Abstract
Photobacterium damselae subsp. damselae, an important pathogen of marine animals, may also cause septicemia or hyperaggressive necrotizing fasciitis in humans. We previously showed that hemolysin genes are critical for virulence of this organism in mice and fish. In the present study, we characterized the hlyA gene product, a putative small β-pore-forming toxin, and termed it phobalysin P (PhlyP), for “photobacterial lysin encoded on a plasmid.” PhlyP formed stable oligomers and small membrane pores, causing efflux of K+, with no significant leakage of lactate dehydrogenase but entry of vital dyes. The latter feature distinguished PhlyP from the related Vibrio cholerae cytolysin. Attack by PhlyP provoked a loss of cellular ATP, attenuated translation, and caused profound morphological changes in epithelial cells. In coculture experiments with epithelial cells, Photobacterium damselae subsp. damselae led to rapid hemolysin-dependent membrane permeabilization. Unexpectedly, hemolysins also promoted the association of P. damselae subsp. damselae with epithelial cells. The collective observations of this study suggest that membrane-damaging toxins commonly enhance bacterial adherence.
INTRODUCTION
Photobacterium damselae subsp. damselae causes severe infections in wild marine animals and in aquaculture. Time and again, septicemia or necrotizing soft tissue infections in humans have also been reported (1–6). In many cases, even radical surgery and antibiotic treatment fail to save the lives of patients. Due to global ocean warming, pathogenic species of the family Vibrionaceae are spreading in the aquatic environment (7, 8). The virulence of P. damselae subsp. damselae toward mice and fish depends on a large plasmid termed pPHDD1 (9). This conjugative element comprises dly and hlyApl, which encode two hemolysins: damselysin (Dly) and HlyApl. Dly is a phospholipase D that until recently was the only known hemolysin of P. damselae subsp. damselae (10–12), whereas HlyApl is a putative pore-forming toxin (PFT) (9). A third, chromosomally encoded hemolysin, HlyAch, present in all hemolytic strains, is 92% identical to HlyApl (13), and it makes a minor contribution to hemolysis and virulence (14). P. damselae subsp. damselae hemolysins are secreted via the type II secretion system (T2SS), which was also shown to be required for virulence and hemolysis (15). In contrast to Dly, HlyA has not been characterized so far.
PFTs are produced by many bacteria. They are secreted as water-soluble molecules which undergo conformational changes upon binding to target membranes, leading to the insertion of oligomeric transmembrane pore complexes (16). Despite a common mode of action, there are significant structural and functional differences between toxins of different families and even among toxins belonging to the same family (17). PFTs may cause direct damage of target cells (18), promote escape from membrane-bound compartments (19, 20), or introduce virulence factors into the cytosol (21) (for recent reviews, see references 22 and 23).
In the present study, we characterized the product of P. damselae subsp. damselae hlyApl and termed it “photobacterial lysin encoded on a plasmid” (phobalysin P [PhlyP]). This toxin exhibits distinct properties as well as features shared by other small β-PFTs, underscoring the diversity of these toxins. The results fuel the idea that hemolysins trigger complex stress responses, and they support an emerging role in bacterial adherence to target cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Culture conditions and conjugative matings used in this study have been described previously (9, 14, 15). The Staphylococcus aureus strains have been described previously (24). Adherence assays with Escherichia coli were performed with DH5α.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| P. damselae subsp. damselae strains | ||
| AR57 | RM-71 derivative, spontaneously rifampin-resistant mutant | 9 |
| AR78 | AR57 with in-frame deletion of dly and hlyApl genes | 9 |
| AR158 | AR57 with in-frame deletion of hlyApl and hlyAch genes | 14 |
| AR119 | AR57 with in-frame deletion of dly and hlyAch genes | 14 |
| AR89 | AR57 with in-frame deletion of dly, hlyApl, and hlyAch genes | 14 |
| AR239 | AR57 with in-frame deletion of pilD gene | 15 |
| AR252 | AR57 with in-frame deletion of pilD and tadV genes | 15 |
| AR263 | AR57 with suicide plasmid pAJR80 inserted into the cheA gene | This study |
| AR264 | AR89 with suicide plasmid pAJR80 inserted into the cheA gene | This study |
| AR272 | AR89 complemented with pAJR38 | This study |
| E. coli strains | ||
| DH5α | Cloning strain; recA | Laboratory stock |
| S17-1 λpir | recA thi pro Δhsd(R− M+)RP4:2-Tc:Mu-Km:Tn7 λpir Tpr Smr | 70 |
| Plasmids | ||
| pSpark | PCR cloning vector; Ampr | Canvax |
| pHRP309 | lacZ reporter plasmid; mob Gmr | 71 |
| pTrcHisA | His6× tag fusion expression vector; Ampr | Invitrogen |
| pTrcHisA-pPhlyP | pTrcHisA with pro-PhlyP | This study |
| pNidKan | Suicide vector, derived from pCVD442; Kmr | 72 |
| pAJR80 | pNidKan containing an internal fragment of the cheA gene | This study |
| pAJR38 | pHRP309 with the hlyApl gene from strain RM-71 | 9 |
| pAJR76 | pHRP309 with the pilD gene from strain RM-71 | 15 |
Mutant construction and gene complementation.
For construction of a cheA mutant, a cheA internal fragment of 1,734 bp was amplified by PCR from P. damselae subsp. damselae strain AR57 by using Kapa Taq DNA polymerase (Kapa) and the primers cheA-Forward (CTTTATTGCAAGTTCGCCCG) and cheA-Reverse (GGTAGGAGCCACTTTCATGA). The resulting product was ligated into the TA vector pSpark (Canvax). Subsequently, the DNA insert was cut out by digestion and cloned into the BamHI site of the suicide vector pNidKan (Table 1), resulting in pAJR80. As a derivative of pCVD442, pNidKan contains the R6K ori, requiring the pir gene product for replication. Insertion of the suicide vector into the chromosome by a single crossover results in a Kmr phenotype. After conjugational matings, P. damselae subsp. damselae Kmr exconjugants were isolated. Disruption of the cheA gene was confirmed by PCR. For hlyApl complementation, pAJR38 was transferred by conjugation to P. damselae subsp. damselae strain AR89 (triple mutant [TM]).
Cloning, expression, and purification of toxins.
Extracellular products (ECPs) of the various P. damselae subsp. damselae strains were obtained by growing the bacteria on cellophane membranes placed on LB agar in petri dishes (14-cm diameter). In our hands, this procedure yielded higher hemolytic titers than those of planktonic cultures. After incubation for ∼60 h at room temperature (RT), bacteria and ECPs were collected by rinsing each cellophane membrane with 2 ml 0.85% NaCl (vol/vol). The various suspensions were adjusted to an optical density at 600 nm (OD600) of 1, and bacteria were removed by centrifugation and subsequent filtration (0.2-μm pore size). Purification of PhlyP was achieved as follows. First, ECPs of P. damselae subsp. damselae ΔhlyAch Δdly (AR119) were prepared as described above. Proteins were precipitated by adding 20 ml 3.3 M ammonium sulfate to 10 ml ECPs and were pelleted by centrifugation. The pellet was resuspended in 56 ml H2O and 4 ml of Bio-Lyte 3/10 ampholyte (Bio-Rad). Proteins were separated by isoelectric focusing (IEF) (15 W; 230 to 550 V) for 4 h at 6 to 8°C. Twenty fractions of 2 to 4 ml were collected. Hemolytic activity focused at pH 5.5 to 6.5; 2 ml of the fraction with the highest hemolytic activity was diluted 1:5 (vol/vol) in binding buffer (50 mM malonic acid, pH 5.5), loaded onto a Mono S column, and eluted at a flow rate of 0.4 ml/min with 15 ml elution buffer (linear gradient ranging from 0 to 1.0 M NaCl in 50 mM malonic acid, pH 5.5). Fractions of 0.5 ml each were collected and tested for hemolytic activity. Fractions 10 and 11 (eluting at ∼250 mM NaCl) were pooled and stored at −70°C in 25 mM malonic acid, pH 5.5, 250 mM NaCl, 50% glycerol. These protein stocks were ∼95% pure and had a concentration of ∼1.0 μg/ml, as estimated by densitometric analysis of silver-stained SDS-PAGE gels and comparison with various amounts of recombinant pVCC as a protein standard, respectively. For Edman degradation, proteins from ECPs were separated by isoelectric focusing. Ethanol (96%) was added to each fraction from the IEF at a ratio of 2:1 (vol/vol) and centrifuged (12,000 × g) in a benchtop centrifuge for 30 min at 4°C. The resulting pellet was resuspended in SDS loading buffer (65 mM Tris-HCl, pH 6.8, 10% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol, 2% [wt/vol] SDS, and bromophenol blue) and separated by SDS-PAGE (12%). Separated proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane and stained with Coomassie brilliant blue. A dominant band with an ∼50-kDa apparent molecular mass was cut out and submitted for custom protein sequencing by Edman degradation. Vibrio cholerae cytolysin was expressed as an N-terminally His6×-tagged protoxin (pVCC) and prepared as described previously (25). In brief, Origami B cells carrying pQE30-pVCC were grown, induced with IPTG (isopropyl-β-d-thiogalactopyranoside), and harvested by centrifugation. Pellets were resuspended in binding buffer (50 mM NaPO4, 300 mM NaCl, 10 mM imidazole, pH 8.0, 1 mg/ml lysozyme, 4 mM Pefabloc), and bacteria were lysed and disrupted by ultrasonication. The supernatant was filtered using 0.2-μm-pore-size filters and applied to Ni-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen); further purification steps were performed according to the Qiagen protocol. Fractions with the highest protein concentrations were pooled and dialyzed in 20 mM Tris and 20 mM NaCl for 24 h at 4°C. Subsequently, dialysates were loaded onto a Mono Q column (GE Healthcare) and eluted at a flow rate of 0.5 ml/min with 20 ml of a linear gradient (20 mM to 500 mM NaCl in 20 mM Tris, pH 8). Again, fractions with the highest protein concentrations/hemolytic activities were pooled, and glycerol was added to a final concentration of 50% (vol/vol) prior to storage at −20°C. The preparation was ∼99% pure as estimated by densitometric analysis of a Coomassie-stained SDS-PAGE gel, and it had a concentration of 1 mg/ml as determined by using the Bio-Rad protein assay. For the production of recombinant pro-PhlyP (pPhlyP), the gene encoding pPhlyP was amplified by PCR from P. damselae subsp. damselae AR57 by using high-fidelity Kapa Taq DNA polymerase (Kapa) and primers PhlyP-Forward (5′-AACTATTCTACACCTGCAGA) and PhlyP-Reverse (5′-TTAACCCCAAATGAGCTAAT). The amplified DNA was purified, digested, and cloned into the BamHI site of pTrcHisA (Invitrogen Life Technologies), resulting in pTrcHisA-pPhlyP, which was transformed into Shuffle Express competent E. coli cells (New England BioLabs [NEB]). Induction was performed according to the NEB protocol. N-terminally His6×-tagged pPhlyP was affinity purified from E. coli cell lysates by using Ni-NTA agarose (Qiagen) following Qiagen protocols. The purity of the material was estimated to be at least 90%, as estimated by densitometric analysis of Coomassie-stained SDS-PAGE gels, and the concentration was estimated to be ∼20 μg/ml as assessed by an anti-His6×Western blot with His6×-tagged pVCC serving as a standard. S. aureus alpha-toxin and the single-amino-acid-exchange D152C mutant were prepared as published elsewhere (see references 26 and 27 and references therein).
Hemolysis.
Hemolysis assays were performed with rabbit erythrocytes (RRCs). The release of hemoglobin was measured in the supernatant by determining the A405. Assays were carried out by mixing serial dilutions of filtered ECPs (stocks contained 3 mg/ml total protein) and washed RRCs. One hemolytic unit is defined as the amount of hemolysin which lyses 50% of RRCs, calculated with a hemolytic standard of fully lysed RRCs (deionized water). ECPs from different mutants were diluted 2-fold in phosphate-buffered saline (PBS) in microtiter plates. To 50 μl of diluted toxin in each well, 50 μl of 5% rabbit erythrocytes was added and subsequently incubated at 37°C for 1 h. To remove cholesterol, RRCs were incubated for 30 min at 37°C with methyl-β-cyclodextrin (MβCD; Sigma-Aldrich), at 0.5, 1.0, or 2.0 mM. To restore cholesterol in membranes, MβCD-treated RRCs were washed with PBS and supplemented with 400 μg/ml water-soluble cholesterol (Sigma-Aldrich). The absorbance of hemoglobin in supernatants was measured at 405 nm by use of an enzyme-linked immunosorbent assay (ELISA) reader.
Osmoprotection.
Assays were performed by exposing RRCs to toxins in the presence or absence of various osmolytes. Purified pVCC or ECPs from strain AR119 (ΔhlyAch Δdly), which produces only PhlyP/HlyApl, were serially diluted in PBS and mixed with RRCs in the absence of osmoprotectants or in the presence of 80 mM sucrose, 80 mM maltotriose, or 10% dextran 4. Samples were incubated at 37°C for 1 h. Subsequently, hemolysis was quantified by measuring the absorbance of supernatants at 405 nm. In some experiments, cells were subsequently pelleted and resuspended in fresh PBS to wash out osmoprotectants; samples were then reread at the time points indicated in the relevant figure.
Transmission electron microscopy (TEM) of PhlyP-treated erythrocyte ghosts.
For preparation of PhlyP-treated erythrocyte ghost membranes, 5 × 108 washed RRCs/ml in osmoprotection buffer (20 mM Tris-HCl, pH 7.0; 0.1% bovine serum albumin [BSA]; 30 mM dextran 4) (28) were incubated with 3.75 μg/ml of PhlyP for 1 h at RT. Subsequently, RRCs were washed in osmoprotection buffer and lysed in 5 mM sodium phosphate, pH 8. RRC membranes were washed twice and then resuspended in 100 μl of PBS. Droplets of the PhlyP-loaded ghost preparation were applied to carbon-coated Formvar films mounted on electron microscope nickel grids and exposed to glow discharge immediately before analysis. Absorbed samples were negatively stained with ammonium molybdate or uranyl acetate, and specimens were examined with a Zeiss EM 902 instrument.
Cells and culture conditions.
HaCaT cells (non-virally transformed human keratinocytes) (29) were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 GlutaMAX-I medium with 10% fetal calf serum, 1% HEPES buffer, 1% penicillin-streptomycin in a humidified incubator with 5% CO2 at 37°C. AB.9 zebra fish cells (30) were cultured in DMEM GlutaMAX-I medium with 15% fetal calf serum, 1% HEPES buffer, 1% penicillin-streptomycin in a humidified incubator with 5% CO2 at 28°C. All media and additives were obtained from Life Technologies.
Antibodies and chemicals.
Primary antibodies against eIF2α or S6K were obtained from Cell Signaling Technology. Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Santa Cruz Biotechnology and Cell Signaling Technology. Anti-puromycin antibody was from Merck Millipore. Chemicals were purchased from Sigma-Aldrich if not stated otherwise.
ATP measurements.
HaCaT cells were seeded in 96-well plates at a density of 2 × 104 cells/well. After 24 h, the medium was replaced with diluted ECPs, purified toxins, or medium controls. ATP in cell lysates was measured luminometrically using a firefly luciferase-based assay as described elsewhere (31).
Staining with trypan blue.
ECPs (final dilution, 1:100) of the strains indicated in the figures were added to cell suspensions containing 5 × 105 HaCaT cells/ml. One milliliter of cell suspension was mixed with 10 μl of each of the ECPs to be tested. The mixture was incubated at 37°C for 1 h. Subsequently, cells were centrifuged and resuspended in 100 μl of PBS; 50 μl of treated cells was mixed with 50 μl of 0.4% trypan blue. Stained and unstained cells were counted in a Neubauer chamber.
PI influx assay and flow cytometry.
To assess membrane perforation, toxin-treated cells were incubated with propidium iodide (PI; 50 μg/ml) for 1 min and then fixed. Nuclei were stained with Hoechst 33342 (Cell Signaling Technology), and cells were analyzed by wide-field fluorescence microscopy. The number of sub-G1 events in toxin-treated cell populations or controls was determined by staining ethanol-fixed cells with PI and subsequent analysis by flow cytometry using a FACScan instrument (BD) as described previously (32). Ethanol treatment was omitted for measuring toxin-dependent PI influx into cells.
Flame photometry for measuring K+ ions.
Cellular K+ was quantified by flame photometry as described previously (33). In brief, cells were washed three times with ice-cold K+-free choline buffer. Cells were subsequently lysed by incubation for 30 min in choline buffer–0.5% Triton X-100 at RT on a shaker. Lysates were analyzed for K+ with an M401 flame photometer (Sherwood, United Kingdom) using propane gas.
Western blots.
Cells were lysed directly in loading buffer (65 mM Tris, 10% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol, 2% [wt/vol] SDS, and bromophenol blue) and heated for 5 min at 95°C. Proteins were separated by SDS-PAGE (10%) and electroblotted onto a nitrocellulose membrane. After blocking for 1 h at RT in skim milk in TBST (20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% [vol/vol] Tween 20), the membrane was incubated with a primary antibody, washed three times in TBST, and incubated with an HRP-conjugated second antibody for 1 h at RT. After three washing steps, bound antibody was detected by an enhanced chemiluminescence (ECL) assay (Roche Applied Science).
Fluorescence microscopy and DIC microscopy.
Cells grown on glass coverslips were incubated or not with ECPs or bacteria as detailed in the figure legends. After incubation, cells were washed in PBS and subsequently fixed with 2% paraformaldehyde in PBS for 10 min at RT. For staining of mitochondria or F-actin in cells, we used the MitoTracker mitochondrion-selective probe (Molecular Probes, Invitrogen) or Alexa 488 Fluor-conjugated phalloidin, respectively; in the case of F-actin staining, cells were permeabilized with 0.1% Triton X-100 for 10 min prior to application of the probe. Coverslips were mounted on slides with Fluoprep (bioMérieux SA), and samples were examined in a Zeiss Axiovert 200M epifluorescence microscope equipped with a Plan Apochromat 100×/1.4-numerical-aperture oil-immersion differential interference contrast (DIC) objective. For DIC microscopy, a Zeiss POL filter set was used. Digital images were acquired with a Zeiss Axiocam camera. Image processing was done using Zeiss AxioVision software rel. 4.8 and Adobe Photoshop.
Adherence assays.
HaCaT cells were seeded at a density of 4 × 105/well in six-well plates, with or without coverslips, for microscopy- or culture-based assays, respectively. The next day, bacteria (100 μl/well of a suspension in exponential growth phase, i.e., OD600 = 0.4) were recovered by centrifugation, and the pellet was resuspended in medium containing ECPs from different P. damselae subsp. damselae strains, S. aureus alpha-toxin, mutant (D152C) alpha-toxin, or pVCC. Subsequently, these suspensions were added to cells, and the cocultures were incubated for 15 min at 37°C. Next, cells were washed twice before they were harvested and resuspended in 500 μl PBS. Dilutions of each sample were prepared, plated onto LB agar plates, and incubated overnight at 25°C. Finally, colony counts were assessed by visual inspection.
Motility measurements.
For motility determinations, bacteria were stabbed into semisolid LB plates containing 0.22% agar. Growth diameters around the puncture site were measured. Ten plates were evaluated for each condition.
Statistics.
Data displayed are derived from ≥3 independent experiments; in the figures, columns show mean values, and error bars indicate standard errors (SE). The statistical significance of differences between two mean values was assessed using two-sided, unpaired Student's t test. Analysis of variance (ANOVA) and Tukey's post hoc analysis were employed for multiple comparisons. In the figures, degrees of significance are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001 (P values of ≤0.05 were assumed to indicate significance).
RESULTS
PhlyP forms small membrane pores.
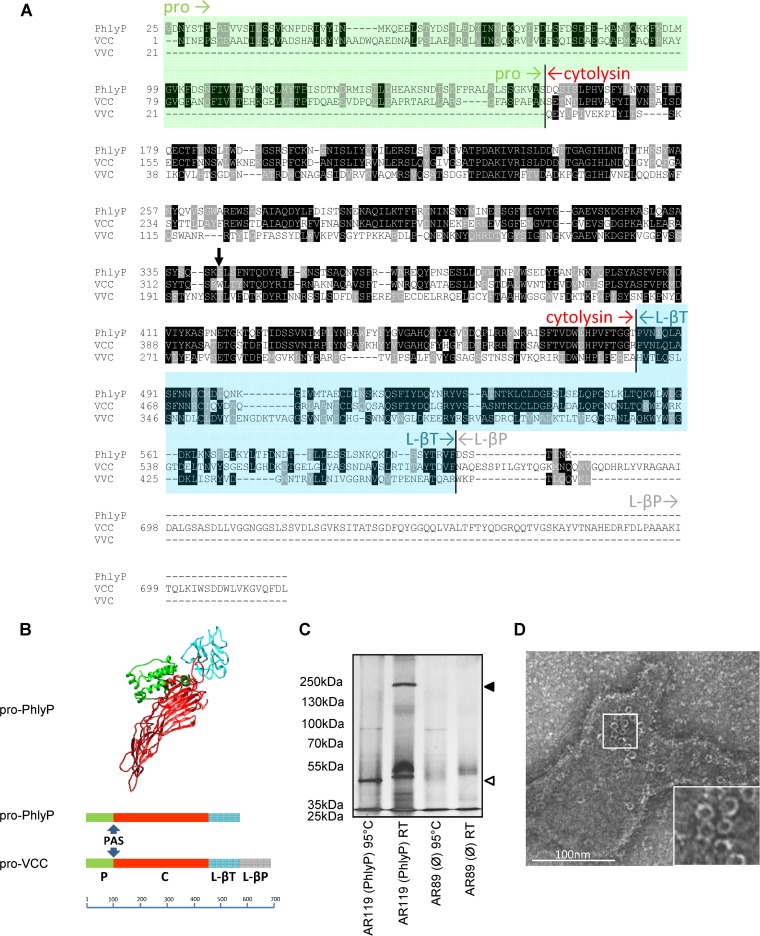
Previously, we noted that highly hemolytic strains of P. damselae subsp. damselae are particularly virulent (9). Further, the data suggested that hlyApl plays major roles in both virulence and hemolytic activity (14). Sequence comparison suggested that hlyApl encodes a hemolysin related to Vibrio cholerae cytolysin (VCC), leaving unmatched, however, an additional lectin domain present in pro-VCC (pVCC) (9) (Fig. 1A). Also, homology-based structure prediction with the aid of I-TASSER (34) inferred a structure similar to that of pVCC (Fig. 1B). To obtain experimental evidence that PhlyP is in fact a small β-PFT, we incubated ECPs from P. damselae subsp. damselae strain AR119 (Δdly ΔhlyAch; produces only HlyApl) with erythrocyte ghosts and separated firmly bound proteins by SDS-PAGE. ECPs from strain AR119 yielded a 50-kDa species and one with a high apparent molecular mass (250 kDa), both of which were absent from samples treated with ECPs of the triple mutant strain AR89 (Δdly ΔhlyAch ΔhlyApl; TM). At RT, the high-molecular-mass band resisted SDS and trypsin digestion (Fig. 1C), and it was lost only after heating to 95°C in SDS. Thus, PhlyP, similar to other small β-PFTs, appears to form stable oligomers (28, 35). Transmission electron microscopy of pore complexes formed by PhlyP on red cell membranes (“ghosts”) revealed typical circular structures associated with membranes (Fig. 1D).
FIG 1.
PhlyP, a new β-pore-forming toxin. (A) Alignment of amino acid sequences of pro-PhlyP, pro-VCC, and VVC (the prodomain of VVC is not contiguously expressed with the hemolysin domain and the lectin domain). Sequences were aligned by use of ClustalW 2.0 (68) and displayed with version 3.21 of BOXSHADE, written by K. Hofmann and M. Baron (http://www.ch.embnet.org/software/BOX_form.html). The prodomain of PhlyP is shaded in green, and the L-βT domain is shown in blue. The cytolysin domain is labeled in red, and the L-βP domain is labeled in gray. The black arrow points at W318 of pVCC. (B) (Top) Similarity-based structure prediction for pPhlyP, performed by using the I-TASSER server (34). The C score for alignment of mature PhlyP and VCC was 0.99; the score for the protoxins was negative due to the lack of the L-βP domain in pPhlyP. The model as shown here was created with the aid of UCSF Chimera (http://www.cgl.ucsf.edu/chimera). (Bottom) Comparison of domain structures of pPhlyP and pVCC. The scale bar shows numbers of amino acid residues. PAS, proteolytic activation site. (C) Erythrocyte ghosts were incubated with the indicated ECPs, treated with trypsin, and separated by SDS-PAGE, and proteins were silver stained. The filled triangle on the right indicates the presumed PhlyP oligomer, and the open triangle indicates the monomer. (D) Rabbit erythrocyte ghosts were loaded with PhlyP (ECPs of strain AR119) and analyzed by TEM.
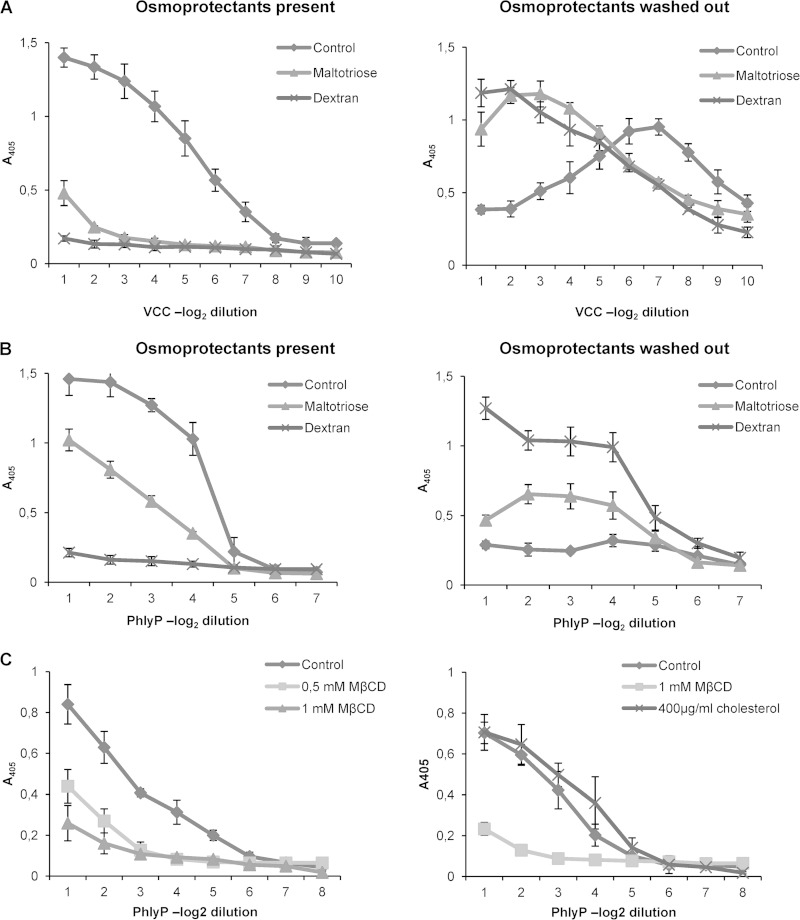
To approximate pore size, we next performed osmoprotection experiments (36). PFTs induce an uncontrolled influx of water into red cells, which ultimately leads to lysis. However, when osmolytes with hydrodynamic diameters (HDs) above the effective pore size are present, cells are protected by retention of water in the extracellular space. The differential ability of various osmolytes to suppress lysis allows an approximation of pore size, as previously shown for pVCC (37). Osmoprotection assays were carried out with ECPs from the PhlyP-producing strain AR119; pVCC served as a control. Although pVCC-dependent hemolysis was significantly reduced by maltotriose (molecular mass, 504.4 g mol−1; HD, ∼1.2 nm) (Fig. 2A, left panel), inhibition of PhlyP-dependent hemolysis was inefficient (Fig. 2B, left panel). In contrast, dextran 4, which has an HD of ∼3 nm, protected cells equally well against either of these toxins. When RRCs which had been treated with PFTs in the presence of an active osmoprotectant (and thus were protected from lysis) were pelleted and resuspended in PBS, hemolysis occurred after incubation for 1 h at 37°C, indicating that osmoprotectants had not prevented binding of toxins to RRCs (Fig. 2A and B, right panels). The results suggested that PhlyP creates pores with an effective diameter of >1.2 and <3.0 nm, which are thus wider than those formed by VCC.
FIG 2.
PhlyP forms small membrane pores, and its hemolytic activity requires cholesterol in cell membranes. (A and B) Osmoprotection experiments were performed with pVCC (A) and PhlyP (ECPs of AR119) (B). The left graphs show hemolysis in the presence of osmoprotectants, and the right graphs show hemolysis after washout of osmoprotectants. Data show mean values ± SE (n = 3). (C) RRCs were depleted of cholesterol by using methyl-β-cyclodextrin (MβCD) and were challenged with ECPs of strain AR119 (left). Replenishment of cholesterol was conducted by adding soluble cholesterol to cholesterol-depleted RRCs (right). Data are mean values ± SE (n = 3).
Cholesterol is required for PhlyP-dependent hemolysis.
As exemplified by VCC, cholesterol-dependent cytolysins (CDCs) (38) are not the only PFTs that require cholesterol in target cell membranes to function properly (39). When cholesterol was extracted from RRCs by use of methyl-β-cyclodextrin (MβCD), we observed that PhlyP failed to lyse cells (Fig. 2C, left panel), suggesting that PhlyP requires cholesterol in order to cause hemolysis. The prediction that the effect of MβCD was actually a consequence of cholesterol depletion was confirmed by adding back cholesterol to depleted RRCs (Fig. 2C, right panel), as this fully restored the ability of PhlyP to cause hemolysis.
Extracellular products of Photobacterium damselae subsp. damselae contain active PhlyP.
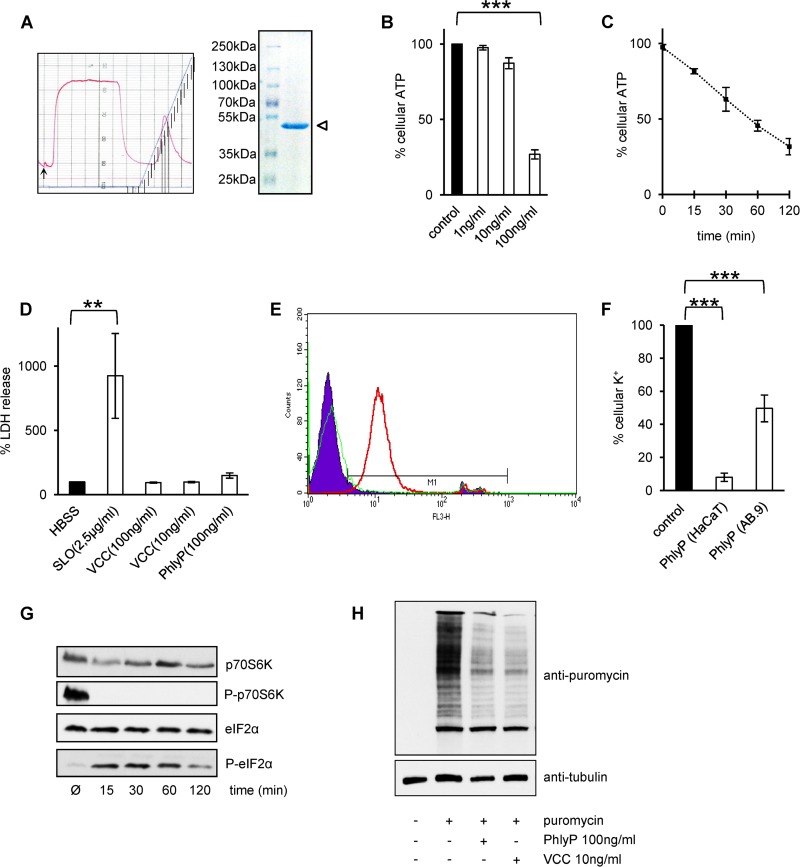
Proteases in the extracellular environment (40) or located in the host cell membrane (41) process pVCC to yield mature, active VCC, which oligomerizes and forms heptameric transmembrane pores (25). Sequence analysis indicated that hlyApl encodes a small β-PFT related to VCC (9) that is also produced as a protoxin, but cleavage sites of pVCC (25) are not conserved in pPhlyP. However, secretion products of strain AR119 contained hemolytic activity which was not fully blocked by low temperature or inhibitor of tumor necrosis factor alpha processing 2 (TAPI-2), a metalloprotease inhibitor that inhibits processing of pVCC to VCC by cellular metalloproteases (41) (see Fig. S1A and B in the supplemental material). Therefore, it appeared likely that these ECPs contained mature PhlyP. Indeed, preparative isoelectric focusing (IEF), which has been employed to purify VCC (28), followed by ion-exchange chromatography allowed us to isolate the hemolytic activity from ECPs of strain AR119. It focused at a pI of ∼5.5, eluted as a single peak from a Mono S column (Fig. 3A, left panel), and had an apparent molecular mass of ∼50 kDa as determined by SDS-PAGE (Fig. 3A). The band was cut out from gels and subjected to Edman degradation, which revealed the sequence N terminus-valine-alanine-serine-aspartic acid-glutamine-C terminus, matching positions 155 to 159 of the amino acid sequence predicted from in silico translation of hlyApl. The corresponding protein is expected to comprise 447 amino acids and to have a molecular mass of 50.47 kDa, in line with its electrophoretic migration velocity (Fig. 3A).
FIG 3.
P. damselae subsp. damselae extracellular products contain mature PhlyP. (A) The left panel shows the elution profile from the Mono S column; note the single peak in the right half of the profile, which contained the hemolytic activity. The right panel shows an SDS-PAGE gel with purified PhlyP (two peak fractions from the elution profile), indicated by an open triangle. (B) ATP was measured after incubation of HaCaT cells with various concentrations of purified PhlyP for 2 h. Data are mean values ± SE (n = 3). ***, P ≤ 0.001 in two-sided, unpaired Student's t test. (C) Results of ATP assays after incubation of HaCaT cells with purified PhlyP (100 ng/ml) for various times. Data shown are percentages of the level in untreated controls and are mean values ± SE (n = 3). (D) HaCaT cells were treated for 10 min with the indicated toxins, and lactate dehydrogenase (LDH) was measured in supernatants. Control, saline without toxin (HBSS). Data are mean values ± SE (n = 3). **, P ≤ 0.01 in two-sided, unpaired Student's t test. (E) HaCaT cells in suspension were incubated with PhlyP (100 ng/ml) for 4 min, PI (50 μg/ml) was added, and samples were analyzed after 1 min at 37°C by flow cytometry. FL3H, fluorescence intensity (log) for PI; counts, number of events per channel. Red line, PhlyP; green line, VCC; filled area, untreated cells. (F) Cellular K+ levels were determined by flame photometry in HaCaT or AB.9 cell lysates after exposure of cells to PhlyP. Cells were incubated with purified PhlyP (100 ng/ml) (HaCaT cells) or ECPs from strain AR119 (produces only PhlyP) (AB.9 cells). Intracellular levels of potassium were determined after 10 min. Data are percentages of the levels in untreated controls and are mean values ± SE (n ≥ 3). ***, P ≤ 0.001 in two-sided, unpaired Student's t tests. (G) HaCaT cells were treated with purified PhlyP (100 ng/ml) for the indicated times at 37°C and were analyzed by Western blotting for phosphorylation of p70S6K or eIF2α. (H) HaCaT cells were incubated with purified PhlyP (100 ng/ml) or VCC (10 ng/ml) for 10 min at 37°C. Subsequently, cells were incubated with 10 μg/ml puromycin (1 h at 37°C), which is incorporated into nascent proteins (69). Finally, cells were analyzed by Western blotting using antibodies directed against puromycin.
Purified PhlyP is cytotoxic to nucleated cells.
Cytotoxic activity of P. damselae subsp. damselae has been described previously (42), but the roles of individual P. damselae subsp. damselae hemolysins in this context have not been investigated. Purified PhlyP provoked a marked, time- and dose-dependent drop of intracellular ATP in HaCaT cells (Fig. 3B and C). In contrast to the large pore-forming protein streptolysin O, no significant leakage of lactate dehydrogenase (LDH) (140 kDa) was observed with PhlyP (Fig. 3D). Notably, however, PhlyP, but not VCC, rapidly caused a significant influx of PI (Fig. 3E), in line with the differential behavior of these toxins in osmoprotection assays.
Loss of potassium ions is a hallmark of PFT action and is held responsible for many of their downstream effects (27, 33, 43, 44). PhlyP caused a loss of intracellular K+ in HaCaT cells and also in AB.9 cells from zebra fish (Fig. 3F). Phosphorylation of eIF2α and inactivation of mTORC1 are important consequences of the PFT-dependent loss of intracellular K+ from cells (27), both leading to attenuation of translation (33, 45, 46). The Western blot in Fig. 3G documents that PhlyP leads to dephosphorylation of p70S6K, a substrate of TORC1, and to hyperphosphorylation of eIF2α. Consistently, incorporation of puromycin in growing polypeptide chains was markedly reduced, indicating that PhlyP inhibits protein synthesis (Fig. 3H).
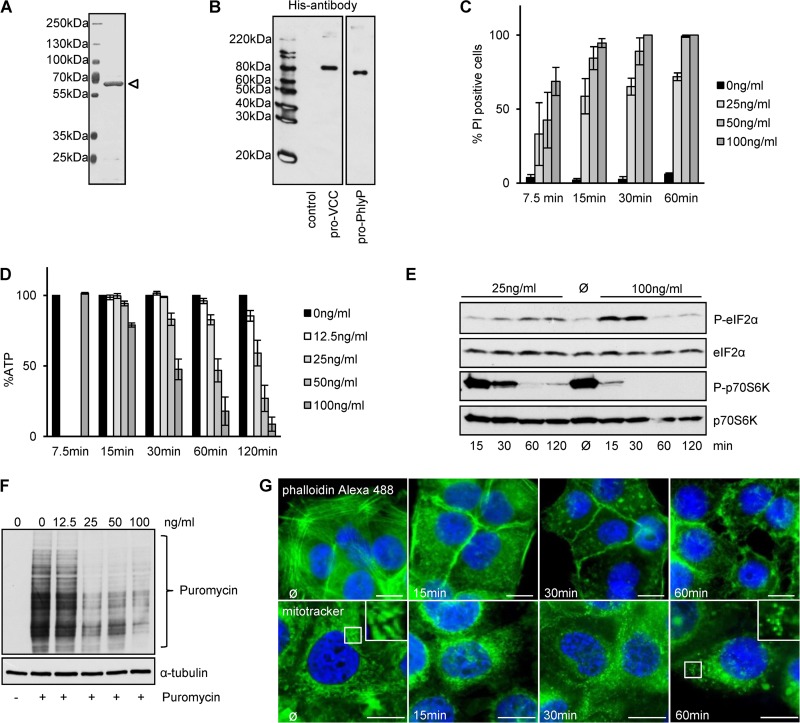
Recombinant pro-PhlyP recapitulates cytotoxic effects.
In order to provide evidence that the cytotoxicity of PhlyP did not depend on factors potentially copurified from P. damselae subsp. damselae ECPs, we sought to perform experiments with a heterologously expressed toxin. To this end, we cloned and expressed the protoxin (pPhlyP) as a His6× fusion protein in E. coli (Fig. 4A and B) to analyze the dose and time dependency of various effects of heterologously expressed, affinity-purified material on HaCaT cells. The earliest change that was consistently observed was an influx of PI (∼50% positive cells after 7.5 min of incubation with 100 ng/ml pPhlyP) (Fig. 4C), followed by a drop of ATP (Fig. 4D), depolymerization/redistribution of F-actin, morphological alterations of mitochondria (Fig. 4G) similar to what had been observed for listeriolysin (47), and (de)phosphorylation of S6K and eIF2α (Fig. 4E), all commencing at ∼15 min. By 1 h, at a toxin concentration of 100 ng/ml, incorporation of puromycin—an indicator of ongoing protein synthesis—was largely inhibited (Fig. 4F). After 2 h, a significant increase in sub-G1 events was seen, indicating irreversible DNA damage (see Fig. S1C in the supplemental material).
FIG 4.
Recombinant pro-PhlyP (pPhlyP) reproduces cytotoxic effects observed with native PhlyP. (A) SDS-PAGE analysis of affinity-purified N-terminally His6×-tagged pPhlyP (indicated by an open triangle). (B) Purified N-terminally His6×-tagged pVCC and pPhlyP were analyzed by Western blotting with anti-His antibodies. (C) HaCaT cells were incubated as indicated with different doses of pPhlyP (recombinant) for the indicated times and then stained with PI. The graph shows percentages of PI-positive cells out of the total cell number (mean values ± SE [n = 3]). (D) HaCaT cells were incubated with the indicated doses of His6×-tagged pPhlyP. Cellular ATP levels were determined at the indicated times. Data are percentages of the values in untreated controls and are mean values ± SE (n ≥ 5 for 15, 30, 60, and 120 min; n = 4 for 7.5 min). (E) HaCaT cells were incubated or not with 25 ng/ml or 100 ng/ml His6×-tagged pPhlyP for the indicated times. Lysates were analyzed by Western blotting for P-eIF2α, eIF2α, P-p70S6K, and p70S6K. (F) HaCaT cells were incubated or not with different doses of His6×-tagged pPhlyP for 1 h. Subsequently, cells were incubated or not for 1 h at 37°C with 10 μg/ml puromycin, which is incorporated into nascent proteins (69). Cells were analyzed by Western blotting for puromycin and α-tubulin. (G) HaCaT cells were incubated for the indicated times with 100 ng/ml His6×-tagged pPhlyP and then either washed, fixed, and treated with 0.1% Triton X-100 prior to staining with phalloidin-Alexa 488 (upper panels) or washed and treated with MitoTracker Green FM (200 nM) for 30 min at 37°C (lower panels). Bars = 20 μm.
Photobacterium damselae subsp. damselae causes rapid hemolysin-dependent damage.
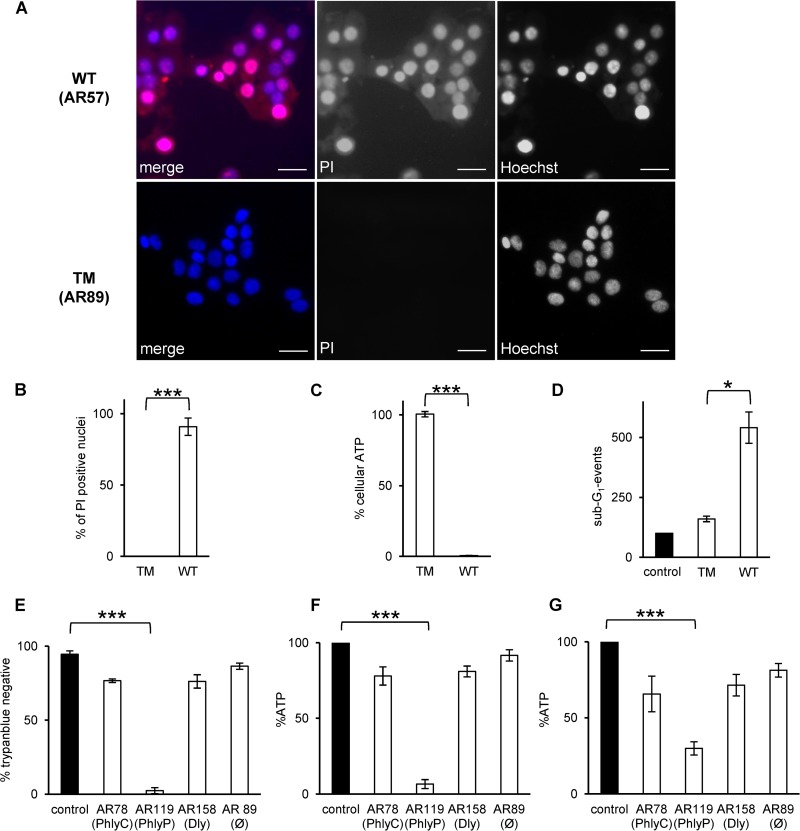
To evaluate the cytotoxicity of PhlyP and bacteria in context, we infected cultures of epithelial cells with P. damselae subsp. damselae strains expressing all or none of the three known hemolysins, or one hemolysin only. First, HaCaT cells infected with P. damselae subsp. damselae were stained with PI, which is excluded from intact cells. Infection with the wild-type (WT) strain, encoding the three known hemolysins (14), led to permeabilization of the majority of cells within 30 min (Fig. 5A, upper panels); considerable permeabilization was already noticed even after 15 min (data not shown). In contrast, keratinocytes infected with the TM, in which all three hemolysin genes are disrupted (14), did not stain with PI (Fig. 5A, lower panels). Incubation of HaCaT cells with ECPs of the WT strain, but not the TM strain, recapitulated the effect of infection on membrane permeability (Fig. 5B) and caused a loss of cellular ATP (Fig. 5C); complementation of the TM strain with pAJR38 (yielding AR272) restored the ability to reduce ATP levels (see Fig. S1D in the supplemental material). Moreover, the frequency of sub-G1 events increased (Fig. 5D). Next, we extended coculture experiments to measure the effects of ECPs from double mutant strains encoding only one of the three known hemolysins each (Table 1). Among these strains, AR119, which expresses only PhlyP, yielded the strongest effects on membrane integrity (Fig. 5E) and cellular ATP levels (Fig. 5F). Notably, ECPs of AR119 also induced a loss of ATP in AB.9 cells from zebra fish (Fig. 5G).
FIG 5.
P. damselae subsp. damselae toxins cause fulminant membrane damage. (A) HaCaT cells were infected with washed wild-type (WT) or triple mutant (TM) P. damselae subsp. damselae at a multiplicity of infection (MOI) of 1:30, incubated for 30 min at 37°C, stained for 1 min with PI (50 μg/ml), and subsequently fixed and stained with Hoechst 33342. Bars = 50 μm. Representative images are shown. (B) HaCaT cells were incubated for 8 min with ECPs (6 μg/ml total protein) from the WT or TM strain and stained as described for panel A, and PI-positive cells in digital microscopic images were enumerated. The graph shows mean values ± SE for 4 independent experiments. ***, P ≤ 0.001 in two-sided, unpaired Student's t test. (C) HaCaT cells were incubated with ECPs of the wild-type or triple mutant strain. After 1 h, the cellular ATP level was determined. Data are mean values ± SE (n = 4). ***, P ≤ 0.001 in two-sided, unpaired Student's t test. (D) Cells were incubated with normal medium or ECPs for 48 h and then processed for flow cytometric analysis of DNA content. Columns show numbers of sub-G1 events. Data shown are mean values ± SE (n = 3). *, P ≤ 0.05 in two-sided, unpaired Student's t test. (E) HaCaT cells were incubated for 1 h at 37°C with ECPs containing single toxins, as indicated. Control, medium only. Cells were stained with trypan blue (TB), and TB-positive and -negative cells were counted in a Neubauer chamber. Data are mean values ± SE (n = 3). ***, P ≤ 0.001 in two-sided, unpaired Student's t test. HaCaT (F) and AB.9 (G) cells were treated as described for panel E prior to measurement of ATP. Graphs show mean values ± SE (n = 3). ***, P ≤ 0.001 in two-sided, unpaired Student's t test.
Hemolysins promote bacterial adherence.
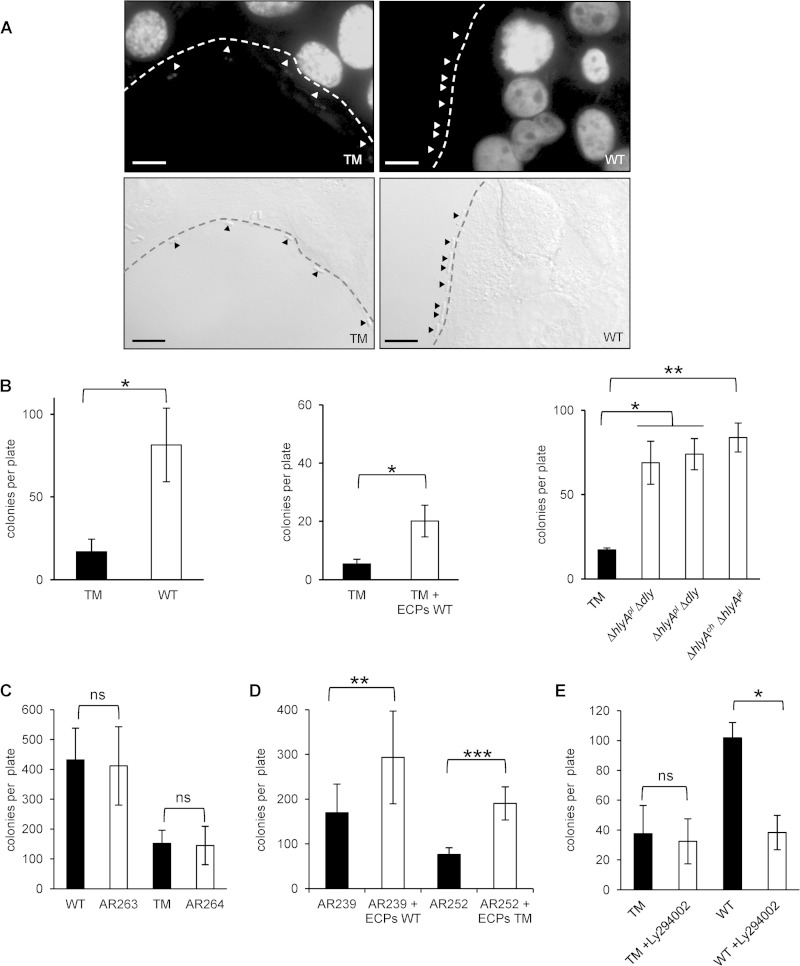
When analyzing mammalian cells infected with P. damselae subsp. damselae by differential interference contrast microscopy, we made the fortuitous observation that P. damselae subsp. damselae WT bacteria decorated the surfaces of HaCaT cells, whereas comparably few bacteria of the TM strain adhered to cells (Fig. 6A).
FIG 6.
Hemolysins promote bacterial adherence to target cells. (A) HaCaT cells were infected with the P. damselae subsp. damselae WT (AR57) strain or TM (AR89) strain. The distribution of bacteria was analyzed by differential interference contrast microscopy. Bars = 10 μm. (B) Results of adherence assays based on CFU counts. (Left) HaCaT cells infected with P. damselae subsp. damselae TM or WT. (Middle) HaCaT cells infected with P. damselae subsp. damselae TM and treated (white columns) or not (black column) with ECPs obtained from the WT strain (6 μg/ml total protein). (Right) HaCaT cells infected with the TM (black column) or double mutant strains, each expressing one of the known hemolysins only (white columns). Data shown are mean values ± SE (n = 3). * (left and middle panels), P ≤ 0.05 as determined by two-sided, unpaired Student's t test; * and ** (right panel), P ≤ 0.05 and P ≤ 0.01, respectively, as determined by ANOVA. (C) Results of adherence assays based on CFU counts. The bars show mean numbers of CFU (±SE [n = 3]) determined for samples of HaCaT cells infected with P. damselae subsp. damselae WT or TM (black) and their respective cheA mutants. ns, nonsignificant, i.e., P ≥ 0.05, in two-sided, unpaired Student's t tests. (D) HaCaT cells were infected with P. damselae subsp. damselae AR239 (ΔpilD) or AR252 (ΔpilD ΔtadV) in the presence of ECPs from the WT strain. For determination of the numbers of adherent AR239 and AR252 bacteria, a 10-fold larger volume of the harvested coculture than that of all other strains analyzed here was plated for CFU counts. Data shown are mean values ± SE (n = 3). ** and ***, P ≤ 0.01 and P ≤ 0.001, respectively, in two-sided, unpaired Student's t tests. (E) HaCaT cells were infected with the TM and WT strains in the presence of Ly294002 (1 μM). Data are mean values ± SE (n = 3). ns, nonsignificant; *, P ≤ 0.05 in two-sided, unpaired Student's t test.
To verify this unexpected result by using an independent approach and to quantify the effect, we enumerated bacteria adhering to cells by a culture-based assay. Severalfold larger numbers of bacteria (CFU) were obtained with WT bacteria than with TM bacteria, suggesting that hemolysins conferred adherence to cells (Fig. 6B). Addition of ECPs of the WT strain to TM bacteria reconstituted the adherent phenotype. Using double mutant bacteria, we found that each of the three known hemolysins was capable of enhancing bacterial adherence to cells (Fig. 6B).
In order to clarify whether hemolysins of P. damselae subsp. damselae also promoted adherence of other bacteria to HaCaT cells, we tested the effect of WT ECPs on adherence of E. coli. The results obtained with this combination suggested that the activity of ECPs did not depend on some P. damselae subsp. damselae-specific, nonsecreted factor(s) (see Fig. S2A, left panel, in the supplemental material). Next, we asked whether purified pVCC could also enhance adherence of E. coli to HaCaT cells; the confirmatory results are shown in Fig. S2A, right panel.
Up to this point, all adherence assays had been performed with E. coli or P. damselae subsp. damselae, i.e., Gram-negative, motile bacteria. Flagellated bacteria may move along concentration gradients of nutrients or ions (48), which might form when the nutrients or ions leak from target cells of PFTs. To investigate whether the apparent hemolysin-dependent attachment of bacteria to cells depended on bacterial chemotaxis, we extended our experiments to include S. aureus, a Gram-positive, nonmotile species. As shown in Fig. S2B in the supplemental material, ECPs of P. damselae subsp. damselae, purified pVCC, and alpha-toxin all enhanced the adherence of an alpha-toxin-negative S. aureus strain, suggesting that hemolysin-dependent adherence did not depend on bacterial chemotaxis but not excluding a role of chemotaxis in the case of hemolysin-dependent association of motile bacteria with target cells. Therefore, we deleted the cheA gene from the P. damselae subsp. damselae WT and TM strains. CheA is a histidine kinase that senses changes in the concentration of nutrients through transmembrane chemoreceptors and leads to flagellar motor switching (49). Among the three cheA genes carried on the Vibrio cholerae chromosome, only cheA2 is directly related to chemotaxis (50). Interestingly, only one cheA homologue was found to be carried on the P. damselae subsp. damselae chromosome. Although deletion of cheA decreased the spread of both the P. damselae subsp. damselae WT and TM strains on semisolid agar plates (see Fig. S2C), as expected, no significant effect on the association of P. damselae subsp. damselae with HaCaT cells was observed (Fig. 6C). This indicated that the enhanced association of bacteria and target cells was independent of chemotaxis. Hence, it likely reflected increased adherence. Bacterial adherence commonly involves interactions between pili and host cells. The role of type IV pili in adherence to host cells has been demonstrated for many Gram-negative bacteria (51–53). Deletion of the P. damselae subsp. damselae prepilin peptidase gene pilD severely affects hemolysin secretion and prevents pilus formation (15). In addition to pilD, the gene encoding TadV, another prepilin peptidase, which belongs to the tight adherence (tad) cluster carried on pPHDD1, is involved in the production of type IV pili (54). TadV partially compensates for PilD and is responsible for the residual release of hemolytic activity observed with ΔpilD mutants (15). Therefore, we investigated whether exogenous hemolysins are able to promote the association of target cells with P. damselae subsp. damselae strains lacking either pilD or both pilD and tadV. As expected, ΔpilD bacteria showed a marked (∼10-fold) decrease of adherence, and complementation of the ΔpilD mutant with the pilD gene restored the ability to produce pili and large hemolytic halos (15). Although the number of pilus-negative bacteria adhering to target cells was much reduced, the addition of WT ECPs increased adherence of both the ΔpilD single mutant and the ΔpilD ΔtadV double mutant (Fig. 6D). The fold increase was similar to that for pilus-expressing wild-type bacteria, indicating that the adherence-enhancing effect of hemolysins did not depend on pili.
Next, we wished to address the question of whether the effect of membrane-damaging toxins on the increased adherence of bacteria depended on active responses of target cells. The observation that an alpha-toxin with a single amino acid mutation, with a far lower hemolytic activity than that of wild-type toxin, exerted a weaker effect on adherence than that of the wild-type toxin (see Fig. S2B, right panel, in the supplemental material) indicated that membrane perforation is involved. This would be expected, for instance, if signaling pathways triggered by membrane perforation played a role. By phosphorylating phosphatidylinositols at the 3′-OH position of the inositol ring, phosphatidylinositol 3-kinases (PI3Ks) modulate the composition and function of biological membranes of eukaryotic cells in response to many types of stress. Therefore, we investigated the effect of low-molecular-weight inhibitors of PI3Ks on toxin-dependent bacterial adherence. Three functional classes of PI3Ks are distinguished based on their preferred substrates. ZSTK474, a class I/II-selective PI3K inhibitor, did not reduce hemolysin-dependent adherence (data not shown), but Ly294002, which also inhibits class III PI3K, led to a significant inhibition of adherence (Fig. 6E). Therefore, hemolysin-dependent increases of bacterial adherence to target cells appear to depend on class III PI3K-mediated cellular responses.
DISCUSSION
We report here the primary characterization of PhlyP, a major contributor to the virulence of P. damselae subsp. damselae (14). PhlyP is encoded on a plasmid that is found in ∼20% of environmental isolates of P. damselae subsp. damselae (13). It is conceivable that the plasmid spreads to other bacterial species (9). Because P. damselae subsp. damselae has the potential to cause serious infections, it is relevant to elucidate the basis of its virulence. The characterization of PhlyP represents an important step toward this goal. We showed that PhlyP is a small β-PFT, with distinct properties, which exerts multiple toxic effects on nucleated target cells. Further, the results led to the conclusion that hemolysins are general promoters of bacterial adherence to target cells.
The observation that PhlyP is a small pore-forming protein was predicted from nucleic acid sequences. Several lines of experimental evidence, including functional (osmoprotection), morphological (circular structures in electron micrographs), and biochemical (SDS-stable complexes) evidence, support this contention. Also, in line with previous observations with V. cholerae cytolysin (VCC), cholesterol depletion in red cells reduced the lytic activity of PhlyP. Although PhlyP thus shares typical characteristics with other members of the small β-PFT family, there are substantial differences even between PhlyP and VCC, the closest known ortholog, with 50% identity at the amino acid level. First, the PhlyP transmembrane channel is wider than the VCC channel, in line with the fact that the narrow point in the VCC β-barrel is formed by a heptad of tryptophan residues (W318) (55), whereas the corresponding position in PhlyP is a serine (S341), which is less bulky. A second distinguishing feature of PhlyP that deserves mentioning is the absence of a second lectin domain. VCC contains two contiguous lectin domains: the so-called β-trefoil domain and the more C-terminal β-prism domain (55). Deletion of the β-prism domain virtually eliminated hemolytic activity (56), which led to the conclusion that the β-trefoil domain in VCC is inactive (57). Although devoid of the β-prism domain, PhlyP applied at nanomolar concentrations kills mammalian cells, suggesting that its trefoil domain is active. In support of this, an amino acid substitution in the β-trefoil domain renders PhlyP substantially less hemolytic, but the activity is fully recovered by replacing the defective domain with the wild-type sequence (13). Notably, PhlyP's overall structure resembles that of Vibrio vulnificus cytolysin (VVC) in that it too lacks a C-terminal lectin domain, but the primary sequences of PhlyP and VCC are more closely related (57), underscoring the remarkable diversification of small β-PFTs.
Loss of membrane integrity after attack by PhlyP was followed by a broad range of toxic effects in HaCaT cells, all of which might contribute to the severe consequences of infection by P. damselae subsp. damselae. The loss of ATP (Fig. 3B and 4D) and the change in shape of mitochondria (Fig. 4G) suggest that target cells experience metabolic stress. Modulation of actin and cytokeratin filaments (Fig. 4G; see Fig. S2D in the supplemental material) might affect epithelial integrity and possibly change adhesive properties (58, 59). Interestingly, direct interaction between a PFT and actin has been reported (60). Sustained attenuation of translation via inactivation of mTORC1 and/or hyperphosphorylation of eIF2α will ultimately lead to cell death (45, 61). Notably, phosphorylation of eIF2α is critically involved in regulation of autophagy, a conserved cellular recycling pathway known to be induced by PFTs, although its role in this context remains incompletely understood. The implication in bacterial adherence of PI3K, which is an established master regulator of autophagy, raises the intriguing question of whether autophagy and adherence are mechanistically linked.
The effect of hemolysins on the association of bacteria with cells was unexpected, but a directed search of the literature showed that the phenomenon is not unprecedented (62–65). Our findings add important new information by showing (i) that hemolysins increase the association of Gram-positive or Gram-negative bacteria with mammalian cells, (ii) that the effect does not depend on chemotaxis, (iii) that it is independent of pili, and (iv) that it depends on an active response of target cells, which is apparently under the control of class III PI3Ks. Because the virulence of bacteria correlates with levels of adherence to cultured cells (66, 67), the results call for further studies along these lines.
Supplementary Material
ACKNOWLEDGMENTS
A.J.R. was supported by a postdoctoral grant awarded by FEMS and by grants from the Ministry of Economy and Competitiveness (MINECO) of Spain, cofunded by the FEDER Programme from the European Union, to M.L.L. (grant AGL2012-39274-C02-01) and C.R.O. (grant AGL2013-48353-R). G.V.H. and M.H. received support from the University Medical Center Mainz, and Q.Q. received support from the Chinese Scholarship Council. Parts of this work were done in partial fulfilment of the doctoral thesis of Q.Q.
We thank Rudolf E. Leube for generously providing AK13-1 cells, Fatima Boukhallouk for valuable technical tips, and Monica Wiedmann for critical comments on the manuscript. We gratefully acknowledge generous support by Andreas Diefenbach, University Medical Center Mainz.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00277-15.
REFERENCES
- 1.Álvarez JR, Lamba S, Dyer KY, Apuzzio JJ. 2006. An unusual case of urinary tract infection in a pregnant woman with Photobacterium damsela. Infect Dis Obstet Gynecol 2006:80682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarridge JE, Zighelboim-Daum S. 1985. Isolation and characterization of two hemolytic phenotypes of Vibrio damsela associated with a fatal wound infection. J Clin Microbiol 21:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodell KH, Jordan MR, Graham R, Cassidy C, Nasraway SA. 2004. Rapidly advancing necrotizing fasciitis caused by Photobacterium (Vibrio) damsela: a hyperaggressive variant. Crit Care Med 32:278–281. doi: 10.1097/01.CCM.0000104920.01254.82. [DOI] [PubMed] [Google Scholar]
- 4.Kim HR, Kim JW, Lee MK, Kim JG. 2009. Septicemia progressing to fatal hepatic dysfunction in an cirrhotic patient after oral ingestion of Photobacterium damsela: a case report. Infection 37:555–556. doi: 10.1007/s15010-009-9049-8. [DOI] [PubMed] [Google Scholar]
- 5.Yamane K, Asato J, Kawade N, Takahashi H, Kimura B, Arakawa Y. 2004. Two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. J Clin Microbiol 42:1370–1372. doi: 10.1128/JCM.42.3.1370-1372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivas AJ, Lemos ML, Osorio CR. 2013. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol 4:283. doi: 10.3389/fmicb.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alter T, Appel B, Bartelt E, Dieckmann R, Eichhorn C, Erler R, Frank C, Gerdts G, Gunzer F, Huhn S, Neifer J, Oberheitmann B, Strauch E. 2011. Vibrio infections from food and sea water. Introducing the “VibrioNet.” Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54:1235–1240. [DOI] [PubMed] [Google Scholar]
- 8.Vezzulli L, Colwell RR, Pruzzo C. 2013. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol 65:817–825. doi: 10.1007/s00248-012-0163-2. [DOI] [PubMed] [Google Scholar]
- 9.Rivas AJ, Balado M, Lemos ML, Osorio CR. 2011. The Photobacterium damselae subsp. damselae hemolysins damselysin and HlyA are encoded within a new virulence plasmid. Infect Immun 79:4617–4627. doi: 10.1128/IAI.05436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothary MH, Kreger AS. 1985. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infect Immun 49:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreger AS, Bernheimer AW, Etkin LA, Daniel LW. 1987. Phospholipase D activity of Vibrio damsela cytolysin and its interaction with sheep erythrocytes. Infect Immun 55:3209–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutter DL, Kreger AS. 1990. Cloning and expression of the damselysin gene from Vibrio damsela. Infect Immun 58:266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas AJ, Labella AM, Borrego JJ, Lemos ML, Osorio CR. 2014. Evidence for horizontal gene transfer, gene duplication and genetic variation as driving forces of the diversity of haemolytic phenotypes in Photobacterium damselae subsp. damselae. FEMS Microbiol Lett 355:152–162. doi: 10.1111/1574-6968.12464. [DOI] [PubMed] [Google Scholar]
- 14.Rivas AJ, Balado M, Lemos ML, Osorio CR. 2013. Synergistic and additive effects of chromosomal and plasmid-encoded hemolysins contribute to hemolysis and virulence in Photobacterium damselae subsp. damselae. Infect Immun 81:3287–3299. doi: 10.1128/IAI.00155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivas AJ, Vences A, Husmann M, Lemos ML, Osorio CR. 2015. Photobacterium damselae subsp. damselae major virulence factors Dly, plasmid-encoded HlyA, and chromosome-encoded HlyA are secreted via the type II secretion system. Infect Immun 83:1246–1256. doi: 10.1128/IAI.02608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacovache I, van der Goot FG, Pernot L. 2008. Pore formation: an ancient yet complex form of attack. Biochim Biophys Acta 1778:1611–1623. doi: 10.1016/j.bbamem.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Tilley SJ, Saibil HR. 2006. The mechanism of pore formation by bacterial toxins. Curr Opin Struct Biol 16:230–236. doi: 10.1016/j.sbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Bhakdi S, Tranum-Jensen J. 1986. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog 1:5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard JL, Berche P, Sansonetti P. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun 52:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamon MA, Ribet D, Stavru F, Cossart P. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol 20:360–368. doi: 10.1016/j.tim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Krantz BA, Finkelstein A, Collier RJ. 2006. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol 355:968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Los FC, Randis TM, Aroian RV, Ratner AJ. 2013. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev 77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy SK, O'Riordan MX. 2013. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins (Basel) 5:618–636. doi: 10.3390/toxins5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jursch R, Hildebrand A, Hobom G, Tranum-Jensen J, Ward R, Kehoe M, Bhakdi S. 1994. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect Immun 62:2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson R, Gouaux E. 2005. Crystal structure of the Vibrio cholerae cytolysin (VCC) pro-toxin and its assembly into a heptameric transmembrane pore. J Mol Biol 350:997–1016. doi: 10.1016/j.jmb.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 26.Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S. 2009. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett 583:337–344. doi: 10.1016/j.febslet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M. 2010. Pro-autophagic signal induction by bacterial pore-forming toxins. Med Microbiol Immunol 199:299–309. doi: 10.1007/s00430-010-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitzer A, Palmer M, Weller U, Wassenaar T, Biermann C, Tranum-Jensen J, Bhakdi S. 1997. Mode of primary binding to target membranes and pore formation induced by Vibrio cholerae cytolysin (hemolysin). Eur J Biochem 247:209–216. doi: 10.1111/j.1432-1033.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- 29.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badakov R, Jazwinska A. 2006. Efficient transfection of primary zebrafish fibroblasts by nucleofection. Cytotechnology 51:105–110. doi: 10.1007/s10616-006-9018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugwitz U, Bobkiewicz W, Han SR, Beckmann E, Veerachato G, Shaid S, Biehl S, Dersch K, Bhakdi S, Husmann M. 2006. Pore-forming Staphylococcus aureus alpha-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell Microbiol 8:1591–1600. doi: 10.1111/j.1462-5822.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 32.Husmann M, Dersch K, Bobkiewicz W, Beckmann E, Veerachato G, Bhakdi S. 2006. Differential role of p38 mitogen activated protein kinase for cellular recovery from attack by pore-forming S. aureus alpha-toxin or streptolysin O. Biochem Biophys Res Commun 344:1128–1134. doi: 10.1016/j.bbrc.2006.03.241. [DOI] [PubMed] [Google Scholar]
- 33.González MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG. 2011. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol 13:1026–1043. doi: 10.1111/j.1462-5822.2011.01600.x. [DOI] [PubMed] [Google Scholar]
- 34.Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer M, Weller U, Messner M, Bhakdi S. 1993. Altered pore-forming properties of proteolytically nicked staphylococcal alpha-toxin. J Biol Chem 268:11963–11967. [PubMed] [Google Scholar]
- 36.Lobo AL, Welch RA. 1994. Identification and assay of RTX family of cytolysins. Methods Enzymol 235:667–678. doi: 10.1016/0076-6879(94)35180-5. [DOI] [PubMed] [Google Scholar]
- 37.Lohner S, Walev I, Boukhallouk F, Palmer M, Bhakdi S, Valeva A. 2009. Pore formation by Vibrio cholerae cytolysin follows the same archetypical mode as beta-barrel toxins from gram-positive organisms. FASEB J 23:2521–2528. doi: 10.1096/fj.08-127688. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert RJ. 2010. Cholesterol-dependent cytolysins. Adv Exp Med Biol 677:56–66. doi: 10.1007/978-1-4419-6327-7_5. [DOI] [PubMed] [Google Scholar]
- 39.Harris JR, Palmer M. 2010. Cholesterol specificity of some heptameric beta-barrel pore-forming bacterial toxins: structural and functional aspects. Subcell Biochem 51:579–596. doi: 10.1007/978-90-481-8622-8_21. [DOI] [PubMed] [Google Scholar]
- 40.Nagamune K, Yamamoto K, Naka A, Matsuyama J, Miwatani T, Honda T. 1996. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect Immun 64:4655–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valeva A, Walev I, Weis S, Boukhallouk F, Wassenaar TM, Endres K, Fahrenholz F, Bhakdi S, Zitzer A. 2004. A cellular metalloproteinase activates Vibrio cholerae pro-cytolysin. J Biol Chem 279:25143–25148. doi: 10.1074/jbc.M313913200. [DOI] [PubMed] [Google Scholar]
- 42.Fouz B, Barja JL, Amaro C, Rivas C, Toranzo AE. 1993. Toxicity of the extracellular products of Vibrio damsela isolated from diseased fish. Curr Microbiol 27:341–347. doi: 10.1007/BF01568958. [DOI] [Google Scholar]
- 43.Bischofberger M, Iacovache I, van der Goot FG. 2012. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12:266–275. doi: 10.1016/j.chom.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Kloft N, Busch T, Neukirch C, Weis S, Boukhallouk F, Bobkiewicz W, Cibis I, Bhakdi S, Husmann M. 2009. Pore-forming toxins activate MAPK p38 by causing loss of cellular potassium. Biochem Biophys Res Commun 385:503–506. doi: 10.1016/j.bbrc.2009.05.121. [DOI] [PubMed] [Google Scholar]
- 45.Kloft N, Neukirch C, von Hoven G, Bobkiewicz W, Weis S, Boller K, Husmann M. 2012. A subunit of eukaryotic translation initiation factor 2alpha-phosphatase (CreP/PPP1R15B) regulates membrane traffic. J Biol Chem 287:35299–35317. doi: 10.1074/jbc.M112.379883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemaitre B, Girardin SE. 2013. Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nat Rev Microbiol 11:365–369. doi: 10.1038/nrmicro3029. [DOI] [PubMed] [Google Scholar]
- 47.Stavru F, Cossart P. 2011. Listeria infection modulates mitochondrial dynamics. Commun Integr Biol 4:364–366. doi: 10.4161/cib.4.3.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sourjik V, Wingreen NS. 2012. Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol 24:262–268. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 50.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol 3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paranjpye RN, Strom MS. 2005. A Vibrio vulnificus type IV pilin contributes to biofilm formation, adherence to epithelial cells, and virulence. Infect Immun 73:1411–1422. doi: 10.1128/IAI.73.3.1411-1422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn HP. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa: a review. Gene 192:99–108. doi: 10.1016/S0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 53.Kallstrom H, Blackmer Gill D, Albiger B, Liszewski MK, Atkinson JP, Jonsson AB. 2001. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell Microbiol 3:133–143. doi: 10.1046/j.1462-5822.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 54.Tomich M, Planet PJ, Figurski DH. 2007. The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol 5:363–375. doi: 10.1038/nrmicro1636. [DOI] [PubMed] [Google Scholar]
- 55.De S, Olson R. 2011. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc Natl Acad Sci U S A 108:7385–7390. doi: 10.1073/pnas.1017442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazumdar B, Ganguly S, Ghosh AN, Banerjee KK. 2011. The role of C-terminus carbohydrate-binding domain of Vibrio cholerae haemolysin/cytolysin in the conversion of the pre-pore beta-barrel oligomer to a functional diffusion channel. Indian J Med Res 133:131–137. [PMC free article] [PubMed] [Google Scholar]
- 57.Kaus K, Lary JW, Cole JL, Olson R. 2014. Glycan specificity of the Vibrio vulnificus hemolysin lectin outlines evolutionary history of membrane targeting by a toxin family. J Mol Biol 426:2800–2812. doi: 10.1016/j.jmb.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knutton S, Baldwin T, Williams PH, McNeish AS. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun 57:1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batchelor M, Guignot J, Patel A, Cummings N, Cleary J, Knutton S, Holden DW, Connerton I, Frankel G. 2004. Involvement of the intermediate filament protein cytokeratin-18 in actin pedestal formation during EPEC infection. EMBO Rep 5:104–110. doi: 10.1038/sj.embor.7400038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hupp S, Förtsch C, Wippel C, Ma J, Mitchell TJ, Iliev AI. 2013. Direct transmembrane interaction between actin and the pore-competent, cholesterol-dependent cytolysin pneumolysin. J Mol Biol 3:636–646. doi: 10.1016/j.jmb.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakrabarti S, Liehl P, Buchon N, Lemaitre B. 2012. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe 12:60–70. doi: 10.1016/j.chom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Krawczyk-Balska A, Bielecki J. 2005. Listeria monocytogenes listeriolysin O and phosphatidylinositol-specific phospholipase C affect adherence to epithelial cells. Can J Microbiol 51:745–751. doi: 10.1139/w05-058. [DOI] [PubMed] [Google Scholar]
- 63.Lucas EA, Billington SJ, Carlson P, McGee DJ, Jost BH. 2010. Phospholipase D promotes Arcanobacterium haemolyticum adhesion via lipid raft remodeling and host cell death following bacterial invasion. BMC Microbiol 10:270. doi: 10.1186/1471-2180-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seitz M, Baums CG, Neis C, Benga L, Fulde M, Rohde M, Goethe R, Valentin-Weigand P. 2013. Subcytolytic effects of suilysin on interaction of Streptococcus suis with epithelial cells. Vet Microbiol 167:584–591. doi: 10.1016/j.vetmic.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Vadia S, Arnett E, Haghighat AC, Wilson-Kubalek EM, Tweten RK, Seveau S. 2011. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog 7:e1002356. doi: 10.1371/journal.ppat.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cifrian E, Guidry AJ, O'Brien CN, Marquardt WW. 1995. Effect of alpha-toxin and capsular exopolysaccharide on the adherence of Staphylococcus aureus to cultured teat, ductal and secretory mammary epithelial cells. Res Vet Sci 58:20–25. doi: 10.1016/0034-5288(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 67.Dunn KL, Virji M, Moxon ER. 1995. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb Pathog 18:81–96. doi: 10.1016/S0882-4010(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 68.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt EK, Clavarino G, Ceppi M, Pierre P. 2009. SUnSET, a non-radioactive method to monitor protein synthesis. Nat Methods 6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 70.Herrero M, De Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J Bacteriol 172:6557–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parales RE, Harwood CS. 1993. Construction and use of a new broad-host range lacZ transcriptional fusion vector, pHRP309, for Gram− bacteria. Gene 133:23–30. doi: 10.1016/0378-1119(93)90220-W. [DOI] [PubMed] [Google Scholar]
- 72.Mouriño S, Osorio CR, Lemos ML. 2004. Characterization of heme uptake cluster genes in the fish pathogen Vibrio anguillarum. J Bacteriol 186:6159–6167. doi: 10.1128/JB.186.18.6159-6167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.