Abstract
We previously identified the protein Tet38 as a chromosomally encoded efflux pump of Staphylococcus aureus that confers resistance to tetracycline and certain unsaturated fatty acids. Tet38 also contributes to mouse skin colonization. In this study, we discovered a novel regulator of tet38, named tetracycline regulator 21 (TetR21), that bound specifically to the tet38 promoter and repressed pump expression. A ΔtetR21 mutant showed a 5-fold increase in tet38 transcripts and an 8-fold increase in resistance to tetracycline and fatty acids. The global regulator MgrA bound to the tetR21 promoter and indirectly repressed the expression of tet38. To further assess the full role of Tet38 in S. aureus adaptability, we tested its effect on host cell invasion using A549 (lung) and HMEC-1 (heart) cell lines. We used S. aureus RN6390, its Δtet38, ΔtetR21, and ΔmgrA mutants, and a Δtet38 ΔtetR21 double mutant. After 2 h of contact, the Δtet38 mutant was internalized in 6-fold-lower numbers than RN6390 in A549 and HMEC-1 cells, and the ΔtetR21 mutant was internalized in 2-fold-higher numbers than RN6390. A slight increase of 1.5-fold in internalization was found for the ΔmgrA mutant. The growth patterns of RN6390 and the ΔmgrA and ΔtetR21 mutants within A549 cells were similar, while no growth was observed for the Δtet38 mutant. These data indicate that the Tet38 efflux pump is regulated by TetR21 and contributes to the ability of S. aureus to internalize and replicate within epithelial cells.
INTRODUCTION
Efflux mechanisms are widely recognized as major contributors to resistance to many classes of antimicrobial agents via a diverse group of transporters also called efflux pumps (1, 2). These pumps are implicated in a variety of physiological roles, including the extrusion of drugs and other natural substrates (3, 4). Variations in the expression of efflux pumps can be influenced by several factors, such as antibiotics, natural compounds, and environmental conditions. Examples of such induction phenomena were reported in the expression of the efflux pump genes norA, norB, and tet38 of Staphylococcus aureus in response to low free iron, acidity/low-oxygen conditions, and tetracycline and fatty acids, respectively (5–7). Recently, the resistance-nodulation-division (RND) family efflux pump FarE of the fatty acid resistance system farR-farE of S. aureus was reported to be induced by linoleic and arachidonic acids (8). The overexpressed FarE efflux pump extrudes and confers resistance to these two fatty acids. FarE expression is controlled by the regulator FarR, a member of the AcrR family of regulators (8). Several efflux pumps, such as CmeAB of Campylobacter jejuni and AcrAB of Escherichia coli, have also been shown to have dual functions, conferring resistance to antimicrobial compounds and contributing to the bacterium's ability to invade and survive in host cells or specific host environments (9, 10).
In our previous studies, we demonstrated that Tet38, a chromosomally encoded efflux pump of S. aureus, causes resistance to tetracycline and fatty acids and contributes to the ability of S. aureus to colonize mouse skin, as well as survive in the environment of an abscess (7, 11). We also demonstrated that the global regulator MgrA was an indirect negative regulator of tet38 expression (12). The regulation of tet38 expression and its role in S. aureus colonization are not well understood. S. aureus expresses agr-, sar-, and sae-dependent surface components, such as fibronectin-binding proteins, that enable it to adhere to and invade the host cells by binding to the host cell α5β1 integrins (13–15). The interaction of S. aureus with host cells provokes the rearrangement of the host cell actin cytoskeleton, which leads to the internalization of the pathogen into these cells (16). S. aureus is internalized in epithelial cells in a time- and dose-dependent manner. This invasion and the subsequent bacterial intracellular replication result in cell apoptosis (17–19). Although fibronectin-binding proteins have been shown to be important for the internalization of S. aureus into epithelial cells (13, 20), mutants lacking these proteins had residual binding and internalization capabilities, suggesting that other factors were also involved. We show here that Tet38 also contributes to both internalization and survival within epithelial cells and that its expression is regulated by, in addition to MgrA, a newly characterized transcriptional regulator, tetracycline regulator 21 (TetR21), which also mediates the induction of tet38 by tetracycline and select fatty acids.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains were cultivated in Trypticase soy broth (TSB) (Difco, Sparks, MD) at 37°C, unless otherwise stated. E. coli strains were grown at 37°C in Luria-Bertani (LB) broth containing ampicillin (100 μg/ml). Bacterial strains and plasmids are listed in Table 1.
TABLE 1.
Bacterial strains, plasmids, and cell lines used in this study
| Strain, plasmid, or cell line | Genotype or relevant characteristic(s) | Reference or source |
|---|---|---|
| S. aureus strains | ||
| RN6390 | 8325-4 wild type | 22 |
| QT1 | 8325-4 mgrA::cat or ΔmgrA | 25 |
| QT5 | 8325-4 norB::cat | 12 |
| QT7 | 8325-4 tet38::cat or Δtet38 | 12 |
| QT21 | 8325-4 ΔtetR21 | This study |
| QT22 | QT7(pLI50-tet38) | This study |
| QT23 | 8325-4 tet38::cat ΔtetR21 | This study |
| RN6390(pLI50-tet38) | tet38 overexpressor | This study |
| QT1(pLI50-mgrA) | Complementing QT1 strain | This study |
| QT21(pLI50-tetR21) | Complementing QT21 strain | This study |
| MW2 | CA-MRSA (USA400 lineage) | 11 |
| MW2ΔtetR21 | CA-MRSA mutant, ΔtetR21 | This study |
| MW2Δtet38 | CA-MRSA mutant, Δtet38 | This study |
| E. coli strains | ||
| BL21(DE3) | E. coli B F− dcm ompT hsdS(rB− mB−) gal λ(DE3) | Stratagene |
| Top10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL(Strr) endA1 λ− | Invitrogen |
| DH10B | E. coli for cloning | 23 |
| BL21(DE3)(pTrcHisA-mgrA) | MgrA overexpressor | 25 |
| BL21(DE3)(pTrcHisA-tetR21) | TetR21 overexpressor | This study |
| Plasmids | ||
| pTrcHisA | Cloning and His tag-expressing vector in E. coli | Invitrogen |
| pIMAY | E. coli/S. aureus temp-sensitive plasmid | 23 |
| Cell lines | ||
| A549 | ATCC CCL-185, human lung adenocarcinoma | ATCC |
| HMEC-1 | Immortalized human dermal microvascular endothelial cell line | 27 |
Antibiotics, chemical compounds, and MICs.
Lysostaphin, tetracycline, chloramphenicol, erythromycin, ciprofloxacin, norfloxacin, anhydrotetracycline, lysostaphin, linoleic acid, palmitoleic acid, palmitic acid, and undecanoic acid were from Sigma Chemical Co., St. Louis, MO. Ampicillin and isopropyl-β-d-thiogalactopyranoside (IPTG) were from Fisher Scientific, Pittsburgh, PA. Protease inhibitor tablets were from Life Technologies, Grand Island, NY. The MICs of fatty acids and antibiotics were determined by TSB microdilution as described previously (21).
Induction of tet38 expression by fatty acids.
Cultures of S. aureus RN6390 and its mutants were inoculated from an overnight culture and incubated until the optical density at 600 nm (OD600) reached 0.5 (∼2.5 to 3 h). Linoleic acid, palmitoleic acid, palmitic acid, undecanoic acid, or tetracycline at concentrations of 0.5-fold, 0.25-fold, and 0.10-fold MIC for each strain were added to the culture, followed by incubation at 37°C for 1 h. At intervals of 10 min, 1 ml of the culture was collected and centrifuged at 15,000 × g in a microcentrifuge. An identical bacterial culture without fatty acid or tetracycline was incubated in parallel to serve as a control. Bacteria in cultures without antibacterial compounds grew slightly faster (OD600 of 0.60 after 3 h of growth) than the induced cultures. Bacteria were lysed, and RNA prepared for real-time quantitative reverse transcription (qRT-PCR) as previously described (22).
Real-time qRT-PCR.
Total S. aureus RNA was extracted from lysostaphin-treated cells using the RNeasy midi kit (Qiagen, Valencia, CA). cDNAs were synthesized using the Verso cDNA synthesis kit (Thermo Scientific, ABgene, Epsom, Surrey, United Kingdom), followed by real-time qRT-PCR assays using EvaGreen dye and the CFX96 real-time system (Bio-Rad, Hercules, CA). Primers designed for the qRT-PCR assays were synthesized at Tufts University, Boston, MA, and are listed in Table 2. The housekeeping gene gmk was used as an internal control. All samples were analyzed in triplicate, and expression levels normalized against gmk gene expression, which remained unchanged following exposure to fatty acids and tetracycline. The assays were repeated with three independent biological samples. Statistical analyses were performed based on the Student t test to determine the significance of the gene expression values.
TABLE 2.
Primers used in this study
| Purpose and primer or amplified gene | Direction | Sequence | Fragment size (bp) |
|---|---|---|---|
| Real-time qRT-PCR | |||
| gmk | Forward | TCAGGACCATCTGGAGTAGGTAAAG | 108 |
| Reverse | TTCACGGATTTGACGTGTTG | ||
| mgrA | Forward | AGCTGAAGCGACTTTGTCAGATGC | 110 |
| Reverse | AGCGTGAACGTTCCGAAGTCGA | ||
| tetR21 | Forward | ATTTTACTTACATTACGAGGATAAA | 100 |
| Reverse | GCGTAACAAATCAAATTGAGTATATG | ||
| tet38 | Forward | ATGAATGTTGAATATTCTAA | 106 |
| Reverse | TGGCTACAGAAATCAAT | ||
| Internal region SA0131-tet38 (SA0132) of S. aureus N315 | 200 | ||
| SA0131 | Forward | AGGTGTGTTCACAGTGAGCG | |
| tet38 | Reverse | CATCAGCAATGGCTACAGAA | |
| tetR21 cloning | 624 | ||
| tetR21-F(BamHI) | TATTTGGATCCTTGAAAGAAGATAGGCGAa | ||
| tetR21-R(EcoRI) | AGCGAGTAGAATTCTTAAGTTAGTGAATa | ||
| Promoter binding assays | |||
| Biotinylated-tet38PF | TACAATAATTTTTACTCAAA | 240 | |
| tet38-R | ATTGGTTTAATGTGTGGTGT | ||
| Biotinylated-norBPF | ATAAGGTAAGATAACTAGCA | 150 | |
| norB-R | ATCTCTATTTGCCTCCCTATA | ||
| Biotinylated-tetR21PF | TTTACCAATGTTAACATTTT | 320 | |
| tetR21-R | ACCTCCTTGTGTTATTGAAC | ||
| Biotinylated-mgrAPF | TACCGAATTCATTCATGATG | 320 | |
| mgrA-R | CTCCAGACATACTATCCGTT | ||
| S. aureus adhesins | |||
| fnbA-F | ATCCGCCGAACAACATACCT | 110 | |
| fnbA-R | TGAAAAGGTTAAAGCAGTGG | ||
| fnbB-F | GGAGCGGCCTCAGTATTCTT | 114 | |
| fnbB-R | AGCTGAACTCCCACTTTCC |
Restriction enzymes are underlined.
Transcript levels of fnbA and fnbB of S. aureus in vitro cultures.
We designed primers for real-time qRT-PCRs for the fnbA and fnbB genes based on DNA sequences of the published genome of S. aureus NCTC8325. RNAs were extracted from S. aureus strain RN6390 and mutant strains QT7 and QT21. Primers were synthesized by Tufts Core Facility (Table 2).
Construction of a tetR21 mutant.
We constructed a tetR21 in-frame deletion mutant based on the technique described by Monk et al. (23). The following primers were synthesized by the Tufts DNA Core Facility: forward primer 21AF/KpnI, 5′-ATCAGGTACCTTAACCCATTTAATTTTATTAGA-3′ (the KpnI restriction site is underlined); reverse primer 21BR, 5′-CTTCTTTCAAAAATAAATCAACCTCCTTGT-3′; forward primer 21CF, 5′-ACAAGGAGGTTGATTTATTTTTGAAAGAAGTAAGTAATACTCGCTTTTCC-3′, with the complementary sequence of primer 21BR (underlined); and reverse primer 21DR/SacI, 5′-TTTTGAGCTCAAATTAATATCACGCTTAGGTAAATT-3′ (the SacI restriction site is underlined). Primers 21AF/KpnI and 21BR were used to amplify a 429-bp sequence upstream from the open reading frame (ORF) SA2165, and a 459-bp downstream sequence was amplified by the pair 21CF and 21DR/SacI. The two PCR products were diluted 20-fold, and 1 μl of each was used as the template for a second PCR with primers 21AF/KpnI and 21DR/SacI. The 888-bp PCR product carried a deleted SA2165 gene with KpnI and SacI restriction sites in the flanking end regions. The PCR product was cloned into plasmid pIMAY and transformed into E. coli DH10B. The transformants were grown at 37°C on LB plates containing chloramphenicol at 10 μg/ml. The construct pIMAY-SA2165Δ was extracted and reintroduced into S. aureus strain RN4220 and then into S. aureus RN6390 for subsequent allelic exchange. S. aureus transformants were grown at 28°C in the presence of chloramphenicol at 10 μg/ml. DNA sequencing was performed at each transformation step to verify the plasmid construction. We named the new construct pIMAY-tetR21Δ. To integrate the construct pIMAY-tetR21 into the chromosome, the S. aureus RN6390 transformants were diluted between 10-fold and 1,000-fold and then plated with chloramphenicol at 10 μg/ml and grown at 37°C. We verified the absence of extrachromosomal plasmid by PCR using primers designed from the plasmid pIMAY. Colonies were selected, plated on brain heart infusion (BHI) agar plates supplemented with anhydrotetracycline at 1 μg/ml, and grown at 28°C for 48 h. Chloramphenicol-sensitive colonies were selected and verified by PCR of the flanking regions of ORF SA2165 and by DNA sequencing. The deletion mutant was named QT21.
We constructed the tet38-, mgrA-, and tetR21-complemented strains by introducing the plasmids pLI50-tet38, pLI50-mgrA, and pLI50-tetR21 into the mutants QT7, QT1, and QT21, respectively. The tet38-complemented strain was named QT22.
Construction of a Δtet38 ΔtetR21 double mutant by phage ϕ85 transduction.
tet38::cat from donor QT7 was transferred into the recipient tetR21 mutant by phage transduction using phage ϕ85, as previously described (12). The phage titer was 108 PFU/ml. Colonies of interest were selected on Trypticase soy agar (TSA) plates containing sodium citrate (10 μg/ml) and chloramphenicol (5 μg/ml) and were characterized by restriction mapping and Southern hybridization analysis. The double mutant was named QT23.
DNA mobility shift analyses.
Primers designed to amplify the putative promoter regions of norB, tet38, tetR21, and mgrA are listed in Table 2. The sense primers were biotinylated by the Tufts University Core Facility (Boston, MA). The promoter of mgrA was designed based on data published by Ingavale et al. (24).
To obtain the TetR21 protein for the gel shift binding assays, the tetR21 gene was amplified from the genome of S. aureus RN6390 using primers tetR21-F and tetR21-R (Table 2). The PCR product was then cloned into plasmid pTrcHisA for protein expression as previously done for the MgrA protein (25). DNA sequencing was carried out to verify the DNA sequence of the cloned gene.
Purification of His-tagged TetR21 used Ni affinity chromatography. After column loading with cell lysate, the column was first washed with buffer A (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5% glycerol) containing 10 mM imidazole, and TetR21-His was then eluted with buffer A containing 100 mM imidazole. SDS-polyacrylamide gel electrophoresis (PAGE) indicated 95% homogeneity of the purified TetR21 protein. Purified TetR21 (200 ng, 7.2 pmol) was mixed with biotin-labeled promoter DNAs (4 ng, 20 fmol), and the gel mobility shift assays were carried out using a LightShift chemiluminescence electrophoretic mobility shift assay (EMSA) kit (Pierce, Rockford, IL), as recommended by the manufacturer. Following incubation, the binding mixture was analyzed by 5% nondenaturing PAGE as previously described (25). The optimum quantities of proteins and DNA were determined and subsequently used in assays determining the effects of tetracycline and fatty acids on the ability of TetR21 to bind to the tet38 promoter.
Tetracycline and fatty acids at concentrations 10-fold below their MICs were mixed with the TetR21 protein in buffer A supplemented with protease inhibitors (1 tablet per 50 ml of buffer A) (Life Technologies, Grand Island, NY) for 60 min prior to the gel shift binding assays with tet38 promoter. The protease inhibitor cocktail contained inhibitors of major proteases, such as serine, cysteine, and aspartic acid proteases, and aminopeptidase. The TetR21 protein used as a control without incubation with drugs was submitted to the same 60-min incubation with buffer A supplemented with protease inhibitors. To monitor any degradation of TetR21 following preincubation with drugs, the proteins were submitted to SDS-PAGE in parallel with the gel shift binding assays.
Invasion assays.
Invasion assays were performed using the ATCC CCL-185 human lung adenocarcinoma cell line A549 and the immortalized human microvascular endothelial cell line HMEC-1 (26, 27). The S. aureus parental strain RN6390 was compared with mutants QT1, QT5, QT7, and QT21. The tet38-complemented strain QT22 and the tet38 overexpressor RN6390(pLI50-tet38) were used as additional controls for the effect of tet38 on bacterial internalization. Strain MW2 was compared with RN6390 and with mutants MW2Δtet38 and MW2ΔtetR21 to verify the effects of tet38 on the clinical isolate and its mutants (Table 1). The assays were based on the technique described by Cheung and Bayles (28).
Bacterial preparation.
A 20-ml fresh culture of S. aureus was prepared from an overnight culture and grown to an OD600 of 0.4. The bacteria were washed twice with Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Fisher Scientific, Waltham, MA), referred to hereinafter as assay medium. The bacterial pellet was resuspended in a 20-ml volume of fresh assay medium.
Cell culture preparation.
A549 cells were cultured in assay medium in a 75-ml tissue culture flask until 90% confluence, as previously described by the manufacturer (ATCC), and were then seeded into 24-well plates (Costar) in assay medium and grown again to 90% confluence.
The HMEC-1 cells were cultured in the endothelial cell basal medium MCDB 131 supplemented with 10 mM l-glutamine and 10% fetal bovine serum (FBS) (Fisher Scientific, Waltham, MA).
Internalization assays.
Infections were initiated at a multiplicity of infection (MOI) of 10:1 (106 washed bacteria/105 cells), using bacteria in logarithmic growth phase (OD600 of 0.4). The 24-well plates were quickly centrifuged for 1 min at 500 × g to allow bacterial adhesion to the cell monolayer. The bacterium-cell mixtures were incubated at 37°C in 5% CO2 for 120 min. At intervals of 30, 60, and 120 min, infected monolayer cells in corresponding wells were washed twice with assay medium to remove residual nonadherent bacteria and then incubated for 60 min at 37°C in assay medium with 200 μg/ml gentamicin. The plates were washed again with fresh medium, and the epithelial cells were lysed with 250 μl of Triton X-100 (1×). The bacteria were diluted in phosphate-buffered saline (PBS) and plated on LB agar plates, and colony counts were performed to determine the numbers of viable intracellular bacteria.
Quantitation of internalized bacteria.
We carried out the experiments using the techniques described by Pfortner et al. with some modifications (29). Bacterial internalizations were performed as described above. After 45 min of bacterium-epithelial cell contact, gentamicin (200 μg/ml) was added to each well, followed by incubation at 37°C to eliminate the extracellular bacteria. For samples obtained at each time point from 0.5 h to 4 h, gentamicin was removed, and the cells were washed twice with assay medium and then lysed with 250 μl of Triton X-100 (1×), and colony counts were performed.
RESULTS
Identification of the tetR21 gene of S. aureus.
We searched the genome of S. aureus strain N315 for possible regulator(s) of the tet38 efflux pump and found two putative ORFs (SA2165 and SA2358) that were annotated as hypothetical proteins that showed similarity with the TetR transcriptional regulator family. We proceeded with the ORF SA2165, which encoded a putative protein with 207 amino acid residues and a predicted molecular mass of 24.5 kDa (GenBank accession number BAB43467). This hypothetical protein carries two regions that show amino acid motifs similar to the two conserved domains of the TetR transcriptional regulator family (pfam00440), which are the HTH-XRE superfamily and the TetR-C-8 superfamily. The first region (residues K20 to D60) was similar to the helix-turn-helix (HTH) portion of the N-terminal domain (E value of 4.27e−06), and the second region (residues N92 to W165) was similar to the transcriptional regulator C-terminal domain (E value of 1.44e−07) of the tetracycline regulator family. The SA2165 protein shows an overall similarity to the AcrR cluster of proteins (E value of 2.89e−12). All the E values reported from the BLAST search were minimal, which indicated a significant level of similarity between ORF SA2165 and the TetR regulator family. The AcrR protein is a repressor that regulates the expression of the multidrug efflux pump acrAB genes of E. coli under global stress conditions (30). Based on this similarity, we renamed ORF SA2165 as TetR21. Identical tetR21 genes are found in the genomes of over 3,000 S. aureus genomes already sequenced, including strain NCTC8325. The tetR21 gene from the genome of RN6390 (NCTC8325-4 background) was cloned, and the encoded protein expressed from plasmid pTrcHisA.
TetR21 affects the susceptibility of S. aureus to antibiotics and fatty acids.
We previously showed that overexpression of tet38 from plasmid pLI50-tet38 caused 5- to 8-fold increases in the MICs of palmitoleic and undecanoic acids and a 16-fold increase in the MIC of tetracycline (7). Relative to the MIC for the parental strain, the MIC for the tet38 mutant QT7 had a limited, 2-fold change for all compounds except norfloxacin and ciprofloxacin, suggesting limited expression of tet38 under basal conditions in vitro. Deletion of the tetR21 gene in the mutant QT21 resulted in 8-fold increases in the MICs of tetracycline and chloramphenicol, a 4-fold increase in the MIC of erythromycin, and 2-fold and 3-fold increases in the MICs of palmitoleic and undecanoic acid, respectively. Twofold increases in the MICs of norfloxacin and ciprofloxacin were also seen (Table 3).
TABLE 3.
Susceptibility of S. aureus strains to tetracycline, fatty acids, quinolones, chloramphenicol, and erythromycin
| Strain | MIC (μg/ml) ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Undecanoic acid | Palmitoleic acid | Linoleic acid | NOR | CIP | TET | CHL | ERY | |
| RN6390 | 500 | 500 | 1,000 | 0.5 | 0.25 | 0.06 | 4 | 0.06 |
| QT1 (ΔmgrA) | 1,500 | 1,500 | 2,500 | 4 | 2 | 4 | 4 | 0.06 |
| QT7 (Δtet38) | 250 | 250 | 500 | 0.5 | 0.25 | 0.03 | 2 | 0.03 |
| QT21 (ΔtetR21) | 1,500 | 1,000 | 2,000 | 1 | 0.5 | 0.5 | 32 | 0.25 |
| QT23 (Δtet38 ΔtetR21) | 250 | 500 | 1,000 | 1 | 0.5 | 0.06 | 32 | 0.25 |
| RN6390(pLI50) | 500 | 500 | 1,000 | 0.5 | 0.25 | 0.06 | 4 | 0.06 |
| QT1(pLI50) | 1,500 | 1,500 | 2,500 | 4 | 2 | 4 | 4 | 0.06 |
| QT1(pLI50-mgrA) | 500 | 500 | 1,000 | 0.5 | 0.25 | 0.06 | 4 | 0.06 |
| QT21(pLI50) | 1,500 | 1,000 | 2,000 | 1 | 0.5 | 0.5 | 32 | 0.25 |
| QT21(pLI50-tetR21) | 500 | 500 | 1,000 | 0.5 | 0.25 | 0.06 | 4 | 0.06 |
NOR, norfloxacin; CIP, ciprofloxacin; TET, tetracycline; CHL, chloramphenicol; ERY, erythromycin.
The double mutant QT23 (Δtet38 ΔtetR21) showed MICs of tetracycline and fatty acids with values similar to those of the tet38 mutant QT7. The MICs of chloramphenicol, erythromycin, ciprofloxacin, and norfloxacin remained the same as those of the tetR21 mutant QT21. These data suggest that TetR21 may regulate tet38 expression, the product of which confers resistance to tetracycline and fatty acids, but may also affect the expression of other determinants controlling the susceptibility to chloramphenicol and erythromycin.
Effect of tetR21 on the expression of tet38.
The effect of tetR21 on the transcript levels of tet38 was measured by real-time qRT-PCR using total RNAs from strain RN6390 and mutants QT7 (Δtet38), QT1 (ΔmgrA), and QT21 (ΔtetR21). Without induction by fatty acids or tetracycline, the transcript levels of tet38 increased 5-fold in both mutant QT1 and mutant QT21 compared to its level in RN6390 (Table 4). These data suggested that tet38 expression was under the control of MgrA and TetR21. No change was found in the transcript levels of tetR21 or mgrA in mutant QT1 and mutant QT21, respectively (Table 4). We further evaluated the transcriptional variation of other efflux pump genes in the absence of an intact TetR21 and found that the transcript levels of norA, norC, norD, mepA, and abcA were not affected in mutant QT21. One exception was norB, which showed a 2-fold increase in its transcripts (data not shown).
TABLE 4.
Differences in the tet38, tetR21, and mgrA transcript levels in S. aureus strains following exposure to fatty acids and tetracycline
| Strain, compound (MIC [μg/ml]) | Fold MIC used for induction | Relative expression (mean fold change ± SD) ofa: |
||
|---|---|---|---|---|
| tet38 | tetR21 | mgrA | ||
| RN6390 (wild type) | No induction | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Tetracycline (0.06) | 0.5 | 2.8 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.1 |
| 0.25 | 3.1 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 | |
| 0.10 | 4.0 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Palmitoleic acid (500) | 0.5 | 2.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.3 |
| 0.25 | 2.5 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.1 | |
| 0.10 | 4.0 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 | |
| Undecanoic acid (500) | 0.5 | 2.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| 0.25 | 1.5 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| 0.10 | 4.1 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Linoleic acid (1,000) | 0.5 | 1.8 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| 0.25 | 2.5 ± 0.5 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| 0.10 | 3.0 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.1 | |
| Palmitic acid (2,000) | 0.5 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| 0.25 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| 0.10 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 | |
| QT1 (ΔmgrA) | No induction | 5.0 ± 0.5 | 0.9 ± 0.1 | |
| Tetracycline (4.0) | 0.5 | 5.2 ± 0.4 | 0.8 ± 0.2 | |
| 0.25 | 5.1 ± 0.4 | 0.7 ± 0.2 | ||
| 0.10 | 5.0 ± 0.3 | 0.9 ± 0.1 | ||
| Palmitoleic acid (1,500) | 0.5 | 5.0 ± 0.2 | 0.9 ± 0.4 | |
| 0.25 | 5.1 ± 0.4 | 1.0 ± 0.1 | ||
| 0.10 | 5.2 ± 0.5 | 1.2 ± 0.3 | ||
| Undecanoic acid (1,500) | 0.5 | 5.0 ± 0.5 | 1.1 ± 0.1 | |
| 0.25 | 4.5 ± 0.3 | 0.8 ± 0.3 | ||
| 0.10 | 5.1 ± 0.4 | 0.9 ± 0.1 | ||
| Linoleic acid (2,500) | 0.5 | 5.0 ± 0.4 | 0.9 ± 0.1 | |
| 0.25 | 5.1 ± 0.3 | 1.1 ± 0.1 | ||
| 0.10 | 5.1 ± 0.4 | 1.0 ± 0.2 | ||
| Palmitic acid (5,000) | 0.5 | 5.0 ± 0.2 | 1.0 ± 0.1 | |
| 0.25 | 5.0 ± 0.2 | 1.0 ± 0.2 | ||
| 0.10 | 5.0 ± 0.1 | 1.0 ± 0.2 | ||
| QT21 (ΔtetR21) | No induction | 5.0 ± 0.5 | 1.0 ± 0.1 | |
| Tetracycline (0.5) | 0.5 | 4.8 ± 0.5 | 1.0 ± 0.1 | |
| 0.25 | 5.1 ± 0.3 | 1.0 ± 0.2 | ||
| 0.10 | 5.0 ± 0.4 | 1.0 ± 0.1 | ||
| Palmitoleic acid (1,000) | 0.5 | 4.5 ± 0.4 | 1.0 ± 0.1 | |
| 0.25 | 4.5 ± 0.3 | 1.0 ± 0.2 | ||
| 0.10 | 5.0 ± 0.5 | 1.0 ± 0.2 | ||
| Undecanoic acid (1,500) | 0.5 | 4.8 ± 0.2 | 1.0 ± 0.1 | |
| 0.25 | 4.5 ± 0.3 | 1.0 ± 0.1 | ||
| 0.10 | 4.8 ± 0.3 | 1.0 ± 0.1 | ||
| Linoleic acid (2,000) | 0.5 | 5.0 ± 0.4 | 1.0 ± 0.1 | |
| 0.25 | 4.5 ± 0.5 | 1.0 ± 0.1 | ||
| 0.10 | 4.8 ± 0.3 | 1.0 ± 0.1 | ||
| Palmitic acid (5,000) | 0.5 | 5.0 ± 0.2 | 1.0 ± 0.2 | |
| 0.25 | 4.8 ± 0.4 | 1.0 ± 0.2 | ||
| 0.10 | 5.0 ± 0.4 | 1.0 ± 0.1 | ||
| RN6390(pLI50) | No induction | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Tetracycline | 0.10 | 3.9 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Palmitoleic acid | 0.10 | 4.0 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Undecanoic acid | 0.10 | 3.8 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Linoleic acid | 0.10 | 2.6 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Palmitic acid | 0.10 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| QT1(pLI50-mgrA) | No induction | 1.0 ± 0.2 | 1.0 ± 0.2 | 6.0 ± 0.5 |
| Tetracycline | 0.10 | 3.5 ± 0.4 | 1.0 ± 0.1 | 6.0 ± 0.4 |
| Palmitoleic acid | 0.10 | 3.7 ± 0.4 | 1.0 ± 0.1 | 6.0 ± 0.4 |
| Undecanoic acid | 0.10 | 3.2 ± 0.4 | 1.0 ± 0.1 | 6.0 ± 0.5 |
| Linoleic acid | 0.10 | 2.2 ± 0.4 | 1.0 ± 0.1 | 6.0 ± 0.3 |
| Palmitic acid | 0.10 | 1.0 ± 0.1 | 1.0 ± 0.1 | 6.0 ± 0.2 |
| QT21(pLI50-tetR21) | No induction | 1.0 ± 0.3 | 5.0 ± 0.2 | 1.0 ± 0.4 |
| Tetracycline | 0.10 | 3.5 ± 0.3 | 5.0 ± 0.5 | 1.0 ± 0.1 |
| Palmitoleic acid | 0.10 | 3.7 ± 0.4 | 5.0 ± 0.3 | 1.0 ± 0.1 |
| Undecanoic acid | 0.10 | 3.0 ± 0.3 | 5.0 ± 0.3 | 1.0 ± 0.2 |
| Linoleic acid | 0.10 | 2.0 ± 0.2 | 5.0 ± 0.5 | 1.0 ± 0.1 |
| Palmitic acid | 0.10 | 1.0 ± 0.1 | 5.0 ± 0.5 | 1.0 ± 0.1 |
The relative gene expression is determined as the ratio of gene transcripts of mutant versus wild-type strains and the ratio of transcripts with versus without induction by fatty acids or tetracycline. The gmk gene was used as an internal control. Each assay was done in triplicate, and RNAs were collected from three independent biological samples. All values represent the means of three independent experiments. Values in boldface represent differences that are statistically significant based on Student's t test (P < 0.05). The differences between wild-type and mutant genes and the increases in transcript levels for gene expression after induction versus without induction were calculated.
To assess the effects of fatty acids on the transcript levels of tet38, tetR21, and mgrA, we exposed S. aureus RN6390, QT1, and QT21 to linoleic, palmitoleic, and undecanoic acids at 0.5-, 0.25-, and 0.10-fold MIC for each strain for up to 1 h. In RN6390, at 10-fold below the MIC, the tet38 transcripts increased 4-fold with palmitoleic and undecanoic acids and 3-fold with linoleic acid and showed no change with palmitic acid (Table 4). The transcript levels of tetR21 and mgrA of RN6390 remained unchanged after induction with all lipids. The mutants QT1 (ΔmgrA) and QT21 (ΔtetR21) showed approximately the same levels of mgrA, tetR21, and tet38 transcripts with or without induction (Table 4). No induction of tet38, tetR21, or mgrA was found with exposure to palmitic acid at any of the three below-MIC concentrations.
Similarly, exposure of RN6390 to tetracycline at 10-fold below the MIC led to an increase of 4-fold in tet38 transcripts. Exposure of QT1 and QT21 to tetracycline at 10-fold below the MIC did not substantially change the transcript levels of tet38, mgrA, or tetR21 compared to the levels in the absence of tetracycline (Table 4). We chose the 10-fold-below-MIC concentrations of tetracycline and fatty acids for the gel shift assays to prevent any unintended damage to the TetR21 protein during the incubation period. These amounts of tetracycline and fatty acids would be sufficient to assess the effect of each compound on the TetR21-tet38 promoter binding. Additional induction assays were carried out using complemented QT1 and QT21 strains, and the results indicated that the mutants exhibited gene expression patterns similar to that of RN6390 (Table 4). Statistical analyses were performed based on the Student t test to determine the significance of the gene expression values.
Gel mobility shift assays.
DNA-protein gel mobility shift assays were carried out using the TetR21 purified protein and the promoters of efflux pump genes as previously described (12, 31–34). The putative tet38 promoter is located within a 240-bp DNA intergenic region separating the S. aureus strain N315 ORF SA0130 (encoding a putative trehalose operon transcriptional regulator, on the complementing DNA strand) and the two N315 ORFs SA0131 and SA0132 (tet38). SA0131 encodes a putative purine nucleoside phosphorylase that is separated from tet38 by 6 nucleotides. RT-PCR using primers encompassing the internal region of the two genes (Table 2) generated a 200-bp PCR product, indicating that the two adjacent ORFs share the same promoter region and are cotranscribed. The upstream 240-bp region of DNA carries a putative promoter, based on a search using the Neural Network Promoter Prediction server (35).
TetR21 protein bound to biotinylated tet38 promoter, resulting in a band shift, and was displaced by excess unlabeled tet38 promoter DNA. Nonspecific herring sperm DNA in 100-fold excess, in contrast, did not affect the TetR21-mediated tet38 promoter band shift, showing the specificity of TetR21 binding to tet38 promoter (Fig. 1A). TetR21 also bound to the norB promoter but did not bind to the promoters of efflux pump genes norA, norC, norD, and abcA (Fig. 1B). Additional gel mobility shift assays were carried out with TetR21 and MgrA proteins and tetR21 and mgrA promoters. The tetR21 promoter was based on prediction by the Neural Network Promoter Prediction server, and the mgrA promoter was based on the primer extension data published by Ingavale et al. (24). The results showed that TetR21 and MgrA each bound to the tetR21 promoter (Fig. 1C). In contrast, TetR21 did not bind to the mgrA promoter and MgrA did not bind to tet38 promoter (data not shown). The absence of binding of MgrA to tet38 promoter was also reported in our previous study (12).
FIG 1.
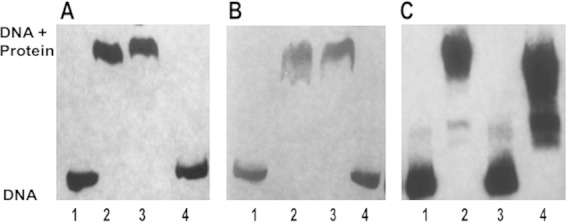
Binding of TetR21 to promoter DNA of tet38, norB, and tetR21 and binding of MgrA to tetR21 DNA. (A) Gel mobility shift analyses of the interactions of the purified TetR21 protein with the biotinylated tet38 promoter. Protein (200 ng, 7.2 pmol) and DNA (4 ng, 20 fmol) were in contact for 30 min at room temperature, followed by electrophoresis through a 5% acrylamide gel. Competing unlabeled herring sperm DNA (nonspecific, 100-fold excess) and unlabeled tet38 promoter (specific, 100-fold excess) were used to determine the specificity of the promoter binding assay. Lane 1, tet38 promoter; lane 2, tet38 + TetR21; lane 3, tet38 + TetR21 + 100-fold herring DNA; lane 4, tet38 +TetR21 + 100-fold unlabeled tet38. (B) Gel mobility shift analyses of the interactions of the purified TetR21 protein with the biotinylated norB promoter. Competing unlabeled herring sperm DNA (nonspecific, 100-fold excess) and unlabeled norB promoter (specific, 100-fold excess) were used to determine the specificity of the norB promoter binding. Lane 1, norB promoter; lane 2, norB + TetR21; lane 3, norB + TetR21 + 100-fold herring DNA; lane 4, norB + TetR21 + 100-fold unlabeled norB. (C) Gel mobility shift analyses of the interactions of the purified TetR21 and MgrA protein with the biotinylated tetR21 promoter. The same procedure was carried out as described above. Lane 1, tetR21 promoter; lane 2, tetR21 + TetR21; lane 3, tetR21 promoter; lane 4, tetR21 + MgrA.
Effects of tetracycline and palmitoleic acid on TetR21 binding to tet38 promoter.
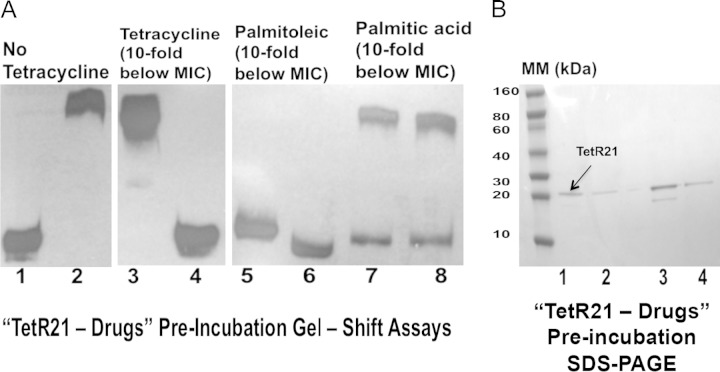
Tetracycline has been shown to disrupt the binding between other TetR regulators and the gene promoters they regulate (36). We assessed the ability of tetracycline and palmitoleic acid, both substrates of Tet38, to prevent the binding of TetR21 to tet38 promoter. Palmitoleic acid at 0.10-fold MIC induced the highest level of tet38 transcript under the conditions used in this study (Table 4). We used the MIC for the wild-type RN6390 and preincubated TetR21 with tetracycline (0.006 μg/ml or 1.8 μM) for a period of 60 min. At 60 min, 200 ng (7.2 μM) of protein was applied to gel mobility shift assays with tet38 promoter. The control protein was treated in the same manner as the tested protein. We found that tetracycline at this level prevented TetR21 binding to tet38 promoter after 60 min of preincubation (Fig. 2A).
FIG 2.
Effects of tetracycline, palmitoleic acid, and palmitic acid on the tet38-TetR21 complex. (A) tet38 promoter and TetR21 protein gel shift binding assays. TetR21 was preincubated with tetracycline, palmitoleic acid, or palmitic acid prior to being in contact with the tet38 promoter. At 60 min, 200 ng of TetR21 protein was removed and used in a gel shift assay with tet38 promoter. The drug concentrations (10-fold below the MICs) were as follows: tetracycline, 0.006 μg/ml (1.8 μM); palmitoleic acid, 50 μg/ml (200 μM); palmitic acid, 200 μg/ml (780 μM). Lane 1, tet38 DNA; lane 2, tet38 + TetR21; lane 3, tet38 + TetR21 with no tetracycline; lane 4, TetR21 + tetracycline for 60 min before addition of tet38 DNA; lane 5, tet38 DNA; lane 6, TetR21 + palmitoleic acid for 60 min before addition of tet38 DNA; lane 7, TetR21 + palmitic acid for 60 min before addition of tet38 DNA; lane 8, tet38 + TetR21 with no fatty acid. (B) TetR21 protein before and after incubation with drugs and SDS-PAGE protein gels. The protein gel results indicated absence of major protein degradation following incubation with tetracycline or fatty acids for 60 min. Lane 1, purified TetR21 protein; lane 2, TetR21 incubated with tetracycline (1.8 μM); lane 3, TetR21 incubated with palmitoleic acid (200 μM); lane 4, TetR21 incubated with palmitic acid (780 μM). A smaller protein band appeared in the sample in lane 3, which suggests minor degradation after incubation of TetR21 with palmitoleic acid. The amount of intact protein was still sufficient to produce a mobility shift in the tet38 DNA.
We repeated the same experiments with palmitoleic acid at 50 μg/ml (200 μM). TetR21 was preincubated with fatty acids for 60 min prior to being in contact with tet38 promoter for a gel mobility shift assay. As was found with tetracycline, palmitoleic acid disrupted the TetR21-tet38 promoter complex after 60 min of preincubation (Fig. 2A). Palmitic acid was used as a negative control since this saturated fatty acid did not affect tet38 expression, and preincubation of TetR21 with palmitic acid (780 μM) for 60 min did not affect the formation of the TetR21-tet38 promoter complex (Fig. 2A). In parallel, TetR21 protein (200 ng, 7.2 μM each assay) was incubated with tetracycline, palmitoleic acid, and palmitic acid individually and then separately applied to SDS-PAGE followed by Coomassie blue staining to assess the integrity of the protein. No major protein degradation was found, indicating that tetracycline and these fatty acids did not result in degradation of the TetR21 protein under these conditions (Fig. 2B). A similar experiment with undecanoic acid at a concentration of 50 μg/ml (268 μM) could not be interpreted because TetR21 was degraded despite the presence of protease inhibitors.
Effects of tet38, tetR21, and mgrA on S. aureus internalization, survival, and replication within host cells.
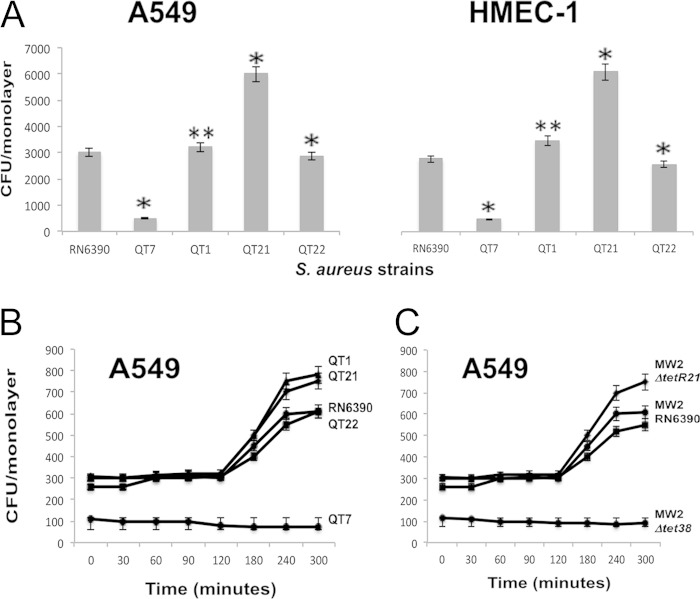
Our previous work demonstrated that the Tet38 efflux pump affected the ability of S. aureus to colonize mouse skin. To determine whether Tet38 affects the ability of S. aureus to invade epithelial and endothelial cells, we developed invasion assays using epithelial cell line A549 and endothelial cell line HMEC-1. S. aureus parental strain RN6390 was compared with mutants QT1 (ΔmgrA), QT7 (Δtet38), and QT21 (ΔtetR21) to assess their ability to invade and survive in these cell lines. All strains used in these assays grew similarly in TSB (data not shown). The complemented strain QT22 (QT7 with plasmid pLI50-tet38) was added to the assays to validate the effect of Tet38 on the internalization process. After 2 h of contact between cells and bacteria, we treated the cells with gentamicin at 200 μg/ml, washed them with PBS, disrupted the cells with Triton X-100, and plated the internalized bacteria on LB agar. We found that QT7, which lacks a functional Tet38, had 6-fold less viable bacteria within A549 cells than the parental strain RN6390. In contrast, QT21, which lacks a functional TetR21 regulator, exhibited 2-fold more viable bacteria within A549 cells than RN6390. The QT1 mutant, which lacks functional MgrA, was similar (1.5-fold) to the wild-type RN6390 in the number of viable bacteria within A549 cells. We found a similar pattern using the HMEC-1 endothelial cell line, which showed a 5-fold-lower and a 2-fold-greater number of internal bacteria for QT7 and QT21, respectively. The complemented strain QT22 showed the same level of intracellular bacteria as RN6390 for both cell lines (Fig. 3A). Thus, tet38 contributes to the ability of S. aureus to survive in epithelial and endothelial cells.
FIG 3.
Internalization and survival of S. aureus within epithelial and endothelial cells. (A) Internalization and survival of S. aureus within A549 and HMEC-1 cells. The internalization assays were carried out as described in Materials and Methods with a bacterial multiplicity of infection (MOI) of 10:1. After 2 h of internalization, cell monolayers were treated with gentamicin (200 μg/ml), detached, and lysed, and colony counts (CFU/monolayer) were performed. All values are the mean results ± standard deviations (SD) from three independent experiments. *, statistically significantly different from the wild type as determined by Student's t test (P < 0.05); **, not statistically significantly different. S. aureus strains were RN6390 (wild type), QT7 (Δtet38), QT1 (ΔmgrA), QT21 (ΔtetR21), and QT22 (Δtet38 complemented). A549, lung adenocarcinoma epithelial cells; HMEC-1, human microvascular endothelial cells. (B) Intracellular replication of S. aureus strains in A549 cells. Gentamicin was added after 45 min of contact between bacteria and A549 cells as described in Materials and Methods. Intracellular survival assays of S. aureus were carried out over a period of 300 min. Viable bacterial counts for strains RN6390, QT1, QT21, and QT22 increased after 120 min and stabilized at 5 h. Viable bacterial counts for strain QT7 at first remained unchanged and then decreased slightly over the course of 5 h. All values are the mean results ± SD from three independent experiments. (C) Intracellular replication of clinical S. aureus strains in A549 cells. Clinical strain MW2 and mutants MW2Δtet38 and MW2ΔtetR21 show the same pattern of intracellular replication as strains RN6390, QT7 and QT21, shown in panel B. All values are the mean results ± SD from three independent experiments.
We followed the intracellular replication of S. aureus over time after treatment of A549 cells with gentamicin to eliminate the remaining extracellular bacteria. Gentamicin remained in the medium over the course of the extended incubation and was washed away prior to cell lysis for colony counts. Based on the results of the initial experiments, we determined that 45 min would allow sufficient bacteria inside the A549 cells for subsequent enumeration. At 45 min of contact with an MOI of 10:1, the amounts of internalized bacteria of strains RN6390, QT1, and QT21 were similar (300 to 305 bacteria) and were 3-fold higher than the internalized amount of mutant QT7 (108 bacteria) (Fig. 3B). These data indicated that the MgrA and TetR21 regulators did not influence the initial internalization of S. aureus into A549 cells but that the absence of the Tet38 efflux pump resulted in an early lower level of survival. Bacterial intracellular replication was then monitored over 5 h. The numbers of intracellular bacteria remained stable for all strains for up to 120 min. Between 120 and 240 min, the numbers of bacteria of strains RN6390, QT1, and QT21 increased between 2-fold and 2.5-fold, followed by a plateau period, whereas the bacterial numbers of QT7 decreased slightly over the full time period. The complemented strain QT22, which carried the plasmid-cloned tet38 in QT7, showed a growth pattern similar to that of RN6390 (Fig. 3B). We carried out the assay using the tet38 overexpressor RN6390(pLI50-tet38) and found that this overexpressor generated a number of CFUs per monolayer similar to that of the QT21 mutant (720 versus 750 CFU/monolayer, respectively; data not shown in Fig. 3B). The ΔnorB efflux pump mutant QT5 was also tested in this assay, and it behaved similarly to RN6390, indicating that NorB did not play a role in bacterial intracellular replication (data not shown). S. aureus MW2 and its mutants were added to the intracellular replication assays to assess the effects of tet38 in other S. aureus strains. We found that MW2Δtet38 survived 6-fold less efficiently than MW2, and MW2ΔtetR21 replicated as efficiently as MW2 (Fig. 3C). The complemented strain MW2Δtet38(pLI50-tet38) recovered the ability to internalize inside host cells, and the mutant MW2ΔtetR21 showed a 2.5-fold increase in internalization (data not shown). These data, taken together, indicate that Tet38 contributes to the ability of S. aureus to survive within epithelial cells. The findings demonstrate an effect on intracellular replication over time. Furthermore, the stable numbers of viable bacteria of mutant and parental strains over several hours and the presence of differences in numbers of viable intracellular bacteria between mutant and parental strains as early as 45 min suggest that Tet38 may also have an effect on internalization itself.
tet38 and tetR21 do not affect the expression of fnbA and fnbB adhesins.
Since fibronectin-binding proteins mediate the adhesion and invasion of S. aureus in several cell types, including epithelial and endothelial cells (13), we carried out quantitative real-time RT-PCRs using primers designed from genes fnbA and fnbB and total RNAs extracted from RN6390, QT7, and QT21 to assess potential differences in the expression of these adhesins. The transcript levels of fnbA and fnbB remained unchanged between the three S. aureus strains RN6390, QT7, and QT21. These data indicated that, under these conditions, tet38 and tetR21 did not affect the expression of fnbA and fnbB.
DISCUSSION
tet38 is highly conserved among S. aureus strains, based on published genomes of both methicillin-susceptible and -resistant strains. We previously found that Tet38 conferred resistance to tetracycline and certain unsaturated fatty acids, such as linoleic, palmitoleic, and undecanoic acids, but not to palmitic acid or the polyamine spermidine (7).
In this study, we identified a new TetR-like regulator of the tet38 efflux pump gene that we named TetR21. The tetR21 mutant exhibited a 5-fold increase in tet38 transcripts, which correlated with an 8-fold increase in resistance to tetracycline, a 2-fold increase in resistance to palmitoleic acid, and a 3-fold increase in resistance to undecanoic acid. Unexpectedly the tetR21 mutant also exhibited an 8-fold and a 4-fold increase in the MIC of chloramphenicol and erythromycin, respectively. These data suggested that TetR21 acted as a repressor of tet38 expression and may also regulate the expression of other bacterial resistance determinants in addition to Tet38. The nature of these additional resistance determinants regulated by tetR21 is the subject of ongoing investigation.
We then demonstrated that TetR21 directly and specifically bound to the tet38 promoter. Notably, the presence of tetracycline at a concentration 10-fold below its MIC provoked a reduction in the binding of TetR21 to tet38 promoter in gel mobility shift assays, suggesting that the binding of tetracycline to TetR21 resulted in disruption of repressor binding and was responsible for the induction of tet38 expression by tetracycline. The same phenomenon was observed when we used palmitoleic acid but not with palmitic acid (a saturated fatty acid) at 10-fold below the MIC. We have previously shown that the global regulator MgrA negatively regulated the expression of tet38 (12). We demonstrated here that MgrA bound to the promoter of tetR21 but not to that of tet38, suggesting that it functions as a direct regulator of tetR21 expression, thereby contributing to TetR21 repression of tet38 expression. The nature of the role played by MgrA toward tetR21 expression was not clear, since a lack of MgrA did not lead to an obvious change in the level of tetR21 expression in either direction.
Many multidrug efflux pumps in Gram-negative bacteria like E. coli, K. pneumoniae, and E. cloacae are controlled by special regulatory systems that mediate responses to hostile environments (37). In addition, studies of Gram-negative efflux pumps revealed that the expression of several transporters of the RND (resistance-nodulation-division) family of multidrug (MDR) transporters were induced by and transported cellular metabolites as part of the bacterial cell physiology (38–40). Since Tet38 conferred resistance to fatty acids, a natural host defense component, we observed the effects of certain lipids on the gene expression levels of tet38 and its regulators tetR21 and mgrA. Exposure of S. aureus RN6390 to linoleic, palmitoleic, and undecanoic acids led to 4-fold increases in tet38 transcript levels in vitro, while no change was detected for the tetR21 and mgrA transcripts. Furthermore, we found that the tet38 transcript levels remained unchanged after exposure of mgrA and tetR21 mutants to fatty acids, suggesting that induction requires intact MgrA and TetR21 regulators.
To understand further the role played by the Tet38 efflux pump and the effects of TetR21 and MgrA on S. aureus survival in epithelial cells, we carried out invasion assays using human lung epithelial cell and microvascular endothelial cell lines. Compared with the wild-type RN6390, the tet38 mutant QT7 survived in both cell types in 6-fold-lower numbers and was unable to replicate intracellularly. Both defects were restored following complementation of the QT7 strain with a plasmid-cloned wild-type tet38. Notably, both the mgrA and the tetR21 mutant showed similar enhancement of delayed intracellular replication. Since both of these mutants had similarly increased tet38 expression, the difference in the two mutants in enhanced initial survival (the tetR21 mutant had enhanced initial survival, and the mgrA mutant did not) indicates that additional factors that contribute to initial internalization and survival are affected differently by the two regulators. Further evidence for their distinct regulatory profiles is the difference in chloramphenicol and erythromycin resistance seen between the tetR21 and mgrA mutants. The factors that determine the delay in intracellular replication in the parental strain and regulatory mutants are not known, and it is possible that delays may vary with different regulatory mutants and that this could explain our finding of differences between the tetR21 mutant and the parental strain RN6390 at the initial time point in the short-incubation assay (45-min bacterium-cell incubation) (Fig. 3B) and the initial time point in the long-incubation assay (120-min bacterium-cell incubation) (Fig. 3A) (41).
Our data indicated that Tet38 contributed to the process of early cell internalization and survival, as well as intracellular replication of S. aureus in nonprofessional phagocytes, such as epithelial and endothelial cells. Because initial intracellular survival was stable over several hours and because differences between the tet38 mutant and parental strain were seen as early as 45 min, we think it is likely that Tet38 contributed directly to internalization within host cells, as it did for delayed intracellular replication. Although both Tet38 and NorB contributed to bacterial survival in a murine abscess model (11), only Tet38 contributed to survival on mouse skin (7), and only Tet38 contributed to survival in epithelial and endothelial cells. The mechanism by which Tet38 facilitates intracellular survival and replication is not known but may relate to an ability to protect from as-yet-undefined intracellular toxins, which could include antibacterial fatty acids.
A number of efflux pumps have been shown to be important for bacterial survival in host environments. For example, the lack of a functional tripartite transperiplasmic efflux pump can lead to the accumulation of xenobiotic compounds inside the E. coli cells, most notably bile salts, which are present in high concentrations in the native intestinal environment of this organism. Toxin exposure can trigger stress response systems of bacteria (42). Efflux pumps of Salmonella, Campylobacter, Pseudomonas, and E. coli have been shown to facilitate the invasion of and replication within host cells and escape from host defense mechanisms (3, 43). The MacAB transporter of Salmonella enterica also serves to protect the bacterium against reactive oxygen species produced by the host cells (44).
In summary, Tet38 contributes to the ability of S. aureus to survive and replicate within epithelial and endothelial cells, in addition to its contributions to bacterial colonization of skin and survival in an abscess environment, highlighting its important roles in the adaptation of S. aureus to multiple environments. The exact mechanisms by which Tet38 facilitates internalization and intracellular survival are the subject of ongoing investigation. The central regulation of tet38 expression by regulators such as MgrA, which also regulates the expression of other efflux pumps and multiple other cellular functions, further implies an integral role for Tet38 in S. aureus physiology and virulence. TetR21 represents a novel component of the MgrA regulon that also affects other resistance determinants, possibly including additional efflux pumps, thereby adding to the substantial resistance portfolio of this highly successful human pathogen.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants no. R37-AI23988 and P01-AI083214 (M. Gilmore, principal investigator) from the National Institutes of Health to D.C.H. and, in part, by an NSF-ATE grant (no. DUE 1205020) from the National Science Foundation to G.R.B.
REFERENCES
- 1.Nikaido H, Zgurskaya HI. 1999. Antibiotic efflux mechanisms. Curr Opin Infect Dis 12:529–536. doi: 10.1097/00001432-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Webber MA, Piddock LJV. 2003. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 3.Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, Kamihira S, Hancock REW, Speert DP. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med 196:109–118. doi: 10.1084/jem.20020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piddock LJV. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng X, Sun F, Ji Q, Liang H, Missiakas D, Lan L, He C. 2012. Expression of multidrug resistance efflux pump gene norA is iron responsive in Staphylococcus aureus. J Bacteriol 194:1753–1762. doi: 10.1128/JB.06582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truong-Bolduc QC, Bolduc GR, Okumura R, Celino B, Bevis J, Liao CH, Hooper DC. 2011. Implication of the NorB efflux pump in the adaptation of Staphylococcus aureus to growth at acid pH and in resistance to moxifloxacin. Antimicrob Agents Chemother 55:3214–3219. doi: 10.1128/AAC.00289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong-Bolduc QC, Villet RA, Estabrooks ZA, Hooper DC. 2014. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. J Infect Dis 209:1485–1493. doi: 10.1093/infdis/jit660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alnaseri H, Arsic B, Schneider J, Kaiser JC, Scinocca ZC, Heinrichs DE, McGavin MJ. 2015. Inducible expression of a resistance-nodulation-division-type efflux pump in Staphylococcus aureus provides resistance to linoleic and arachidonic acids. J Bacteriol 197:1893–1905. doi: 10.1128/JB.02607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Sahin O, Michel LO, Zhang OJ. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol 48:1609–1619. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y, Onodera Y, Lee JC, Hooper DC. 2008. NorB, an efflux pump in Staphylococcus aureus MW2, contributes to bacterial fitness in abscesses. J Bacteriol 190:7123–7129. doi: 10.1128/JB.00655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol 187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jett BD, Gilmore MS. 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect Immun 70:4697–4700. doi: 10.1128/IAI.70.8.4697-4700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong YQ, Bayer AS, Yeaman MR, van Wamel W, Manna AC, Cheung AL. 2004. Impacts of sarA and agr in Staphylococcus aureus strain Newman on fibronectin-binding protein A gene expression and fibronectin adherence capacity in vitro and in experimental infective endocarditis. Infect Immun 72:1832–1836. doi: 10.1128/IAI.72.3.1832-1836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhuber A, Goerke C, Bayer MG, Doring G, Wolz C. 2003. Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on expression of virulence factors. J Bacteriol 185:6278–6286. doi: 10.1128/JB.185.21.6278-6286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Yurecko RS, Dedhar S, Cleary PP. 2006. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell Microbiol 8:257–266. doi: 10.1111/j.1462-5822.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 17.Jarry TM, Cheung AL. 2006. Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun 74:2568–2577. doi: 10.1128/IAI.74.5.2568-2577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarry TM, Memmi G, Cheung AL. 2008. The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cell Microbiol 10:1801–1814. doi: 10.1111/j.1462-5822.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 19.Kahl BC, Goulian M, van Wamel W, Herrmann M, Simon SM, Kaplan G, Peters G, Cheung AL. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun 68:5385–5392. doi: 10.1128/IAI.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, Nair SP. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun 69:2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak YG, Truong-Bolduc QC, Bin KH, Song KH, Kim ES, Hooper DC. 2013. Association of norB overexpression and fluoroquinolone resistance in clinical isolates of Staphylococcus aureus from Korea. J Antimicrob Chemother 68:2766–2772. doi: 10.1093/jac/dkt286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong-Bolduc QC, Liao C-H, Villet R, Bolduc GR, Estabrooks Z, Taguezem GF, Hooper DC. 2012. Reduced aeration affects the expression of the NorB efflux pump of Staphylococcus aureus by posttranslational modification of MgrA. J Bacteriol 194:1823–1834. doi: 10.1128/JB.06503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingavale SS, Van Wamel W, Cheung AL. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol Microbiol 48:1451–1466. doi: 10.1046/j.1365-2958.2003.03503.x. [DOI] [PubMed] [Google Scholar]
- 25.Truong-Bolduc QC, Zhang X, Hooper DC. 2003. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J Bacteriol 185:3127–3138. doi: 10.1128/JB.185.10.3127-3138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzoni C, Francois P, Huyghe A, Couzinet S, Tapparel C, Charbonnier Y, Renzoni A, Lucchini S, Lew DP, Vaudaux P, Kelley WL, Schrenzel J. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. doi: 10.1186/1471-2164-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidl K, Solis NV, Bayer AS, Hady WA, Ellison S, Klashman MC, Xiong YQ, Filler SG. 2012. Divergent responses of different endothelial cell types to infection with Candida albicans and Staphylococcus aureus. PLoS One 7:e39633. doi: 10.1371/journal.pone.0039633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung AL, Bayles KW. 2007. Tissue culture assays used to analyze invasion by Staphylococcus aureus. Curr Protoc Microbiol Chapter 9:Unit 9C.4. doi: 10.1002/9780471729259.mc09c04s4. [DOI] [PubMed] [Google Scholar]
- 29.Pfortner H, Burian MS, Michalik S, Depke M, Hildebrandt P, Dhople VM, Pane-Farre J, Hecker M, Schmidt F, Volker U. 2014. Activation of the alternative sigma factor SigB of Staphylococcus aureus following internalization by epithelial cells—an in vivo proteomics perspective. Int J Med Microbiol 304:177–187. doi: 10.1016/j.ijmm.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Lee JO, Cho KS, Kim OB. 2014. Overproduction of AcrR increases organic solvent tolerance mediated by modulation of SoxS regulon in Escherichia coli. Appl Microbiol Biotechnol 98:8763–8773. doi: 10.1007/s00253-014-6024-9. [DOI] [PubMed] [Google Scholar]
- 31.Truong-Bolduc QC, Hooper DC. 2010. Phosphorylation of MgrA and its effect on expression of the NorA and NorB efflux pumps of Staphylococcus aureus. J Bacteriol 192:2525–2534. doi: 10.1128/JB.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong-Bolduc QC, Strahilevitz J, Hooper DC. 2006. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus. Antimicrob Agents Chemother 50:1104–1107. doi: 10.1128/AAC.50.3.1104-1107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong-Bolduc QC, Hooper DC. 2007. The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and beta-lactams in Staphylococcus aureus. J Bacteriol 189:2996–3005. doi: 10.1128/JB.01819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Y, Fu Y, Lee JC, Hooper DC. 2012. Staphylococcus aureus NorD, a putative efflux pump coregulated with the Opp1 oligopeptide permease, contributes selectively to fitness in vivo. J Bacteriol 194:6586–6593. doi: 10.1128/JB.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reese MG. 2001. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem 26:51–56. doi: 10.1016/S0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 36.Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev 69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duval V, Lister IM. 2013. MarA, SoxS and Rob of Escherichia coli—global regulators of multidrug resistance, virulence and stress response. Int J Biotechnol Wellness Ind 2:101–124. doi: 10.6000/1927-3037.2013.02.03.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikaido H, Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta 1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul S, Alegre KO, Holdsworth SR, Rice M, Brown JA, McVeigh P, Kelly SM, Law CJ. 2014. A single-component multidrug transporter of the major facilitator superfamily is part of a network that protects Escherichia coli from bile salt stress. Mol Microbiol 92:872–884. doi: 10.1111/mmi.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burse A, Weingart H, Ullrich MS. 2004. NorM, an Erwinia amylovora multidrug efflux pump involved in in vitro competition with other epiphytic bacteria. Appl Environ Microbiol 70:693–703. doi: 10.1128/AEM.70.2.693-703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballal A, Ray B, Manna AC. 2009. sarZ, a sarA family gene, is transcriptionally activated by MgrA and is involved in the regulation of genes encoding exoproteins in Staphylococcus aureus. J Bacteriol 191:1656–1665. doi: 10.1128/JB.01555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosner JL, Martin RG. 2013. Reduction of cellular stress by TolC-dependent efflux pumps in Escherichia coli indicated by BaeSR and CpxARP activation of spy in efflux mutants. J Bacteriol 195:1042–1050. doi: 10.1128/JB.01996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin J, Akiba M, Sahin O, Zhang Q. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob Agents Chemother 49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogomolnaya LM, Andrews KD, Talamantes M, Maple A, Ragoza Y, Vazquez-Torres A, Andrews-Polymenis H. 2013. The ABC-type efflux pump MacAB protects Salmonella enterica serovar Typhimurium from oxidative stress. mBio 4:e00630-13. doi: 10.1128/mBio.00630-13. [DOI] [PMC free article] [PubMed] [Google Scholar]