable at 50°C. However, their relative activities decreases drastically from 50°C to 80°C with time course during which denaturalization of the enzyme had occurred.
Fig. (4).
Effect of Temperature Catechol 2,3-dioxygenase from some bacterial isolates.
Fig. (5).
Thermal stability of Catechol-2,3-dioxygenase activity.
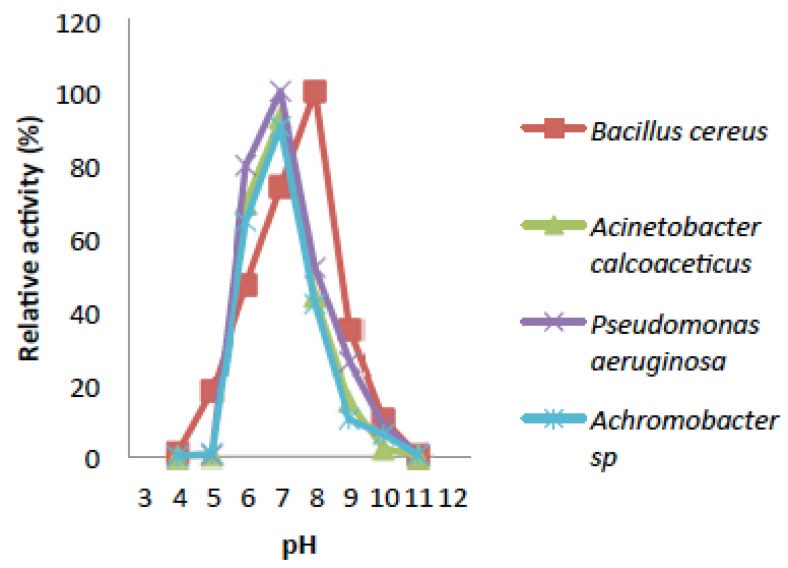
Effect of pH and its Stability on the Activity of Catechol-2,3-dioxygenase
The activity of catechol 2,3-dioxygenase for most of the bacteria were dormant at pH 3.0 and 12 (Fig. 6) while they all showed slight activity within the pH range of (4.0 to 5.0) and (10 to 11), On the other hand, Bacillus cereus showed slight activity at pH 3.0 and it also had significant activity at pH 4.0 to 5.0. Catechol-2,3-dioxygenase from Bacillus cereus exhibited its highest activity of 100% at pH 8.0 which means it is slightly more tolerant to alkaline pH and was still 20% active at pH 9.0 (Fig. 6). Catechol 2,3-dioxygenase obtained from the other three bacterial isolates exhibited activity that is more tolerant to neutral pH and they had lost about 95% of their original relative activity under acidic condition (pH 4.0 to 5.0), and about 55% at pH 9.0 and no activity was observed at pH 12. The pH stability of Catechol-2,3-dioxygenase from B. cereus showed that, it was only partially stable at pH 7.0 while, Acinetobacter was also partially stable at pH 6.0 and 7.0 (Fig. 7). However Catechol 2,3-dioxygenase from Pseudomonas was partially stable at pH 6.0, but was stable at pH 7.0. The Catechol-2,3-dioxygenase from Achromobacter sp. exhibited the highest stability at pH 7.0, while it was partially stable at pH 6.0 and 8.0 with relative activity during the entire time course. The pH stability decreases with incubation period for most of the isolates.
Fig. (6).
Effect of pH on activities of Catechol-2,3-dioxygenase.
Fig. (7).
pH stability of Catechol-2,3-dioxygenase activity.
DISCUSSION
The quality of life is being eroded in the affected area due to the ill effect of oil exploitation. The inhabitants of the oil producing areas of Ilaje local Government Area of Ondo State, which depends on the rivers and farmlands for their livelihood, are suddenly ensnared in a desolated environment that could no longer provide them with relief [13].
This study shows that there was increase in hydrocarbon-degrading bacteria obtained from the contaminated soil. According to [14], it was reported that hydrocarbon degraders in unpolluted environments, usually represent less than 0.1% of the microbial community while in crude-oil polluted environments they constitute up to 100% of the viable microbes which corroborates with the result obtained in Fig. (1). The results obtained from the taxonomic characterization of the isolates revealed different groups of bacterial genera: Pseudomonas, Bacillus, Acinetobacter and Achromobacter. Members of the above mentioned genera have been reported by many scientists as organisms which utilize hydrocarbons as their source of carbon and energy [2, 15-17]. The extreme optimum crude oil degradative ability of Pseudomonas aeruginosa compared to other bacterial isolates from the result is supported by the reports of [17, 18]. In their study, Pseudomonas sp. are shown to be the most common aerobic soil bacteria which possess diverse aliphatic and aromatic hydrocarbon degradative genes, most of which have been shown to be plasmid-borne. On the other hand, the degrading ability observed in Bacillus sp.in this study has also been reported by [18] as bacteria that have high capacity for crude oil degradation. Bacillus sp. was also observed as the predominant isolates of all the crude oil utilizing bacteria from a study on polluted soil samples and it was postulated that Bacillus species are more tolerant to high levels of hydrocarbons in soil due to their resistant endospores [19]. It is conceived that the isolated organisms should have catabolic enzymes for definite biodegradation in the presence of diverse Petroleum hydrocarbons. The obtained results (Fig. 3) from activities of catechol 2,3-dioxygenase (C23O) from the isolates revealed Pseudomonas aeruginosa with an outstanding activity than the other bacterial isolates. This reflects why the organism shows good growth on crude oil. In this study, the variation found in the result of C23O activity, has also been described by [20, 21] in their findings that enzymatic activity of diverse bacterial strains differ over wide ranges; which might be due to the heterogeneity of enzyme structure. The effects of temperature and pH on the activity of catechol 2,3-dioxygenase were determined in this study as they are important factors influencing the biodegradation of PAH by microorganisms. Enzyme activity depends on temperature [22], and optimum temperature for most enzymes could be linked to the temperature of the environment in which microorganisms were isolated [23]. The optimum temperature observed for catechol 2,3-dioxygenase in this study (Fig. 7) was within the range of 30°C to 50°C, with only Bacillus cereus showing its optimum extracellular activity at 50°C. This agrees with the investigation done by [24], who determined the optimum temperature for the activity of the enzyme C23O as 50°C from Bacillus stearothermophilus. However, many enzymes exhibit higher activity at a temperature at which they are unstable [25]. Catechol 2,3-dioxygenase isolated from Pseudomonas aeruginosa in this study were optimally active at 30°C and 40°C, which coincides with [26] who reported catechol 2,3-dioxygenase isolated from Pseudomonas strains ZJF08 and S-47 to be optimally active at 40°C and within 30– 35°C, respectively. However, catechol 2,3-dioxygenase isolated from most of the isolates in this study had lost most of their activity within the temperature range of 70°C to 80°C and this result agrees with [22] who reported that at low temperatures the movement of the molecules is slow and no activity is required to convert the substrate into a product, while at higher temperatures, the thermal movement of the molecules is too high to ensure enzyme conformation, thus causing their denaturation and activity loss. While the thermal stability of catechol 2,3-dioxygenase was found to be within the range of 30°C to 40°C for most of the isolates (Fig. 6). According to [27], it was postulated that temperature had influence by increasing both microbial activity and PAH solubility, and established the rate of degradation to reduce at temperature above 30°C. This indicates the adverse effect of high temperature on cells. The way that enzymes respond to temperature and pH is important in many areas of biotechnology. Enzyme activity affected in several ways by hydrogen ion concentration [22]. One way to be considered is that catalytic reaction requires by this enzyme, with particular catalytic groups in an ionized or non-ionized state, to react with the substrate without modification in its active conformation and the enzymes stability. Consequently, it does not drastically reduce its activity. The specific activity of pH was highest for all four isolates (Fig. 7) at pH 7.0 except for Bacillus cereus that had its optimum relative activity at pH 8.0. This result is in accordance with reports from literature that the pH optimum for this enzyme ranges from 5.5 to 8.2 [28, 29].
Fig. (1).
Isolation and selection of oil degrading bacteria from soil samples, Legends: CS- contaminated soil; NS- Non- contaminated soil.
CONCLUSION
Petroleum degraders have been reported to be endowed with enzyme systems, nutritional capabilities and metabolic activities which enable them to withstand adverse environmental conditions. It is therefore necessary to confirm the isolates that are able to secrete relevant enzymes with highest activity of degrading hydrocarbons. And the degrading ability demonstrated by the microorganisms is a clear indication that they can be used in hydrocarbon degradation. The selected organism have shown their optimum catechol 2,3-dioxygenase activity within the temperature range of 30°C to 50°C during degradation. Hence, thermophilic nature of these bacteria’s enzymes could add further advantage for their use in bioremediation of petroleum contaminated soils during dry season.
ACKNOWLEDGEMENTS
The authors are grateful to the Department of Microbiology, Federal University of Technology, Akure, Ondo State, Nigeria for the laboratory space.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Okerentugba P.O., Ezeronye O.U. Petroleum degrading potentials of single and mixed microbial cultures isolated from rivers and refinery effluent in Nigeria. Afr. J. Biotechnol. 2003;2:288–292. doi: 10.5897/AJB2003.000-1058. [DOI] [Google Scholar]
- 2. Boboye B, Olukunle OF, Adetuyi FC. Degradative activity of bacteria isolated from hydrocarbon polluted site in Ilaje Ondo State. Nig. Afr J Microbiol. 2010;4(23):2485–90. [Google Scholar]
- 3.Okoh A.I. Biodegradation alternative in the cleanup of petroleum hydrocarbon pollutants. Biotechnol Mol Biol Rev. 2006;1:38–50. [Google Scholar]
- 4.Palaniandavar M., Mayilmurugan R. Mononuclear non-hemiron(III) complex as functional models for catechol dioxygenases. C. R. Chim. 2007;10:366–379. doi: 10.1016/j.crci.2007.01.001. [DOI] [Google Scholar]
- 5.Viggiani A., Siani L., Notomista E., Birolo L., Pucci P., Di Donato A. The role of the conserved residues His-246, His-199, and Tyr-255 in the catalysis of catechol 2,3-dioxygenase from Pseudomonas stutzeri OX1. J. Biol. Chem. 2004;279(47):48630–48639. doi: 10.1074/jbc.M406243200. [DOI] [PubMed] [Google Scholar]
- 6.Wei J., Zhou Y., Xu T., Lu B. Rational design of catechol-2, 3-dioxygenase for improving the enzyme characteristics. Appl. Biochem. Biotechnol. 2010;162(1):116–126. doi: 10.1007/s12010-009-8720-y. [DOI] [PubMed] [Google Scholar]
- 7.Fawole M.O., Oso B.A. Laboratory manual of microbiology. Ibadan, Nigeria: Spectrubooks Limited; 2001. p. 127. [Google Scholar]
- 8.Xiao L., Yang L.Y., Yin D.Q. Environmental Microbiology Experimental Techniques. 2004. [Google Scholar]
- 9.Cheesbrough M. District Laboratory practice in Tropical Countries. 2nd ed. United Kingdom: Cambridge Unipress; 2006. p. 143. [DOI] [Google Scholar]
- 10.Holt J.G., Krieg N.R., Sneath P.H., et al. Bergey's Manual of Determinative Bacteriology. Baltimore, USA: William and Wilkins; 1994. pp. 729–759. [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Stoscheck C.M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-P. [DOI] [PubMed] [Google Scholar]
- 13.Celestine A. 2003. [Google Scholar]
- 14.Sarma P.M., Bhattacharya D., Krishnan S., Lal B. Degradation of polycyclic aromatic hydrocarbons by a newly discovered enteric bacterium, Leclercia adecarboxylata. Appl. Environ. Microbiol. 2004;70(5):3163–3166. doi: 10.1128/AEM.70.5.3163-3166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ollivier B., Magot B. Petroleum Microbiology. 2005. [DOI] [Google Scholar]
- 16.Chikere C.B. Bacterial diversity and community dynamics during the bioremediation of crude oil-polluted soil. 2010. [Google Scholar]
- 17.Chikere C.B., Ughala E. Preliminary screening of hydrocarbon utilizing bacteria habouring plasmids. TWOWS Afr Int J Sci Tech. 2011;2:26–36. [Google Scholar]
- 18.Obayori S.O., Salam L.B. Degradation of polycyclic aromatic hydrocarbons: role of plasmids. Sci. Res. Essays. 2010;5:4093–4106. [Google Scholar]
- 19.Ijah U.J., Antai S.P. Removal of Nigerian light crude oil in soil over a 12 month period. Int. Biodeterior. Biodegradation. 2003;51:93–99. doi: 10.1016/S0964-8305(01)00131-7. [DOI] [Google Scholar]
- 20.Huang S.L., Hsu Y.C., Wu C.M., Lynn J.W., Li W.H. Thermal effects on the activity and structural conformation of catechol 2,3-dioxygenase from Pseudomonas putida SH1. J. Phys. Chem. B. 2010;114(2):987–992. doi: 10.1021/jp9078579. [DOI] [PubMed] [Google Scholar]
- 21.Takeo M., Nishimura M., Shirai M., Takahashi H., Negoro S. Purification and characterization of catechol 2,3-dioxygenase from the aniline degradation pathway of Acinetobacter sp. YAA and its mutant enzyme, which resists substrate inhibition. Biosci. Biotechnol. Biochem. 2007;71(7):1668–1675. doi: 10.1271/bbb.70079. [DOI] [PubMed] [Google Scholar]
- 22.Whiteley C.G., Lee J.D. Enzyme technology and biological remediation. Enzyme Microb. Technol. 2006;38:291–316. doi: 10.1016/j.enzmictec.2005.10.010. [DOI] [Google Scholar]
- 23.Atlas R.M., Bartha R. Microbial Ecology: Fundamentals and applications. 4th ed. New York: Benjamin and Cummings Science Publishing; 1998. pp. 523–530. [Google Scholar]
- 24.Fernandez-Lafuente R., Guisan J.M., Ali S., Cowan D. Immobilization of functionally unstable catechol-2,3-dioxygenase greatly improves operational stability. Enzyme Microb. Technol. 2000;26(8):568–573. doi: 10.1016/S0141-0229(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.Eisenthal R., Peterson M.E., Daniel R.M., Danson M.J. The thermal behaviour of enzyme activity: implications for biotechnology. Trends Biotechnol. 2006;24(7):289–292. doi: 10.1016/j.tibtech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Zou Y., Wei J., Jiang T., et al. Characterisation of thermostable catechol 2,3-dioxygenase from phenanthrene degrading Pseudomonas sp. strain ZJF08. Ann. Microbiol. 2007;57:503–508. doi: 10.1007/BF03175346. [DOI] [Google Scholar]
- 27.Jacques R.J., Santos E.C., Bento F.M., et al. Anthracene biodegradation by Pseudomonas sp. isolated from a petrochemical sludge landfarming site. Int. Biodeterior. Biodegradation. 2005;56:143–150. doi: 10.1016/j.ibiod.2005.06.005. [DOI] [Google Scholar]
- 28.Ahuatzi-Cacon D., Ordorica-Morales G., Ruiz-Orday N., et al. Kinetic study of phenol hydroxylase and catechol-1,2-dioxygenase biosynthesis by Candida tropicalis cells grown in different phenolic substrates. World J. Microbiol. Biotechnol. 2004;20:695–702. doi: 10.1007/s11274-004-2622-5. [DOI] [Google Scholar]
- 29.Viggor S., Heinaru E., Künnapas A., Heinaru A. Evaluation of different phenol hydroxylase-possessing phenol-degrading pseudomonads by kinetic parameters. Biodegradation. 2008;19(5):759–769. doi: 10.1007/s10532-008-9180-8. [DOI] [PubMed] [Google Scholar]