Abstract
MdsABC is a Salmonella-specific tripartite efflux pump that has been implicated in the virulence of Salmonella enterica serovar Typhimurium; however, little is known about the virulence factors associated with this pump. We observed MdsABC expression-dependent alterations in the degree of resistance to extracellular oxidative stress and macrophage-mediated killing. Thin-layer chromatography and tandem mass spectrometry analyses revealed that overexpression of MdsABC led to increased secretion of 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS), affecting the ability of the bacteria to invade and survive in host cells. Overexpression of MdsABC and external addition of PSPS similarly rendered the mdsABC deletion strain resistant to diamide. Diagonal gel analysis showed that PSPS treatment reduced the diamide-mediated formation of disulfide bonds, particularly in the membrane fraction of the bacteria. Salmonella infection of macrophages induced the upregulation of MdsABC expression and led to an increase of intracellular bacterial number and host cell death, similar to the effects of MdsABC overexpression and PSPS pretreatment on the mdsABC deletion strain. Our study shows that MdsABC mediates a previously uncharacterized pathway that involves PSPS as a key factor for the survival and virulence of S. Typhimurium in phagocytic cells.
INTRODUCTION
Multidrug efflux pumps are related to the intrinsic resistance of clinically important bacteria to antimicrobial agents (1–3). This mode of resistance is of particular concern for Gram-negative bacteria, which coopt a variety of efflux pumps for the extracellular expulsion of a wide range of antibiotics and toxic compounds. To date, five families of efflux pumps have been classified using sequence homology, including the resistance-nodulation-division (RND), ATP binding cassette (ABC), multidrug and toxic compound exporter (MATE), small multidrug resistance (SMR), and major facilitator superfamily (MFS) classifications (4–7). Among them, RND-type efflux pumps, such as the AcrAB-TolC system, which are present in a wide range of Gram-negative bacteria, including Escherichia coli, Salmonella enterica, and Vibrio vulnificus, are well known for the export of a variety of small molecules, ranging from solvents and dyes to the majority of amphiphilic and lipophilic antibiotics (8–10). However, there are still many uncharacterized efflux pumps that may affect drug resistance and virulence in bacteria.
S. enterica is a Gram-negative pathogen that causes various diseases worldwide in humans and animals (11). It contains a genus-specific MdsABC (multidrug transporter of Salmonella) efflux pump comprised of an inner membrane RND-type transporter, MdsB, a periplasmic membrane-fusion protein, MdsA, and an outer membrane protein, MdsC. This pump system is known to confer resistance to novobiocin and several toxic chemicals, including crystal violet, acriflavine, and rhodamine 6G, as well as to gold stress (12, 13). Although MdsABC is produced at a basal level significantly lower than that of AcrAB-TolC, it is required for full virulence to infect mice and colonize host cells (8). Compared with that of TolC-linked efflux pumps, the overproduction of MdsABC presents a similar resistance to host cell immune responses (14, 15), indicating the involvement of MdsABC in S. enterica pathogenicity.
We aimed to investigate the expression levels of MdsABC in relation to the antimicrobial resistance and virulence of S. Typhimurium. We constructed mdsABC deletion (DT) and overexpression (MT) mutant strains and found that 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS) was hypersecreted from the MdsABC-overexpressing bacteria, which conferred resistance to some antimicrobial dyes and oxidative stress-inducing agents. Diagonal gel electrophoresis showed alterations in the formation of disulfide bonds in membrane and cytoplasmic proteins obtained from diamide-treated bacteria cultivated with or without the addition of PSPS. Further pathological effects of PSPS treatment on Salmonella infection and host cell death were analyzed by flow cytometry. We report here that the induction of MdsABC expression increased the secretion of PSPS, which led to an increase in the resistance and virulence of S. Typhimurium against phagocytosis.
MATERIALS AND METHODS
Strains and culture conditions.
All derivative strains of Salmonella enterica serovar Typhimurium 14028S and plasmids used in this study are shown in Table S1 in the supplemental material. PCR primers used for the cloning of mdsABC and for the construction of the arcAB tolC and mdsABC deletion constructs (ΔacrAB ΔtolC and ΔmdsABC mutants, respectively) are listed in Table S2 in the supplemental material. The details of the gene deletion and cloning procedures are described elsewhere (13). The constructed expression plasmid pMdsABC was used to transform the ΔmdsABC and ΔacrAB ΔtolC strains. Bacterial strains were routinely cultivated in Luria-Bertani (LB) broth containing 100 μg ml−1 kanamycin and 0.1% l-arabinose at 37°C with shaking at 200 rpm. To test bacterial susceptibility to oxidative stress-inducing agents, exponentially grown cells at an optical density at 600 nm (OD600) of 0.8 were serially diluted 10-fold from 100 to 10−5 with sterile phosphate-buffered saline (PBS), and 10-μl aliquots were spotted on LB agar plates containing 100 μg ml−1 kanamycin, 0.1% arabinose, and appropriate concentrations of diamide, H2O2, tert-butylhydroperoxide (t-BOOH), NaNO2, and 1,1′-dimethyl-4,4′-bipyridinium dichloride (Paraquat).
Antimicrobial susceptibility tests.
The MICs of crystal violet, acriflavine, rhodamine 6G, methylene blue, and gentamicin were determined with the S. Typhimurium mutant strains cultivated in Luria-Bertani (LB) broth containing 100 μg ml−1 kanamycin and 0.1% l-arabinose, as described previously (13). All experiments were performed with three independent replicates.
Diagonal gel electrophoresis.
Diagonal gel analysis was performed with soluble and membrane proteins to identify disulfide-forming proteins. The MOPS minimal (mMOPS) medium-cultured bacteria were washed with ice-cold 1×PBS, and centrifuged cells were treated with 100 mM N-ethylmaleimide (NEM) for 1 h at room temperature to block free cysteine thiol groups. Cells were disrupted by mixing them with 3 volumes of 1× PBS cell lysis buffer containing 1% dodecyl-β-d-glucose and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), followed by agitation (1,000 rpm) for 2 h at room temperature and incubation on ice for 2 h to precipitate dodecyl-β-d-glucose-bound membrane proteins. After centrifugation, the soluble fractions were decanted, and precipitated fractions were washed again with ice-cold 1× PBS. The precipitated membrane proteins were solubilized using 2% octyl-β-d-glucose and then centrifuged to remove debris. Using soluble or detergent-solubilized proteins, diagonal gel analysis was performed using nondenaturing 4 to 15% gradient SDS-polyacrylamide gels in the first dimension followed by denaturing 15% SDS-polyacrylamide gel conditions in the second, as described previously (16). Disulfide-forming protein spots located above or below the diagonal curves were excised from the gels and analyzed by tandem mass spectrometry.
RNA extraction and RT-PCR.
To measure the expression levels of mdsABC, tolC, dnaK, and phosphatidylserine (PS) biosynthesis genes in the wild-type (WT), DT, and MT strains, total RNA was extracted from cells in the mid-exponential phase of growth (OD600 of approximately 0.8) without apparent differences in specific growth rates of the three strains. Next, 1 μg RNA was used for cDNA synthesis and PCR using a Prime Script first-strand cDNA synthesis kit (TaKaRa, Seoul, Republic of Korea). For macrophage infection experiments, Salmonella-infected RAW264.7 cells were lysed in 1% Triton X-100 and centrifuged at 3,000 rpm for 10 min to isolate intracellular bacteria. RNA was prepared as described above. Sequences for the reverse transcription-PCR (RT-PCR) primers are given in Table S2 in the supplemental material. The epitope-tagged MdsABC proteins produced by the MT strain were validated by Western blotting with anti-Flag, anti-myc, and antihexahistidine antibodies, as described previously (13).
Lipid analysis.
Lipid analysis was performed with WT, DT, and MT strains that were grown to an OD600 of 1.0 in mMOPS medium containing 0.1% glucose and 0.1% arabinose. Fatty acid methyl ester (FAME) profiles were analyzed using a Hewlett-Packard 6890 gas chromatograph with Microbial Identification software (MIDI, Newark, DE). Cellular lipids were extracted from cells grown in the presence or absence of arabinose by chloroform-methanol-water-acetic acid (20:10:1:1 by volume), and secreted lipids were extracted with an equal volume of n-butanol added to 0.2-μm membrane filtrates of the acidified culture fluids (10 ml of each) spiked with 10 μg of dimyristoyl dl-α-phosphatidylcholine (Sigma, St. Louis, MO) as a reference compound. Cellular and secreted lipid extracts were dried in vacuo and dissolved in methanol for spotting on Polygram Sil G/UV254 thin-layer chromatography (TLC) plates (Macherey-Nagel, Düren, Germany). Two-dimensional TLC of the cellular lipids was conducted using chloroform–methanol–water–28% aqueous ammonia (130:70:8:0.5 by volume) in the first dimension, followed by chloroform-acetone-methanol-acetic acid-water (100:40:20:20:10 by volume) in the second, as described elsewhere (17). One-dimensional TLC of the secreted lipids was performed using the second acidic solvent. Phospholipid spots were sprayed with molybdenum blue staining solution for visualization, and intensities were analyzed using ImageQuant software (GE Healthcare Life Sciences). From silica gels, higher levels of a phospholipid (PL-1) compound were observed in secreted lipid extracts from MdsABC-producing bacteria. This compound was further purified using a reversed-phase C18 column with methanol solvent and analyzed by tandem mass spectrometry.
Tandem mass spectrometry analysis.
Phospholipids, which were purified from the used mMOPS culture medium of the S. Typhimurium mutant (MT) strain overexpressing MdsABC, were analyzed by electrospray/collision-induced dissociation mass spectrometry in negative-ion mode using a Thermo Velos Pro Mass instrument (Thermo Scientific). The dried lipids were dissolved in a 50% methanol–0.1% formic acid solution, and a 1-μl sample was directly injected into a Thermo Velos Pro Mass analyzer operated with an exit voltage of −4 kV. A full-scan survey was performed between m/z 150 and 2000 with the delivery of 50% methanol–0.1% formic acid solution by a syringe pump at a flow rate of 3 μl min−1, followed by data-dependent MSn scans of the 3 most intense ions from the preview survey scans. The ion trap mass spectrometer was operated with the following options: isolation width, ±0.8 m/z; collision energy, 35%; and dynamic exclusion duration, 30 s.
Tryptic peptides of the disulfide-bonded proteins isolated from the diagonal gels were dissolved in 0.4% acetic acid and analyzed on a Thermo Velos Pro Mass analyzer with a nano-liquid chromatography system and a Magic C18AQ column (75 μm by 75 mm). The chromatographic conditions were a 90-min linear gradient from 5% to 40% acetonitrile in 0.1% formic acid buffer solution, followed by a 20-min column wash with 80% acetonitrile and a 10-min reequilibration to the initial buffer condition at a constant flow rate of 0.3 μl min−1. A full-scan survey was performed between m/z 300 and 2000, followed by data-dependent MS2 scans of the 7 most intense ions from the survey scan using the following options: isolation width, ±0.8 m/z; collision energy, 35%; and dynamic exclusion duration, 30 s. The acquired tandem mass spectrometry data were analyzed by the SEQUEST search of Thermo Proteome Discoverer program version 1.3 against the database of S. Typhimurium 14028S proteins (downloaded from GenBank) and the common Repository of Adventitious Proteins (downloaded from URL ftp://ftp.thegpm.org/fasta/cRAP), which are present either by accident or through unavoidable contamination of protein samples. The search options were as follows: average mass (m/z), maximum of 1 miscleavage site of trypsin digestion, precursor mass tolerance of 1.5 Da, fragment mass error of 0.8 Da, and variable modifications such as N-ethylmaleimide modification of free cysteine thiol, iodoacetamide modification of dithiothreitol (DTT)-reduced cysteine thiol, and oxidation of methionine and tryptophan. For the identification of peptides and proteins, peptide spectrum matches from the tandem mass spectrum data were filtered with a probability of >0.99 and a target-decoy false-discovery rate (FDR) of <0.01.
Measurements of bacterial infection and cell death.
The effects of MdsABC on bacterial invasion and survival in macrophages were examined by a modified gentamicin protection assay (18). Briefly, mouse monocyte macrophage RAW 264.7 cells (Korean Cell Line Bank, Seoul, Republic of Korea) were cultivated in RPMI 1640 medium (Welgene, Daegu, Republic of Korea) supplemented with 10% (vol/vol) fetal bovine serum, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. PBS-washed RAW 264.7 cells were infected by S. Typhimurium grown in LB broth containing 0.1% arabinose. The infection was carried out with a multiplicity of infection (MOI) of 100 at 37°C for 2 h under 5% CO2. Infected macrophages were washed twice with prewarmed PBS and then incubated at 37°C for 2 h in fresh medium containing 100 μg ml−1 gentamicin to kill extracellular bacteria. At 2 h and 15 h postinfection, macrophages were washed twice with PBS and disrupted with 1% (vol/vol) Triton X-100 for 5 min. Lysates were serially diluted 10-fold to determine bacterial CFU on LB agar plates containing 100 μg ml−1 kanamycin. To examine the effects of purified PL-1 on bacteria and host cells, the infection media were treated with 50 μM PL-1 dissolved in dimethylformamide (final concentration, 0.5%).
Flow cytometry.
The cytotoxic effects of PL-1 on infected and uninfected macrophages were examined by cytometry using a calibrated BD FACSCalibur flow cytometer. RAW 264.7 cells treated or not treated with 50 μM PL-1 were labeled using a fluorescein isothiocyanate (FITC)-annexin V (Ann V)/propidium iodide (PI) apoptosis detection kit (BD Biosciences) in accordance with the manufacturer's instructions. Annexin V is a calcium-dependent phospholipid-binding protein with a high affinity for externalized phosphatidylserine, and propidium iodide is excluded by living cells. Scatter plots of fluorescence intensities were analyzed to calculate the percentages of the gated cells as follows: viable cells (Ann V− PI−), early apoptotic cells (Ann V+ PI−), and dead cells (Ann V+ PI+). To induce apoptosis, uninfected RAW 264.7 cells were treated with 100 μM etoposide (Calbiochem, San Diego, CA).
Statistics.
All experiments were performed with at least three independent biological replicates, and the results are reported as the mean and standard deviation (SD) of the mean. Statistically significant differences were determined using one-way analysis of variance (ANOVA) and two-tailed/paired t tests, with P values of less than 0.05 or 0.01 considered significant.
RESULTS
MdsABC confers resistance to antimicrobial dyes and oxidative stress-inducing agents.
To examine the ability of MdsABC to export antimicrobial dyes, S. Typhimurium mutants with chromosomal deletions of the acrAB and tolC (ΔacrAB ΔtolC) or mdsABC (ΔmdsABC) genes were transformed with the constructed pMdsABC plasmid, and their MIC profiles of various antimicrobial dyes were compared to that of the wild-type strain containing an empty vector (pKAN6B) (Table 1). The rationale for using these agents is that they are used as antiseptics on bacteria and are potential substrates of MdsABC or AcrAB-TolC efflux pumps (1, 13, 19). Compared with the MIC results, the arabinose-induced expression of the mdsABC genes in the pMdsABC-containing ΔacrAB ΔtolC mutant strain was able to compensate for the ΔacrAB ΔtolC mutation to export to some degree antimicrobial dyes, but not gentamicin, even though the dye MIC values were much lower than those observed with the wild-type and mdsABC deletion mutant strains containing acrAB and tolC genes. The in vitro susceptibility to gentamicin was significantly increased by the deletion of the arcAB and tolC genes, but it was not significantly changed by the deletion or expression of the mdsABC genes. These results imply that MdsABC plays a role in the export of the dyes to certain degrees in the place of AcrAB-TolC, although ArcAB-TolC plays a major role in the export of such dyes and gentamicin.
TABLE 1.
Susceptibility of Salmonella Typhimurium mutant strains to antimicrobials
| Relevant characteristics of strain | MIC (μg ml−1)a |
||||
|---|---|---|---|---|---|
| Crystal violet | Methylene blue | Acriflavine | Rhodamine 6G | Gentamicin | |
| pKAN6B | >64 | >512 | >64 | >256 | 16 |
| ΔmdsABC/pKAN6B | >64 | >512 | >64 | >256 | 16 |
| ΔmdsABC/pMdsABC | >64 | >512 | >64 | >256 | 16 |
| ΔacrAB ΔtolC/pKAN6B | 4 | 32 | 16 | 4 | 4 |
| ΔacrAB ΔtolC ΔmdsABC/pKAN6B | 4 | 32 | 16 | 4 | 4 |
| ΔacrAB ΔtolC ΔmdsABC/pMdsABC | 16 | 256 | 32 | 64 | 4 |
The MICs of antimicrobials to each strain were determined with various concentrations of crystal violet and acriflavine (0, 1, 2, 4, 8, 16, 32, and 64 μg ml−1), rhodamine 6G (0, 4, 8, 16, 32, 64, 128, and 256 μg ml−1), methylene blue (0, 4, 8, 16, 32, 64, 128, 256, and 512 μg ml−1), and gentamicin (0, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg ml−1).
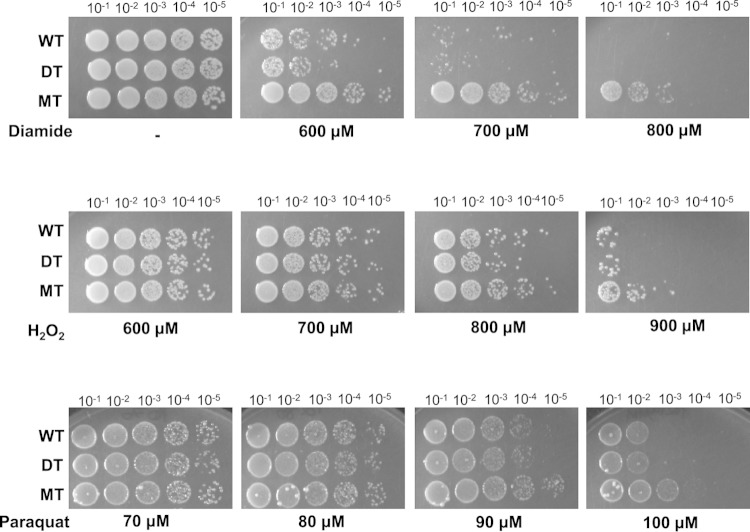
The observed MIC profile of the ΔacrAB ΔtolC mutant exogenously expressing mdsABC was similar to that seen in a previous study of the production of TolC-linked MdsAB by the ΔacrAB ΔmdsC mutant (13), which suggests that MdsC is an alternative outer membrane factor of TolC. Interestingly, forced overproduction of MdsABC in the ΔmdsABC strain (designated MT) resulted in 10- to 1,000-fold higher resistance to oxidative stress-inducing agents, including diamide, H2O2, and Paraquat, than in the control strains harboring pKAN6B (designated WT and DT) (Fig. 1). The induced expression levels of MdsABC in MT cells were about 4-fold higher than the endogenous expression levels in vitro in WT cells measured by quantitative RT-PCR, and the recombinant MdsABC protein production was confirmed by Western blotting with appropriate anti-Flag, anti-myc, and antihexahistidine antibodies (see Fig. S1 in the supplemental material). In contrast, all of the strains used had similar susceptibilities to t-BOOH and NaNO2 (see Fig. S2 in the supplemental material). These observations suggest that the overexpression of MdsABC may lead to an increase of bacterial resistance to diamide and reactive oxygen species (ROS).
FIG 1.
Effects of MdsABC on resistance to oxidative stress-inducing agents. Spot dilution assays of wild-type (WT), mdsABC deletion (DT), and mdsABC overexpression mutant (MT) strains of S. Typhimurium 14028S on LB agar plates containing appropriate concentrations of diamide, H2O2, or Paraquat were performed. Colony growth was observed after 12 to 18 h of incubation at 37°C. Overexpression of MdsABC increased bacterial resistance to diamide, H2O2, and Paraquat.
Infection of macrophages by S. Typhimurium induces the expression of the mdsABC operon.
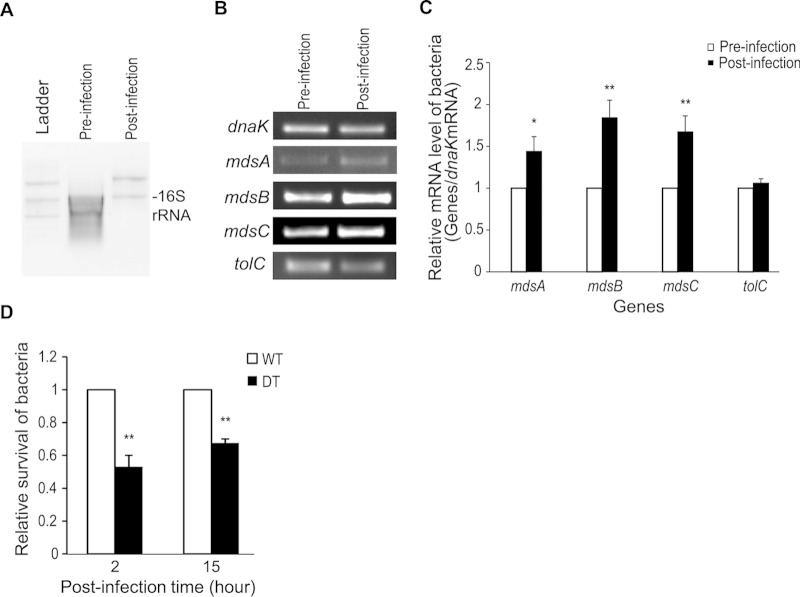
Because it was unclear that the induction of mdsABC expression was promoted in wild-type Salmonella-infected cells, the expression levels of the mdsABC genes were analyzed by quantitative RT-PCR with RNA extracts obtained after infection of RAW 264.7 cells with the WT strain. At 5 h postinfection of the RAW 264.7 cells, the mdsABC expression levels were increased by 1.5- to 2.0-fold compared to preinfection levels, while the transcripts of another MdsAB-assembling partner gene, tolC were nearly constant (Fig. 2A to C). The levels of upregulated MdsABC expression during infection of macrophages with the WT strain were calculated to be about 2-fold lower than those observed with the MT strain exogenously expressing MdsABC, so their effects on resistance to ROS produced during phagocytosis might be smaller than those observed in vitro. The WT strain overexpressing MdsABC in macrophages had the ability to produce 1.5- to 1.9-fold-increased numbers of intracellular bacteria at 2 h and 15 h postinfection, compared to the DT strain lacking mdsABC (Fig. 2D). This indicated that infection of macrophages with wild-type S. Typhimurium led to upregulation of MdsABC, but the lack of MdsABC led to a marked decrease in invasion and proliferation in phagocytic cells. This confirmed the previous suggestion that MdsABC is required for the full virulence of S. Typhimurium (8).
FIG 2.
Expression levels of mdsABC and tolC genes from wild-type S. Typhimurium before and after the infection of macrophages. (A) Total bacterial RNA extracts from Salmonella cells before infection and at 5 h postinfection of RAW 264.7 cells. Bacterial 16S rRNA bands are shown in total RNA extracts. (B) RT-PCR of mdsABC and tolC genes from total RNA extracts obtained from Salmonella cells before infection and 5 h postinfection of RAW 264.7 cells. The mRNA level of the dnaK gene was analyzed in parallel as an internal control to normalize the gene expression levels. (C) Bar graph showing relative expression levels of mdsABC and tolC genes normalized to the expression levels of the dnaK gene in the total bacterial RNA extracts obtained from Salmonella-uninfected (white bars) and Salmonella-infected (black bars) RAW 264.7 cells. The results, reported as the mean ± standard deviation (SD), were calculated from two technical replicates of each RNA extract obtained from three independent macrophage infection experiments. Significant differences analyzed by t tests are shown: *, P < 0.05; **, P < 0.01. (D) Effect of the expression of the mdsABC operon on bacterial invasion and growth in macrophage RAW 264.7 cells. After infection of RAW 264.7 cells with the wild-type (WT) or mdsABC deletion (DT) strain, invasion was measured at 2 h postinfection and bacterial growth (intracellular survival) was measured at 15 h postinfection. The relative levels of invasion and survival are expressed by as ratio of CFU of the DT strain to that of the WT strain (expressed as one unit). Results from three independent macrophage infection assays are reported as the mean ± SD. Statistically significant differences compared to the WT levels are shown with asterisks (**, P < 0.01).
MdsABC causes hypersecretion of phosphatidylserine, conferring bacterial resistance to oxidative stress.
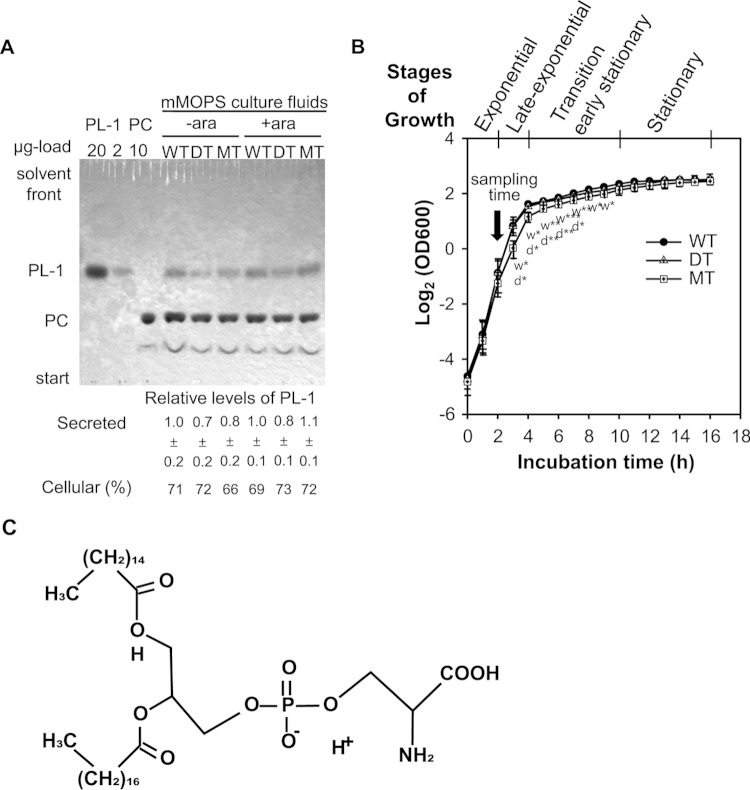
To find an MdsABC-associated factor responsible for bacterial resistance and virulence during macrophage infection, TLC analyses of secreted and cellular lipid components were performed with the strains used for Fig. 1. In this experiment, we found that compared to the MdsABC-deficient bacteria, the MdsABC-expressing bacteria were able to secrete a higher level of a phospholipid compound (PL-1), whereas they had similar levels of cellular phospholipids (Fig. 3A; see Fig. S3 in the supplemental material). When the optical density of cells at 600 nm (OD600) was measured each hour during arabinose-induced batch cultures, the MT strain had significantly decreased growth rates in the late exponential phase and transition phase between 3 h and 9 h, compared to the WT and DT strains, which showed no significant difference in the growth curve (Fig. 3B). Despite the differences in growth rate before entry into stationary phase, the three strains reached similar maximum densities in the stationary phase. The altered growth rate of the MT strain might be due to the overproduction of MdsABC, which was not toxic but rather was responsible for the adaptation process with a compositional change in the cytoplasmic and/or outer membrane of the Gram-negative bacteria, since it led to an increased secretion of PL-1, which can affect various membrane functions by changes not only in phospholipid composition or in membrane properties but also nonmembranous cellular events (20).
FIG 3.
TLC analysis of phospholipids. (A) TLC analysis of phospholipids in cellular and secreted lipid extracts from wild-type (WT), mdsABC deletion (DT), and mdsABC overexpression mutant (MT) strains of S. Typhimurium 14028S grown in the presence or absence of arabinose. Phospholipid spots in silica gels were visualized with molybdenum blue staining solution spray. The percentages of PL-1 resulting from the corresponding cellular phospholipids, as analyzed by two-dimensional TLC and shown in Fig. S3 in the supplemental material, are given below the one-dimensional silica gel of the secreted phospholipids, in which the relative level of PL-1 was calculated by comparison with that in the uninduced WT strain after normalization by spiking of the membrane-filtered culture fluids with dimyristoyl dl-α-phosphatidylcholine (PC), as described in Materials and Methods. (B) Growth curves of WT, DT, and MT strains of S. Typhimurium in the log2 scale. For each strain, the optical density at 600 nm (OD600) was measured every hour from three independent cultures in LB medium containing 100 μg ml−1 kanamycin and 0.1% arabinose at 37°C with shaking at 200 rpm, and results are reported as the mean ± SD. The four characteristic stages of growth from exponential to stationary phase and further investigations at the sampling time of 2 h are shown above the curves. Statistically significant differences in the specific growth rate of the MT strain, analyzed by t tests of OD600 differences for each time compared to those of the WT (w) and DT (d) strains, are shown below the symbols of the MT growth curve, with asterisks indicating the significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Proposed chemical structure of PL-1 (1-palmitoyl-2-stearoyl-phosphatidylserine [PSPS]) identified by tandem mass spectrometry, as shown in Fig. S4 in the supplemental material.
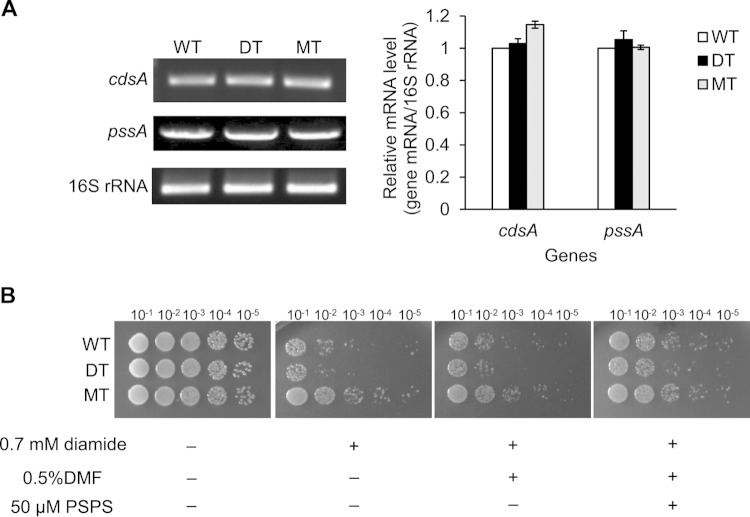
The secreted PL-1 was purified by reversed-phase column chromatography and was identified as 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS) by tandem mass spectrometry (Fig. 3C; see S4 in the supplemental material). When fatty acid methyl esters were analyzed by gas chromatography, two peaks were clearly present, representing palmitate (C16:0) and stearate (C18:0) methyl esters (see Fig. S5 in the supplemental material). The MT strain resulted in a higher level of C16:0 than the WT and DT strains (see Table S3 in the supplemental material). It was unclear whether MdsABC promoted phosphatidylserine (PS) biosynthesis in the bacteria. To assess this possibility, the expression levels of the genes encoding CDP-diacylglycerol synthase (CdsA) and CDP-diacylglycerol:serine O-phosphatidyltransferase (PssA), the enzymatic activities of which are considered to largely contribute to the rate-determining steps of PS biosynthesis in S. Typhimurium (KEGG map00564), were assessed by quantitative RT-PCR with RNA extracts from the WT, DT, and MT strains. The results showed no significant differences in the levels of cdsA and pssA transcripts among the three strains (Fig. 4A), which indicated that the expression of MdsABC did not alter the rate of cellular PS biosynthesis per se.
FIG 4.
Effects of the expression of MdsABC on bacterial biosynthesis of phosphatidylserine and resistance to diamide. (A) Expression levels of the cdsA and pssA genes, coding for CDP-diacylglycerol synthase and CDP-diacylglycerol:serine O-phosphatidyltransferase, respectively, which determine the rate of phosphatidylserine biosynthesis in S. Typhimurium. The 16S rRNA control was measured by semiquantitative RT-PCR with total RNA extracts obtained from exponentially growing cell cultures of the WT, DT, and MT strains in the presence of 0.1% arabinose. Band intensity was quantified by densitometry and normalized based on the level of 16S rRNA. Results from three independent culture experiments are reported as the mean ± SD. (B) Spot dilution assays of S. Typhimurium strains showed an increase in diamide resistance with the addition of 50 μM 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS). Dimethylformamide (DMF) (final concentration, 0.5%) was used as a solvent control.
Although there was a strong relationship between the expression of MdsABC and the secretion of PSPS, it was still unclear whether PSPS played a role in the bacterial defense against oxidative stress. To examine this effect, spot dilution assays were performed after the exposure of the WT, DT, and MT strains to 0.7 mM diamide with or without the addition of purified PSPS at 50 μM, a dose similar to endogenous levels (determined by two-dimensional TLC). Without PSPS treatment, the MdsABC-overexpressing MT strain was more resistant to diamide than the DT and WT strains (Fig. 4B). In the presence of 50 μM PSPS, however, all of the strains were more resistant to diamide than in the absence of PSPS. These results suggest that the hypersecretion of PSPS from the MdsABC-overexpressing bacteria confers resistance to oxidative stress.
Phosphatidylserine reduces the formation of disulfide bonds in membrane-bound proteins with attenuation of superoxide dismutases.
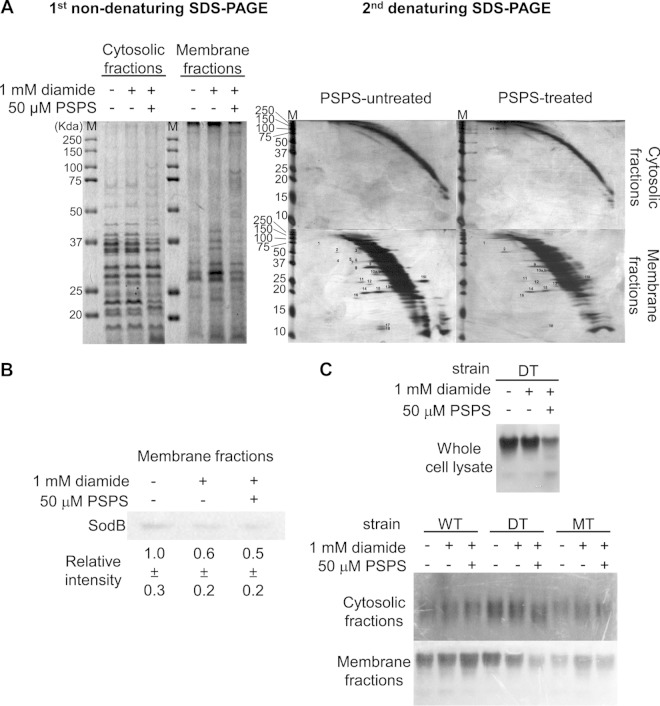
Because diamide promotes the formation of disulfide-bonded proteins via a nonspecific reaction (21), diagonal gel electrophoresis was performed to compare the patterns of disulfide bonds in cytoplasmic and membrane proteins of the MdsABC-deficient DT strain treated or not treated with 50 μM PSPS (Fig. 5A). PSPS treatment markedly reduced the diamide-mediated formation of disulfide-bonded proteins in the membrane fraction compared to the untreated control. In addition, one spot, which formed an intermolecular disulfide bond in alcohol dehydrogenase (AdhE), was found in the cytoplasmic fraction of the bacteria exposed to PSPS. From the 20 spots isolated from the diagonal gels, 19 proteins were identified with high confidence by tandem mass spectrometry (see Table S4 in the supplemental material). From the data for the 19 identified proteins, 8 proteins contained tandem mass spectra that matched the iodoactamide modification (carbamidomethylation) of DTT-reduced cysteine thiol groups at Cys215 of CodA, Cys206 of GlpQ, Cys167 and Cys239 of TalB, Cys88 of SpeB, Cys213 of α-dehydro-β-deoxy-d-glucarate aldolase, Cys70, Cys111, and Cys201 of DeoD, Cys88 of Ppa, and Cys93 of YoaB. The tandem mass spectra of these peptides are shown in Fig. S6 in the supplemental material. Because they did not involve N-ethylmaleimide modification, these cysteine residues, which were detected by DTT reduction followed by iodoacetamide modification, were suggested to primarily form disulfide bonds in membrane-bound proteins under oxidative stress conditions. The other 11 proteins, although they did not have a matched peptide modified by carbamidomethylation, involve reactive cysteine residues near or in catalytic or transient metal-binding sites susceptible to regulation by oxidative modifications. The overall results indicate that PSPS is effective in reducing the formation of disulfide bonds in membrane-bound proteins.
FIG 5.
Effects of PSPS on the reduction of disulfide bond formation and the attenuation of extracellular superoxide dismutases. (A) Fractionation (left panel) and diagonal gel analysis (right panels) of disulfide-bonded proteins in cytosolic and membrane fractions obtained from MdsABC-deficient mutant (DT) cells of S. Typhimurium treated or not treated with 1 mM diamide or 50 μM 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS) purified from the used culture medium of MdsABC-overproducing mutant (MT) cells. Protein spots above or below the diagonal curves are the same as the spot numbers in Table S4 in the supplemental material for the tandem mass spectral data of the proteins and reactive cysteine residues involved in the formation of intramolecular and intermolecular disulfide bonds. (B) Western blot analysis of the superoxide dismutase SodB in the membrane fraction of DT cells treated or not treated with 1 mM diamide or 50 μM PSPS. Relative SodB band intensity was calculated using ImageQuant software version 5.2 (Molecular Dynamics). (C) Activity gel staining of the superoxide dismutases of the WT, DT, and MT cells treated or not treated with 1 mM diamide or 50 μM PSPS. The upper panel shows the attenuation of superoxide dismutase activity in whole-cell lysates of DT cells treated with 50 μM PSPS. When fractionated into cytoplasmic and membrane fractions, the cytoplasmic fractions of the WT, DT, and MT cells (middle panel) showed little or no significant changes in superoxide dismutase activity whether or not they were treated with 1 mM diamide or 50 μM PSPS. The lower panel shows a marked decrease of extracellular superoxide dismutase activity in the membrane fraction of DT cells treated with 50 μM PSPS, which was similar to the level observed in MT cells overproducing MdsABC.
Notably, PSPS treatment resulted in a marked decrease of the disulfide-bonded superoxide dismutase (SOD) SodB in the membrane fraction. With respect to defense against oxidative stress, SodB is generally known as a primary enzyme catalyzing the decomposition of superoxide anion to H2O2 in the cytoplasm. In this study, however, Western blots showed that the DT strain treated with diamide and/or PSPS had significantly decreased SodB in the membrane fraction compared to the untreated strain (Fig. 5B). Moreover, the PSPS-treated DT strain markedly decreased extracellular SOD activity, to a level similar to that observed with forced overproduction of MdsABC, and increased secretion of PSPS from the MT strain (Fig. 5C). These patterns demonstrated that the hypersecretion of PSPS could protect bacteria from oxidative damage through alterations in the bacterial cell envelope, leading to a decrease in extracellular SODs.
Phosphatidylserine enhances the infection, but not killing, of macrophages.
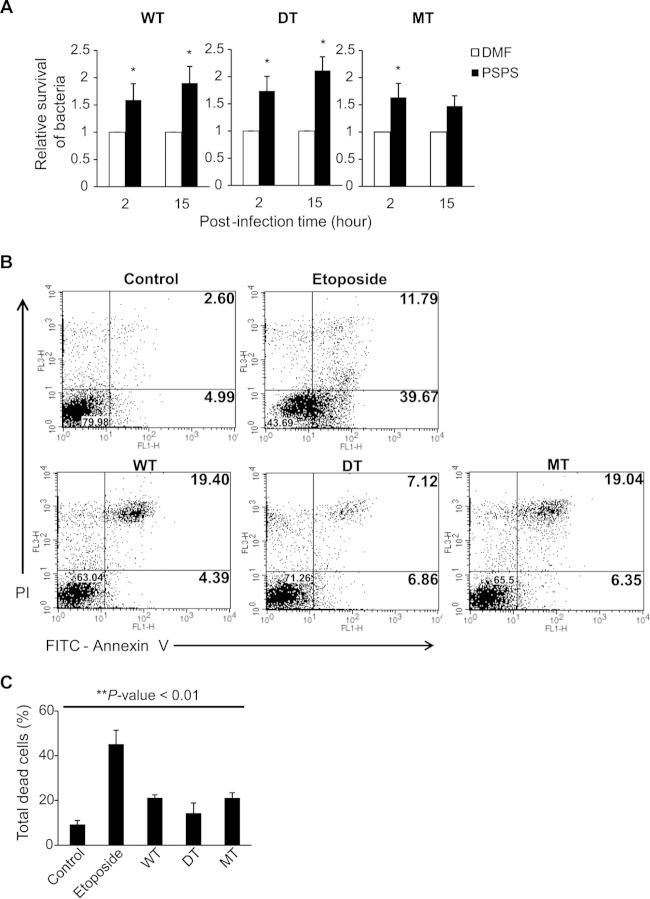
To characterize the pathogenic effects of MdsABC and PSPS, RAW 264.7 cells were infected with the DT, WT, and MT strains in the presence or absence of 50 μM PSPS. As shown Fig. 2D, in the absence of PSPS, the DT strain lacking mdsABC had low invasive potential measured at 2 h postinfection and low growth measured at 15 h postinfection, compared to the wild-type strain overexpressing the mdsABC operon during phagocytosis. In contrast, all of the PSPS-treated bacteria increased invasion and proliferation, by up to 1.7- to 2.1-fold relative to the untreated bacteria (Fig. 6A). The addition of purified PSPS had a relatively lesser effect on the growth of the MT strain, because the hypersecreted PSPS might be sufficient to increase infection of macrophages by the preadapted bacteria.
FIG 6.
Effects of MdsABC and phosphatidylserine on bacterial invasion and survival in macrophage-like RAW 264.7 cells. (A) Effect of 50 μM PSPS on the infection of macrophage RAW 264.7 cells by the WT, DT, and mdsABC-overproducing (MT) bacteria. Bacterial invasion and survival were measured from three independent experiments, as described for Fig. 2D, and results are reported as the mean ± SD. Statistically significant differences compared to the WT levels are shown with asterisks: *, P < 0.05. (B) Scatter plots of infected and uninfected macrophages stained with FITC-annexin V (Ann V) and propidium iodide (PI) at 15 h postinfection. Upper panels, scatter plots of uninfected and etoposide-treated RAW 264.7 cells, which represent normal control and apoptosis-induced macrophages, respectively, under the given experimental conditions. Lower panels, scatter plots of RAW 264.7 cells infected by WT, DT, and MT strains with different expression levels of MdsABC. The percentages of viable cells (Ann V− PI−), dead cells (Ann V+ PI+), and apoptotic cells (Ann V+ PI−) were calculated from scatter plots of PS-binding annexin V (Ann V)- and PI-stained dead cells. (C) Percentages of total dead cells (Ann V+ PI+) analyzed using a calibrated FACS flow cytometer. Results from three independent experiments are reported as the mean ± SD. Statistically significant differences compared to the control were analyzed with general one-way ANOVA and paired t tests, with significance set at a P value of <0.01 (**).
The pathological effects of PSPS on macrophages were evaluated using flow cytometry by staining cells with FITC-annexin V (Ann V) and propidium iodide (PI). During an incubation time period of 15 h, the uninfected RAW 264.7 cells treated with 50 μM PSPS displayed no significant change in the distribution of labeled cells compared to the untreated control. PSPS (50 μM) induced neither apoptosis nor necrosis of the uninfected cells only (data not shown). Both MT and WT strains that overexpressed MdsABC pre- and postinfection similarly showed a marked increase in cell death of the infected macrophages, but they had no significant changes in the apoptosis ratio compared to the uninfected macrophages (Fig. 6B and C). In contrast, the MdsABC-deficient DT strain producing less PSPS than the WT and MT strains induced a moderate effect on cell death of the infected macrophages without any significant change of the apoptosis rate. Remarkably, when treated with 50 μM PSPS, the DT strain markedly increased cell death of the infected macrophages up to ∼19% of the total cells at 15 h postinfection, but there was no significant effect on apoptosis of the infected cells. This is similar to the case with the MT and WT strains overexpressing MdsABC, but they are clearly different from etoposide-treated cells that readily undergo apoptosis.
DISCUSSION
We demonstrated that MdsABC plays a role in the secretion of phosphatidylserine from the cell membranes of S. Typhimurium to the culture medium as well as the export of antimicrobial dyes. In this study, artificially induced overexpression of MdsABC caused an increased secretion of PSPS with a significant temporal decrease in growth rate of the bacteria during late exponential phase and transition phase, which influences changes in the membrane composition as well as numerous cellular events that are known to occur at the end of exponential growth to promote adaptation and proliferation in host cells (22). However, there was no clear relationship between MdsABC expression and growth rate of wild-type S. Typhimurium in vitro in culture medium (data not shown). We found that wild-type S. Typhimurium overexpressed MdsABC during infection of macrophages, consistent with a previous study which suggested that this efflux pump plays a physiological role inside host cells (1). The overproduced MdsABC can complement the acrAB or tolC mutant to a certain degree, which is consistent with previous studies that showed that MdsAB assembles with TolC and confers bacterial resistance to antimicrobial dyes, such as acriflavine, crystal violet, methylene blue, and rhodamine 6G (13). It assists in exuding a wide range of extracellular and intracellular compounds to maintain bacterial homeostasis in concert with TolC-linked tripartite efflux pumps (7, 23, 24). Bacterial efflux pumps have a role in exporting not only abiotics such as antibiotics, detergents, and dyes but also host-derived antimicrobial agents. Furthermore, some transporters are involved in pathogenicity and are suspected to export bacterium-derived toxins, degradative enzymes, and other substrates are suspected to establish infection in the host and to evade host defenses (7).
S. Typhimurium is able to promote invasion and proliferation within the host by increasing resistance to host innate immune responses through the secretion of virulence factors and alterations in the bacterial cell envelope (25). Macrophage-infecting bacteria exploit multiple pathways to cope with host innate immunity and to establish infection in the host (26). Among these pathways, it is most important for intracellular bacteria to induce a protective factor against ROS-induced oxidative stress because phagocytes kill bacteria by stimulating the generation of ROS, causing oxidative damages in the bacteria and potentiating the host antimicrobial defense (27). A recent study argued that disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice (28). While Salmonella is largely killed through and NADPH oxidase-dependent mechanism by generating excess H2O2 in the phagosomes of neutrophils and monocytes, most live Salmonella organisms can reside in the phagosomes of macrophages, with apparently little impact of NADPH oxidase on the generation of H2O2 (28). In addition to the disparate effects of host cells on ROS production, bacterial resistance to oxidative stress allows intracellular bacteria to grow and survive in host cells (29–31). The present study shows that the overexpression of MdsABC leads to a small change in the phenotype of Salmonella by increasing secretion of PSPS but confers some resistance to ROS-producing phagocytosis. Using diagonal gel analysis, we showed that external PSPS played an important role in the ability of the macrophage-infecting bacteria to moderate oxidative damage in membrane-bound proteins with the attenuation of extracellular SODs. This mechanism is intimately linked to the defense mechanism of S. Typhimurium against phagocytosis (32). It is likely that the overexpression of MdsABC during infection of macrophages also alters the relative abundance of membrane efflux proteins which cooperate in the export of a variety of antimicrobial agents and host antimicrobial peptides (19, 33, 34, 35). A previous study showed that the induction of MdsABC transcription is controlled by a MerR-like GolS regulator in response to gold stress (12). The Salmonella-specific MdsABC operon controlled by a divergent MerR regulator may be involved in various mechanisms of pathogenesis as well as in gold resistance.
During the infection of macrophages, expression of the mdsABC operon is upregulated in S. Typhimurium, whereas expression of the tolC transcript remains constant. This implies that the mdsABC operon is regulated independent of TolC. In S. enterica, TolC is highly linked to the expression of chemotaxis genes, motility genes, and SPI-1-encoded type III secretion system genes under the PhoP regulon (35). In another study, we found that the induction of mdsABC expression was strongly related to an increase in the secretion of SPI-1 proteins and cellular stress response proteins in the cytoplasm, but there was no significant alteration in the expression levels of SPI-1 genes and the global regulator phoPQ genes (unpublished data). The induction of mdsABC expression during macrophage infection provides a previously uncharacterized pathway for bacterial resistance and pathogenicity in addition to the AcrAB-TolC and type III secretion systems, although the mechanism is unknown. Our study shows that the upregulation of MdsABC increases secretion of PSPS to promote infection and host cell death as well as resistance to some antimicrobial dyes and defense against diamide-mediated formation of protein disulfide bonds in the membrane fraction. PSPS is a key factor contributing to the MdsABC-mediated pathway for bacterial survival and virulence in phagocytic cells, because the external addition of PSPS enhances the ability of S. Typhimurium to infect and reproduce in macrophages and increases nonapoptotic cell death (necrosis) of the infected cells. It is possible that externalized PSPS on the bacterial surface acts as a ligand for annexin V on macrophages to stimulate phagocytosis without triggering apoptotic signaling (36–38). It may also be helpful for macrophage-infecting bacteria to escape from pathogen-associated, molecular pattern-induced immune responses because phospholipids derived from the inner membrane of bacteria can inhibit the binding of lipopolysaccharide (LPS) to the LPS-binding protein of the host cells (39). The secreted PSPS has the potential to increase the pathogenicity of S. Typhimurium throughout the population; however, it remains unclear whether this potential is shared by other bacteria that utilize similar indole signaling pathways in E. coli and S. Typhimurium (40, 41). Further studies are needed to understand the physiopathological role of PSPS and related compounds in pathogenic or commensal bacteria in host animal environments.
In summary, we find that MdsABC not only plays a certain role in the export of antimicrobial agents but also influences the phenotype of S. Typhimurium by changing the growth rate and membrane composition in phagocytic cells. Overexpression of MdsABC increases secretion of PSPS, enhances infection, and leads to an increase of intracellular bacteria and cell death of the infected macrophages. Macrophage infection experiments with the mdsABC deletion and overexpression mutant strains showed that artificially induced overexpression of MdsABC and external addition of PSPS have a similar potential to promote bacterial infection and mortality of macrophages, as seen during infection with wild-type S. Typhimurium. The PSPS treatment was particularly effective in reducing the formation of protein disulfide bonds in the membrane fraction of S. Typhimurium with the attenuation of the extracellular SOD activities. These findings suggest that PSPS is a potential new virulence factor that may affect microbial defense mechanisms against ROS produced during phagocytosis, and they will provide new insights necessary to understand the potential role of PSPS in host-microbe interactions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hanyoung Jin for assistance with FACS analysis.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2013R1A1A2061369 and 2014R1A2A2A09052791) and by the Strategic Initiative for Microbiomes in Agriculture and Food (914010-04-1-HD020), Ministry of Agriculture, Food, and Rural Affairs, Republic of Korea.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00653-15.
REFERENCES
- 1.Nishino K, Nikaido E, Yamaguchi A. 2009. Regulation and physiological function of multidrug efflux pumps in Escherichia coli and Salmonella. Biochim Biophys Acta 1794:834–843. doi: 10.1016/j.bbapap.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Poole K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin Microbiol Infect 10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies J. 1994. Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 4.Putman M, van Veen HW, Konings WN. 2000. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev 64:672–693. doi: 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saier MHJ, Paulsen IT. 2001. Phylogeny of multidrug transporters. Semin Cell Dev Biol 12:205–213. doi: 10.1006/scdb.2000.0246. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen IT. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr Opin Microbiol 16:446–451. [DOI] [PubMed] [Google Scholar]
- 7.Piddock LJ. 2006. Multidrug-resistance efflux pump—not just for resistance. Nat Rev Microbiol 4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 8.Nishino K, Latifi T, Groisman EA. 2006. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol Microbiol 59:126–141. doi: 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Yeom JH, Seo S, Lee M, Kim S, Bae J, Lee K, Hwang J. 4 March 2015. Functional analysis of Vibrio vulnificus RND efflux pumps homologous to Vibrio cholerae VexAB and VexCD, and to Escherichia coli AcrAB. J Microbiol doi: 10.1007/s12275-015-5037-0. [DOI] [PubMed] [Google Scholar]
- 11.DuPont HL. 2007. The growing threat of foodborne bacterial enteropathogens of animal origin. Clin Infect Dis 45:1353–1361. doi: 10.1086/522662. [DOI] [PubMed] [Google Scholar]
- 12.Pontel LB, Audero ME, Espariz M, Checa SK, Soncini FC. 2007. GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol Microbiol 66:814–825. doi: 10.1111/j.1365-2958.2007.05963.x. [DOI] [PubMed] [Google Scholar]
- 13.Song S, Hwang S, Lee S, Ha NC, Lee K. 2014. Interaction mediated by the putative tip regions of MdsA and MdsC in the formation of Salmonella-specific tripartite efflux pump. PLoS One 9:e100881. doi: 10.1371/journal.pone.0100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartog E, Ben-Shalom L, Shachar D, Matthews KR, Yaron S. 2008. Regulation of marA, soxS, rob, acrAB and micF in Salmonella enterica serovar Typhimurium. Microbiol Immunol 52:565–574. doi: 10.1111/j.1348-0421.2008.00075.x. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido E, Shirosaka I, Yamaguchi A, Nishino K. 2011. Regulation of the AcrAB multidrug efflux pump in Salmonella enterica serovar Typhimurium in response to indole and paraquat. Microbiology 157:648–655. doi: 10.1099/mic.0.045757-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Yu M. 2012. Overexpression of reactive cysteine-containing 2-nitrobenzoate nitroreductase (NbaA) and its mutants alters the sensitivity of Escherichia coli to reactive oxygen species by reprogramming a regulatory network of disulfide-bonded proteins. J Proteome Res 11:3219–3230. doi: 10.1021/pr300221b. [DOI] [PubMed] [Google Scholar]
- 17.Parsons JG, Patton S. 1967. Two-dimensional thin-layer chromatography of polar lipids from milk and mammary tissue. J Lipid Res 8:696–698. [PubMed] [Google Scholar]
- 18.Buckley AM, Webber MA, Cooles S, Randall LP, La Ragione RM, Woodward MJ, Piddock LJ. 2006. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol 8:847–856. doi: 10.1111/j.1462-5822.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 19.Piddock LJV. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baysse C, O'Gara F. 2007. Role of membrane structure during stress signaling and adaptation in Pseudomonas, p 193–224. In Lamos JL, Filloux A (ed), Pseudomonas, vol 5 A model system in biology. Springer, New York, NY. [Google Scholar]
- 21.Pöther DC, Liebeke M, Hochgräfe F, Antelmann H, Becher D, Lalk M, Lindequist U, Borovok I, Cohen G, Aharonowitz Y, Hecker M. 2009. Diamide triggers mainly S thiolations in the cytoplasmic proteomes of Bacillus subtilis and Staphylococcus aureus. J Bacteriol 191:7520–7530. doi: 10.1128/JB.00937-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibarra JA, Knodler LA, Sturdevant DE, Virtaneva K, Carmody AB, Fischer ER, Porcella SF, Steele-Mortimer O. 2010. Induction of Salmonella pathogenicity island 1 under different growth conditions can affect Salmonella-host cell interactions in vitro. Microbiology 156:1120–1133. doi: 10.1099/mic.0.032896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosner JL, Martin RG. 2009. An excretory function for the Escherichia coli outer membrane pore TolC: upregulation of marA and soxS transcription and Rob activity due to metabolites accumulated in tolC mutants. J Bacteriol 191:5283–5292. doi: 10.1128/JB.00507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos MR, Cosme AM, Becker JD, Medeiros JMC, Mata MF, Moreira LM. 2010. Absence of functional TolC protein causes increased stress response gene expression in Sinorhizobium meliloti. BMC Microbiol 10:1471–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst RK, Guina T, Miller SI. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J Infect Dis 179:S326–S330. doi: 10.1086/513850. [DOI] [PubMed] [Google Scholar]
- 26.Antonio Ibarra J, Steele-Mortimer O. 2009. Salmonella—the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol 11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slauch JM. 2011. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol 80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton NA, Schürmann N, Casse O, Steeb AK, Claudi B, Zankl J, Schmidt A, Bumann D. 2014. Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host Microbe 15:72–83. doi: 10.1016/j.chom.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Lahiri A, Lahiri A, Iyer N, Das P, Chakravortty D. 2010. Visiting the cell biology of Salmonella infection. Microbes Infect 12:809–818. doi: 10.1016/j.micinf.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Huanga J, Canadiena V, Lama GY, Steinberga BE, Dinauerc MC, Magalhaesd MA, Glogauer M, Grinstein S, Brumell JH. 2009. Activation of antibacterial autophage by NADPH oxidases. Proc Natl Acad Sci U S A 160:6226–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon BY, Yeom JH, Kim JS, Um SH, Jo I, Lee K, Kim YH, Ha NC. 2014. Direct ROS scavenging activity of CueP from Salmonella enterica serovar Typhimurium. Mol Cells 37:100–108. doi: 10.14348/molcells.2014.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amano F. 2011. SEp22, Salmonella Dps, a key molecule bearing both pathogenicity and resistance to environmental stresses in Salmonella. J Health Sci 57:458–471. doi: 10.1248/jhs.57.458. [DOI] [Google Scholar]
- 33.Eswaran J, Koronakis E, Higgins MK, Hughes C, Koronakis V. 2004. Three's company: component structures bring a closer view of tripartite drug efflux pumps. Curr Opin Struct Biol 14:741–747. doi: 10.1016/j.sbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webber MA, Bailey AM, Blair JMA, Morgan E, Stevens MP, Hinton JCD, Wain AIJ, Piddock LJV. 2009. The global consequence of disruption of the AcrAB-TolC efflux pump in Salmonella enterica includes reduced expression of SPI-1 and other attributes required to infect the host. J Bacteriol 191:4276–4285. doi: 10.1128/JB.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frolov VA, Shnyrova AV, Zimmerberg J. 2011. Lipid polymorphisms and membrane shape. Cold Spring Harb Perspect Biol 3:a004747. doi: 10.1101/cshperspect.a004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhoven B, Schlegel RA, Williamson P. 1995. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. 2004. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell 15:2863–2872. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto M, Asi Y, Ogawa T. 2003. Treponemal phospholipids inhibit innate immune responses induced by pathogen-associated molecular patterns. J Biol Chem 278:44205–44213. doi: 10.1074/jbc.M306735200. [DOI] [PubMed] [Google Scholar]
- 40.Vega NM, Allison KR, Samuels AN, Klempner MS, Collins JJ. 2013. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proc Acad Natl Soc U S A 110:14420–14425. doi: 10.1073/pnas.1308085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair JM, Cloeckaert A, Nishino K, Piddock LJ. 2013. Alternative explanation for indole-induced antibiotic tolerance in Salmonella. Proc Natl Acad Sci U S A 110:E4569. doi: 10.1073/pnas.1318318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.