Abstract
Despite the proven ability of immunization to reduce Helicobacter infection in mouse models, the precise mechanism of protection has remained elusive. In this study, we evaluated the role of inflammatory monocytes in the vaccine-induced reduction of Helicobacter felis infection. We first showed by using flow cytometric analysis that Ly6Clow major histocompatibility complex class II-positive chemokine receptor type 2 (CCR2)-positive CD64+ inflammatory monocytes accumulate in the stomach mucosa during the vaccine-induced reduction of H. felis infection. To determine whether inflammatory monocytes played a role in the protection, these cells were depleted with anti-CCR2 depleting antibodies. Indeed, depletion of inflammatory monocytes was associated with an impaired vaccine-induced reduction of H. felis infection on day 5 postinfection. To determine whether inflammatory monocytes had a direct or indirect role, we studied their antimicrobial activities. We observed that inflammatory monocytes produced tumor necrosis factor alpha and inducible nitric oxide synthase (iNOS), two major antimicrobial factors. Lastly, by using a Helicobacter in vitro killing assay, we showed that mouse inflammatory monocytes and activated human monocytes killed H. pylori in an iNOS-dependent manner. Collectively, these data show that inflammatory monocytes play a direct role in the immunization-induced reduction of H. felis infection from the gastric mucosa.
INTRODUCTION
Helicobacter pylori is a helical, rod-shaped, microaerophilic Gram-negative bacterium. It specifically colonizes the stomach of 50% of the world's population (1, 2). H. pylori infection induces inflammation of the gastric mucosa; however, only 15% of infected individuals develop clinical symptoms (3, 4). These clinical symptoms start with superficial gastritis and may evolve into chronic atrophic gastritis, peptic ulcer disease, intestinal metaplasia, gastric carcinoma, or gastric mucosa-associated lymphoid tissue lymphoma (5). Due to its high prevalence, H. pylori is a major cause of cancer worldwide. It is eradicated by antibiotic therapies, which are expensive and promote resistance (6). Hence, alternative therapies need to be developed to eradicate antibiotic-resistant strains in western countries and to reduce the prevalence of H. pylori infection in developing countries. From this perceptive, the development of a therapeutic and/or preventive vaccine(s) needs to be pursued.
In mice, H. pylori infection can be prevented or cleared by prophylactic and therapeutic vaccinations. Some clinical trials have been conducted in humans and have shown that immunization with H. pylori antigens is safe and immunogenic (7–9); however, the elimination or prevention of H. pylori infection has not been achieved with this approach. To develop an efficient vaccination strategy, a better understanding of the protective mechanisms is critical. Indeed, the running of clinical trials without this knowledge is extremely risky (10).
In animal models of the vaccine-induced reduction of Helicobacter infection, mucosal or systemic vaccinations rely on CD4+ T helper (Th) cells to clear the bacteria from the stomach mucosa (11, 12). The chemokine-dependent recruitment of inflammatory cells into the stomach mucosa is associated with the Th1, Th2, and/or Th17 cell response (13–15). Mast cells and also neutrophils, in some circumstances, have already been shown to play critical roles in the vaccine-induced reduction of Helicobacter infection (16–19); however, the role of monocytes has not been evaluated.
Mouse monocytes are subdivided into two populations. Circulating or patrolling monocytes (Ly6Clow and fractalkine receptor [CX3CR1high]) adhere to and migrate along the luminal side of endothelial cells (20, 21). They play a role in IgG-dependent effector functions (22) and were suggested to contribute to tissue repair in the infracted myocardium (23). The second population of monocytes (Ly6Chigh) is recruited to inflamed tissues during infections. These inflammatory monocytes can give rise to tissue macrophages and tumor necrosis factor alpha (TNF-α)- and inducible nitric oxide synthase (iNOS)-producing dendritic cells (Tip-DCs). Macrophages and Tip-DCs have been shown to play key roles in controlling mucosal pathogens, such as Listeria monocytogenes (24), Toxoplasma gondii (25), and Aspergillus fumigatus (26). The chemokine receptor chemokine receptor type 2 (CCR2), which binds monocyte chemoattractant protein 1 (MCP-1; CCL2) and MCP-3 (CCL7), is expressed by inflammatory monocytes (27). During infections, CCR2 mediates the exit of inflammatory monocytes from the bone marrow and their recruitment to sites of inflammation (28, 29). Depending on the experimental model, other chemokine receptors, such as CX3CR1, CCR6, or CCR1, have also been shown to promote the recruitment of inflammatory monocytes to sites of inflammation (30–33).
In this study, we investigated the role of inflammatory monocytes in the vaccine-induced reduction of H. felis infection. The in vivo study of vaccine-induced protective immune mechanisms is facilitated by the use of H. felis instead of H. pylori. Indeed, only one oral gavage, i.e., one antigen challenge, is necessary to infect mice with H. felis (16). In comparison, H. pylori infection requires two oral gavages at a 1-day interval (16), which leads to some uncertainty about the time of initiation of the protective immune response. During the vaccine-induced reduction of H. felis infection, we observed that a Ly6Clow major histocompatibility complex class II (MHC-II)-positive (MHC-II+) CCR2+ CD64+ inflammatory monocyte population accumulates in the stomach mucosa. To investigate the role of these inflammatory monocytes, they were depleted by infusion of anti-CCR2 depleting antibodies. Vaccinated mice injected with anti-CCR2 antibodies could not reduce the infection after 5 days, whereas vaccinated mice treated with the control antibody could. To determine whether the inflammatory monocytes had a direct or indirect role, we studied their antimicrobial activities. The inflammatory monocytes infiltrating the stomach mucosa of vaccinated and infected mice expressed iNOS/TNF-α and were able to kill H. pylori in vitro in an iNOS-dependent manner. Similarly, activated human monocytes efficiently killed H. pylori in vitro. Collectively, these results demonstrate that inflammatory monocytes play a role in the vaccine-induced reduction of Helicobacter infection.
MATERIALS AND METHODS
Mice.
Female BALB/c OlaHsd mice (6 to 8 weeks old) were purchased from Harlan, Horst, The Netherlands. BALB/c-CCR2−/− mice were provided by H. Gilgenkrantz (34) and bred in our animal facility. This study was approved by the State of Vaud Veterinary Office (authorization no. 836.9). Mice were bred under conventional, non-specific-pathogen-free conditions.
Bacteria and infection.
H. felis strain ATCC 49179 and H. pylori P49 were grown in brain heart infusion (BHI) broth supplemented with 0.25% yeast extract and 10% fetal calf serum (FCS; PAA, Pasching, Austria) under microaerophilic conditions. H. felis infection was performed by orogastric intubation with polyvinyl chloride feeding tubes (catalog number V0104050; ECIMED, Boissy-Saint-Léger, France). The tubing was introduced at a fixed distance of 4.5 cm from the incisors. Mice were treated once with 1 × 108 H. felis cells, administered intragastrically in 200 μl BHI broth.
Immunization and monocyte and neutrophil depletion.
Mice were immunized intranasally 4 times at 1-week intervals with 15 μg of recombinant H. pylori urease (kindly provided by Sanofi-Pasteur, Lyon, France) combined with 5 μg of cholera toxin (Calbiochem, Lucerne, Switzerland). To deplete monocytes in vivo, immunized mice were injected intraperitoneally either with 20 μg of anti-CCR2 depleting monoclonal antibody (MAb; MAb MC-21) (35) or with purified control rat antibody (antibody RTK4530; BioLegend, San Diego, CA) at days −1, 0, 1, 2, 3, and 4 after H. felis infection. Mice were sacrificed on day 5 after bacterial challenge. For neutrophil depletion, 200 μg of anti-Ly6G (MAb 1A8) or control IgG was injected intraperitoneally at days −1, 1, and 4 after H. felis infection. Mice were sacrificed on day 5 after bacterial challenge.
Assessment of Helicobacter colonization and histology.
The rapid urease test (RUT; Jatrox test; Procter & Gamble, Weiterstadt, Germany) was used to assess infection status. RUT values have already been shown to match H. felis colonization scores evaluated either on tissue sections (16) or by flaB quantitative PCR (36). To determine RUT values, stomachs were retrieved and cut along the lesser and greater curves to obtain identical halves. One half was immersed in 500 μl the supplier's suspension and incubated at 37°C for 2 h. Specimens were centrifuged, and the supernatant was used for spectrophotometric quantification at an optical density of 550 nm. The other half was processed for histology and fixed in neutral buffered 10% formalin, embedded in paraffin, and routinely processed. Sections 5 μm thick were stained with hematoxylin and eosin. Pathologists unaware of the treatments administered to the mice scored the histopathologic changes in coded sections of the antral and fundic mucosae. Inflammation scores were calculated by the addition of the inflammatory and atrophy grades determined for the antral and fundic mucosae. The degree of inflammation was defined as the absence (grade 0) or the presence (grade 1, mild; grade 2, moderate; grade 3, severe) of polymorphonuclear leukocytes (acute inflammation) and lymphocytic and plasmacytic cells (chronic inflammation) in the stomach mucosa. Atrophy was characterized by the loss of parietal cells and a decrease in the mucosal thickness and graded.
Preparation of gastric immune cell suspension.
Stomachs were recovered and cut longitudinally in half. After washing (NaCl, 9 g/liter), the stomach halves were cut into small pieces using a scalpel. Stomach tissue fragments were then incubated in phosphate-buffered saline (PBS)–1 mmol/liter EDTA for 20 min under gentle stirring at room temperature. After centrifugation (400 × g, 10 min, 4°C), the tissue fragments were incubated at 37°C for 20 min with stirring (150 rpm) in 10 ml RPMI 1640 (Gibco, Invitrogen Corporation, Carlsbad, CA), 10% FCS (heat inactivated), and 0.5 mg/ml type IV collagenase (catalog number C-5138; Sigma). The tissue suspensions were then passed through 2 mesh strainers (mesh sizes, 70 μm and 40 μm) to separate the cell suspension from the undigested tissue. The cell suspensions were centrifuged at 400 × g for 10 min (4°C), and the recovered cells were washed twice with 20 ml fresh 2% FCS–RPMI 1640. The cells were then resuspended in 10 ml 2% FCS, RPMI 1640, and 5 ml Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), the cell suspension was added to the bottom of the tubes (50 ml; Falcon; BD Biosciences, Bedford, MA, USA), and the gradients were centrifuged for 10 min at 1,024 × g (4°C) without a break. Immune cells were recovered from the interface between Ficoll and 2% FCS–RPMI 1640 and washed twice with 20 ml fresh 2% FCS–RPMI 1640.
Flow cytometry and cell sorting.
Cells were resuspended in fluorescence-activated cell sorter (FACS) buffer (1% bovine serum albumin and 5 mmol/liter EDTA in PBS) and incubated with conjugated monoclonal antibodies in the presence of Fc blockers (catalog number 2.4G2; Becton Dickinson [BD], San Jose, CA). All data acquisition was performed on a Cytomics FC 500 flow cytometer (Beckman Coulter, Brea, CA). We used the following reagents for the staining procedures: anti-CD11b-phycoerythrin (PE; catalog number M1/70; BD, San Jose, CA), anti-Ly6C-fluorescein isothiocyanate (FITC; catalog number AL-21; BD), anti-CD45-biotin (catalog number 30-F11; BD), anti-Ly6G-PE (MAb 1A8; BD), anti-CD11b-peridinin chlorophyll protein-Cy5.5 (catalog number M1/70; BD), anti-CD11c-PE (catalog number HL3; BD), anti-CD11b-FITC (catalog number M1/70; BD), anti-CD64-PE (catalog number 5X54-5/7.1; BioLegend, San Diego, CA), anti-Ly6C-PE-Cy7 (catalog number HK1.4; BioLegend), anti-F4/80-PE-Cy7 (catalog number BM8; BioLegend), anti-MHC-II-FITC (catalog number M5/114.15.2; BioLegend), anti-CD4-PE-Cy7 (catalog number GK1.5; BioLegend), anti-CD115-PE (catalog number AFS98; eBioscience, San Diego, CA), anti-CCR2 MAb (MAb MC-21) (35), anti-rat IgG-Alexa Fluor 488 (catalog number A11006; Molecular Probes), anti-rabbit IgG-Alexa Fluor 488 (catalog number A11037; Molecular Probes, Life Technologies, Logan, UT), rabbit anti-CX3CR1 (catalog number ab8021; Abcam, Cambridge, United Kingdom), and streptavidin-PE-CF594 (BD). For intracellular staining, gastric immune cells were permeabilized, fixed (Cytofix/Cytoperm; BD), and stained with anti-TNF-α PE-Cy7 antibody (catalog number MP6-XT22; BD) and rabbit anti-NOS2 (MAb M19; catalog number sc650; Santa Cruz, Dallas, TX). The secondary antibody was anti-rabbit IgG-Alexa Fluor 488 (catalog number A11037; Molecular Probes). FACS data were analyzed using Kaluza software (Beckman Coulter, Brea, CA). Dead cells were excluded using 7-aminoactinomycin D (Beckman Coulter, Brea, CA). Cell sorting was performed on gastric immune cells extracted from the stomach of vaccinated mice at 5 days postinfection with a BD FACSAria IIu flow cytometer using BD FACSDiva (version 6.1.2) software (BD, San Jose, CA). Magnetically activated cell sorting (MACS) was performed on gastric immune cells extracted from the stomach at 5 days postinfection using anti-CD11b MACS beads (Miltenyi Biotec, Bergisch Gladbasch, Germany).
Quantitative PCR.
RNA extraction was performed on stomach tissue using an RNeasy minikit (Qiagen, Valencia, CA). RNA (1 μg) was reverse transcribed into cDNA using a PrimeScript reverse transcriptase (RT) reagent kit (TaKaRa Bio Inc., Otsu, Japan). PCR amplification was performed on a MyiQ iCycler apparatus (Bio-Rad, Hercules, CA), using 96-well plates (Bio-Rad). The PCR was performed in duplicate with iQ SYBR green Supermix (Bio-Rad). Samples were heated at 95°C for 3 min and then subjected to 35 cycles consisting of denaturation (95°C, 15 s) and primer annealing and extension for 60 s at 60°C (for GAPDH [glyceraldehyde-3-phosphate dehydrogenase] and interleukin-17 [IL-17]), 65°C (for CCL2), or 57°C (for CX3CL1, CCL7, CCL20, and CXCL2). Melt curves of the amplified products were performed to identify the amplicon. The primers used were as follows: for GAPDH (900 nmol/liter, 4 mmol/liter MgCl2), 5′-GCTAAGCAGTTGGTGGTGCA-3′ and 5′-TCACCACCATGGAGAAGGC-3′; for MCP-1 (350 nmol/liter, 3 mmol/liter MgCl2), 5′-CCAGCCTACTCATTGGGATCA-3′ and 5′-CTTCTGGGCCTGCTGTTCA-3′; for IL-17 (200 nmol/liter, 3 mmol/liter MgCl2), 5′-AGCTTTCCCTCCGCATTGA-3′ and 5′-GCTCGAGAAGGCCCTCAGA-3′; for mouse CX3CL1, primers with catalog number QT00128345 from Qiagen (Venlo, Netherlands); for mouse CCL7, primers with catalog number QT00171458 from Qiagen; for CCL20, primers with catalog number QT02326394 from Qiagen; for mouse CXCL2, primers with catalog number QT00113253 from Qiagen; and for CD4, primers with catalog number QT00096166 from Qiagen. Quantification of input cDNA from the unknown samples was performed by including a standard curve. To construct the standard DNA curve, amplicons generated by RT-PCR using the primers described above were purified on silica columns (QIAquick PCR purification; Qiagen) and cloned into the pGEM-T Easy vector (Promega Corp., Madison, WI). Ligated fragments were transformed into Escherichia coli DH5α competent cells, and plasmid DNA was prepared using silica cartridges (Qiagen). The sequence of the cloned amplicons was determined by cycle sequencing. Plasmid DNA concentrations were measured by optical density spectrophotometry, and the corresponding copy numbers were calculated using the following equation: 1 μg 1,000 bp DNA = 9.1 × 1011 molecules. Serial 10-fold dilutions of plasmids ranging from 107 to 102 DNA copies were used to create a standard curve for each PCR run. To minimize interassay variability, all samples analyzed from a single experiment were assayed in the same 96-well plate.
Urease-induced specific proliferation.
A total of 1 × 105 splenocytes labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (37) were stimulated with purified urease (10 μg/ml) in RPMI 1640–10% FCS in 96-well plates. After 4 days of incubation at 37°C, the CFSE dilution was measured by FACS using a Cytomics FC 500 flow cytometer (Beckman Coulter, Brea, CA). The proliferation index was determined using proliferation with concanavalin A (1 μg/ml; catalog number C5275-5MG; Sigma) to normalize urease-specific proliferation.
In vitro killing assay.
H. pylori P49 (0.4 × 106) suspended in 25 μl RPMI 1640–2% fetal calf serum was mixed with 25 μl RPMI 1640–2% fetal calf serum containing 0.4 × 105 sorted cells or lymph node cells and incubated in a 96-well plate for 4 h under microaerophilic conditions. One hundred percent bacterial survival was determined by incubating H. pylori P49 with 25 μl RPMI 1640–2% fetal calf serum alone. After incubation, cell suspensions were diluted 1/2,000. One hundred microliters was plated on an H. pylori selective agar plate (Oxoid, Basingstoke, United Kingdom) in duplicate, and the numbers of CFU were determined 5 days later. For the transwell experiment, H. pylori P49 (1.6 × 106 cells) suspended in 100 μl RPMI 1640–2% FCS was distributed in a 24-well plate. A total of 1.6 × 105 cells in 100 μl RPMI 1640–2% FCS were introduced onto the transwell filter (pore size, 0.4 μm) and incubated for 4 h under microaerophilic conditions. After incubation, the cell suspensions were diluted 1/8,000 and plated as described above to determine the numbers of CFU. To investigate the effect of NO on monocyte killing, we added 1400W (50 μM; Calbiochem, Darmstadt, Germany) (38), a selective inhibitor of iNOS, to the culture medium.
Human peripheral blood monocyte isolation.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation, and CD14+ cells were positively selected by magnetic sorting (antihuman magnetic particles, DM clone MφP9; BD Biosciences, San Jose, CA) as described previously (39). In order to investigate the effect of activation on Helicobacter killing, 1 × 106 monocytes per well in 12-well plates in RPMI 1640 medium (25 mM HEPES, 10% FCS, penicillin [50 U/ml], streptomycin [50 μg/ml]) were incubated overnight in the presence of gamma interferon (IFN-γ; 40 IU/ml; R&D Systems, Minneapolis, MN). Blood samples were obtained at a local blood bank (Centre de Transfusion Sanguine [CTS], Lausanne, Switzerland). The use of the samples used in this study was approved by the Institutional Review Board of CTS, and all subjects gave written informed consent. Only samples from individuals with no signs of HIV, hepatitis A virus, hepatitis B virus, or hepatitis C virus infection were included.
Statistical analysis.
The distribution of the data was compared by Mann-Whitney tests and 2-way analysis of variance using GraphPad software (GraphPad Software, San Diego, CA), with a P value of 0.05 being considered the limit of significance.
RESULTS
Increased numbers of inflammatory monocytes in the stomach mucosa during vaccine-induced reduction of H. felis infection.
In order to evaluate whether inflammatory monocytes play a role in the vaccine-induced reduction of H. felis infection, we first determined by flow cytometry the number of inflammatory monocytes within the stomach mucosa. Gastric immune cell populations extracted from the stomachs of vaccinated and control mice sacrificed on days 3, 5, and 7 after H. felis infection were first stained with monoclonal antibodies against CD45, CD11b, and Ly6C. At these time points, H. felis infection was clearly detectable in the stomachs of control mice (Fig. 1A). On the contrary, in vaccinated mice, the infection was near the limit of detection on day 3 and was undetectable by day 5 (Fig. 1A). The vaccine-induced reduction of H. felis infection was associated with a steady increase in the abundance of CD45+ CD11b+ cells (see Fig. S1A in the supplemental material). Among the CD45+ CD11b+ cells, we distinguished three populations on the basis of Ly6C expression. The first population, Ly6Chigh, corresponded to newly recruited Ly6Chigh Ly6G− MHC-II+ CCR2+ CD64+ inflammatory monocytes (40) (Fig. 1B and F). The second population, Ly6Clow, was composed of a mixture of Ly6Clow Ly6G− MHC-II+ CCR2+ CD64+ inflammatory monocytes (Fig. 1B, E, and F) derived from the recruited Ly6Chigh Ly6G− MHC-II+ CCR2+ CD64+ inflammatory monocytes (41) and of Ly6Clow Ly6G+ MHC-II-negative (MHC-II−) neutrophils (Fig. 1B, D, and E). These cell populations were discriminated according to their MHC-II and Ly6G expression (Fig. 1D) and their morphologies (Fig. 1E). As opposed to the Ly6Chigh/low Ly6G− MHC-II+ CD11b+ inflammatory monocytes that expressed high levels of CCR2 and CD64 and low levels of CD11c and F4/80 (Fig. 1E), the Ly6C− Ly6G− MHC-II+ CD11b+ cells had the morphology of macrophages (see Fig. S1B in the supplemental material) and expressed high levels of CD11c and F4/80. Thus, we concluded that the third population, Ly6C− Ly6G− MHC-II+ CD11b+ F4/80−/+ CD11c+/low CD64−/+ CX3CR1−/+ cells, corresponded to a mixture of dendritic cells (DCs) and macrophages derived from either pre-DCs or Ly6Chigh/low inflammatory monocytes (Fig. 1B and F; see also Fig. S1B in the supplemental material) (41). Unfortunately, under our experimental conditions, CX3CR1 staining was not optimal and did not allow a clear discrimination between CX3CR1high macrophages and CX3CR1low inflammatory monocytes (Fig. 1F). We observed that in vaccinated mice, the reduction in the level of H. felis infection was associated with an increased percentage of CD45+ CD11b+ Ly6Clow cells (Fig. 1C). This cell population peaked at 5 days postinfection (40% ± 5% for vaccinated mice versus 14% ± 5% for control mice [n = 5]; P < 0.001), a time point when H. felis infection was no longer detectable in the stomach mucosa (Fig. 1A). In comparison, in control mice the CD45+ CD11b+ Ly6Clow population, which was also composed of a mixture of Ly6G+ MHC-II− neutrophils and Ly6G− MHC-II+ monocytes (see Fig. S1C and D in the supplemental material), was not increased (Fig. 1C). Overall, the percentages of Ly6Clow inflammatory monocytes and neutrophils were higher in vaccinated mice than control mice (Fig. 1G; see also Fig. S1E in the supplemental material). Taken together, our results show that during the vaccine-induced reduction of H. felis infection, not only Ly6Clow inflammatory monocytes but also neutrophils accumulated in the stomach mucosa.
FIG 1.
Inflammatory monocytes are recruited into the stomach mucosa during the vaccine-induced reduction of H. felis infection. (A) BALB/c mice were intranasally administered (at days 0, 7, 14, and 28) either 30 μg urease plus 10 μg cholera toxin (vaccinated mice) or cholera toxin alone (control mice). At day 42, 5 mice per time point were challenged with H. felis (1 × 108 cells) and sacrificed 2, 3, 5, or 7 days later. At sacrifice, stomachs were recovered and Helicobacter infection status was determined by RUT. Bars represent means with standard deviations. DO550nm, optical density at 550 nm. (B) Flow cytometry analysis of Ly6C expression at the surface of stomach-infiltrating CD45+ CD11b+ cells. These results are representative of those from 3 independent experiments performed on 12 mice. (C) Percentage of CD45+ CD11b+ Ly6Clow cells infiltrating the stomach mucosa of vaccinated and control mice at different time points after infection. For each time point, a total of 5 to 6 mice was analyzed. (D) Flow cytometry analysis of MHC-II and Ly6G expression at the surface of CD45+ CD11b+ Ly6Clow cells infiltrating the stomach mucosa of vaccinated mice on day 5 postinfection. These results are representative of those from 3 independent experiments performed on 13 mice. Q2 and Q4, quadrants 2 and 4, respectively. (E) Morphology of CD45+ CD11b+ Ly6Clow Ly6G− MHC-II+ cells and CD45+ CD11b+ Ly6Clow Ly6G+ MHC-II− cells recovered from the stomach mucosa of vaccinated mice on day 5 postinfection. These results are representative of those from 2 independent experiments performed on 8 mice. (F) Flow cytometry analysis of CCR2, CD64, CX3CR1, CD11c, and F4/80 expression at the surface of CD45+ CD11b+ MHC-II+ Ly6Chigh, CD45+ CD11b+ MHC-II+ Ly6Clow, or CD45+ CD11b+ MHC-II+ Ly6C− cells (R3) infiltrating the stomach mucosa of vaccinated mice on day 5 postinfection. These results are representative of those from 3 independent experiments performed on 7 mice. 7AAD, 7-aminoactinomycin D. (G) Percentage of CD45+ CD11b+ Ly6Clow MHC-II+ or CD45+ CD11b+ Ly6Chigh MHC-II+ inflammatory monocytes and neutrophils infiltrating the stomach mucosa of vaccinated or control mice on day 5 after bacterial infection. The numbers that appear beneath the gates show the percentage of cells within the gate. Each dot represents the results for a single mouse. n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Depletion of neutrophils does not prevent the vaccine-induced reduction of H. felis infection.
To determine whether neutrophils play a role in the vaccine-induced reduction of H. felis infection, we injected an anti-Ly6G depleting antibody. We observed that the injection of anti-Ly6G antibodies depleted circulating neutrophils (data not shown) and stomach neutrophils (Fig. 2A). Neutrophil-depleted and vaccinated mice exhibited reduced levels of H. felis infection on day 5 postinfection comparable to those in neutrophil-sufficient mice (Fig. 2B). This result shows that neutrophils are not essential for the vaccine-induced reduction of Helicobacter infection in BALB/c mice.
FIG 2.
Depletion of neutrophils does not prevent the vaccine-induced reduction of H. felis infection. Vaccinated or control mice were injected on day −1, 1, and 4 days after H. felis infection with 200 μg of anti-Ly6G depleting monoclonal antibody (anti-Ly6G) or with purified irrelevant rat immunoglobulin G antibody (Control IgG). (A) Mice were sacrificed on day 5 after infection to analyze by flow cytometry the percentage of neutrophils (CD45+ CD11b+ MHC-II− Ly6C+ Gr1hi) infiltrating the stomach mucosa. (B) Helicobacter infection status determined by RUT in vaccinated mice injected with anti-Ly6G or control antibodies on day 5 after bacterial challenge. The numbers that appear beneath the gates show the percentage of cells within the gate. Each symbol represents the results for a single mouse. n.s., not significant; *, P < 0.05.
Depletion of CCR2+ cells delays the vaccine-induced reduction of H. felis infection.
To determine whether monocytes play a role in the vaccine-induced reduction of H. felis infection, we injected an anti-CCR2 depleting antibody. Most of the monocyte-depleted and vaccinated mice could not reduce infection on day 5 (Fig. 3A), whereas vaccinated mice treated with the control antibody could (n = 17, P < 0.001) (Fig. 3A). Remarkably, we observed that the injection of the anti-CCR2 antibodies depleted circulating inflammatory monocytes (see Fig. S2B in the supplemental material) and stomach Ly6Chigh inflammatory monocytes (Fig. 3B). As expected, the anti-CCR2 injection only partially reduced the percentage of CD45+ CD11b+ Ly6Clow cells (50% ± 7% for vaccinated mice injected with the Ig control [n = 14] versus 33% ± 3% for vaccinated mice injected with anti-CCR2 [n = 6]; P < 0.001) (Fig. 3B). Since we observed that Ly6Clow Ly6G+ MHC-II− CD45+ CD11b+ neutrophils do not express CCR2 (see Fig. 2SC in the supplemental material) and that Ly6Clow Ly6G− MHC-II+ CD64+ CD45+ CD11b+ inflammatory monocytes express high levels of CCR2 (Fig. 1F; see also Fig. 2SC in the supplemental material), one can postulate that most of the remaining CD45+ CD11b+ Ly6Clow cells in anti-CCR2-treated mice are neutrophils. Moreover, anti-CCR2 injection did not impact the H. felis colonization of control mice (see Fig. S2A in the supplemental material). Collectively, these data suggest that inflammatory monocytes play a role in the vaccine-induced reduction of H. felis infection.
FIG 3.
Depletion of inflammatory monocytes delays the vaccine-induced reduction of H. felis infection. (A) Vaccinated or control mice were injected on day −1, 0, 1, 2, 3, and 4 after bacterial challenge with 20 μg of anti-CCR2 depleting monoclonal antibody (anti-CCR2) or with purified irrelevant rat immunoglobulin G antibody (Ig Control). Mice were sacrificed on day 5 after infection to analyze by flow cytometry the percentage of CD45+ CD11b+ Ly6Chigh and CD45+ CD11b+ Ly6Clow cells infiltrating the stomach mucosa. Each symbol represents the results for a single mouse. (B) Helicobacter infection status determined by the rapid urease test of vaccinated mice injected with anti-CCR2 or control antibodies on day 5 after bacterial challenge. (C) Levels of IL-17 mRNA expression in the stomach mucosa of vaccinated or control mice injected with anti-CCR2 or control antibodies on day 5 after bacterial challenge. Each symbol represents the results for a single mouse. (D) Inflammation score of the stomach mucosa of vaccinated or control mice injected with anti-CCR2 or control antibodies on day 5 after bacterial challenge. The numbers that appear beneath the gates show the percentage of cells within the gate. Each symbol represents the results for a single mouse. n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Anti-CCR2 injection did not impact the CD4+ T cell protective response against H. felis.
We observed that 10 to 12% of the CD4+ T cell population from the stomach mucosa of vaccinated and infected mice expressed CCR2 (see Fig. S2C in the supplemental material). Since the vaccine-induced reduction of Helicobacter infection relies on CD4+ IL-17-positive (IL-17+) Th cells to clear the bacteria from the stomach mucosa (15, 19), we analyzed the impact of the anti-CCR2 injection on CD4+ T cell responses in vaccinated and infected mice. We first observed that the anti-CCR2 injection did not significantly decrease the expression of CD4 mRNA (793 ± 774 relative units and 643 ± 683 relative units, respectively, for isotype-treated mice and anti-CCR2-treated mice). In addition, anti-CCR2 treatment did not decrease the amount of mRNA encoding IL-17 (Fig. 3C). Moreover, IL-17-dependent inflammatory processes associated with the vaccine-induced reduction of Helicobacter infection, such as recruitment of neutrophils into the stomach mucosa, were not affected by anti-CCR2 injection (Fig. 3D; see also Fig. S2D in the supplemental material). Finally, the anti-CCR2 injection did not decrease the urease-induced splenic T cell proliferation observed in vaccinated mice on day 5 postinfection (see Fig. S2E in the supplemental material). Collectively, these data suggest that the anti-CCR2 treatment did not impact the CD4+ T cell protective response and associated neutrophil recruitment.
Vaccination increases chemokine expression.
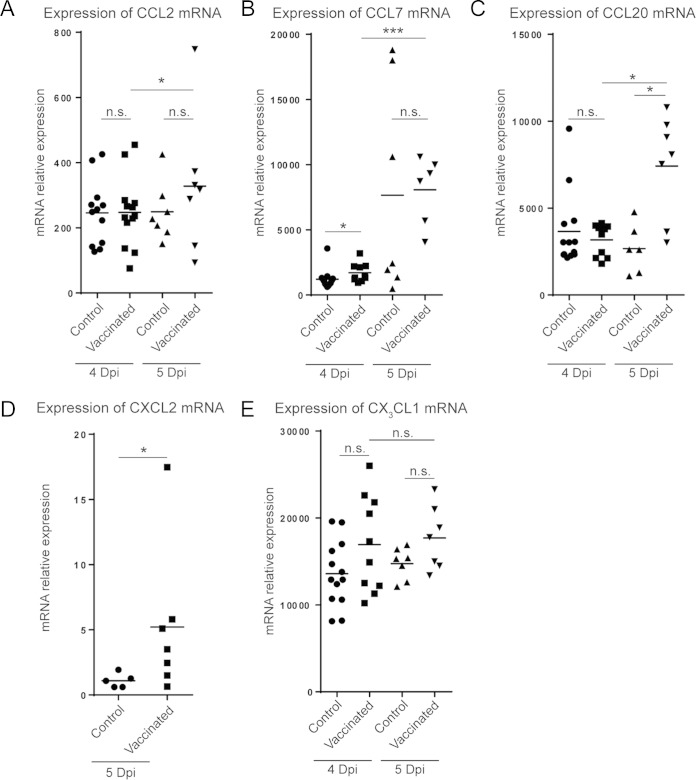
Considering that inflammatory monocytes accumulated in the stomach mucosa of vaccinated and infected mice (Fig. 1G), we next looked for the expression of chemokines possibly involved in their recruitment. We first studied the levels of expression of CCL2, a ligand of CCR2. Although CCL2 mRNA was expressed in the stomach mucosa of vaccinated mice on days 4 and 5 after H. felis infection, its expression was not statistically significantly increased compared to that in the stomach mucosa of control mice (Fig. 4A). This observation suggests that CCL2 does not play a major role in inflammatory monocyte recruitment to the stomach mucosa within this context. However, we found that the levels of mRNA of CCL7, another ligand for CCR2, were significantly increased on day 4 postinfection in vaccinated mice compared to the levels in control mice (Fig. 4B). Interestingly, the levels of mRNA of CCL20 (Fig. 4C), the ligand of CCR6, and CXCL2 (Fig. 4D), the ligand of CXCR2, were increased in vaccinated mice compared to the levels in control mice on 5 day postchallenge. The levels of mRNA of CX3CL1, the ligand of CX3CR1 which was highly expressed by stomach monocytes (Fig. 1F), were not statistically significantly increased in vaccinated mice compared to those in control mice on day 4 and 5 after bacterial challenge (Fig. 4E). Taken together, these results suggest that inflammatory monocyte recruitment to the stomach mucosa during the vaccine-induced reduction of H. felis infection is likely mediated by several chemokines, including CCL20, CCL7, and CXCL2.
FIG 4.
Chemokine expression levels in the stomach mucosa during the vaccine-induced reduction of H. felis infection. Vaccinated or control mice were infected with H. felis and sacrificed 4 or 5 days later. The levels of expression of CCL2 (A), CCL7 (B), CCL20 (C), CXCL2 (D), and CX3CL1 (E) mRNA in the stomach mucosa were quantified by quantitative RT-PCR. Each symbol represents the results for a single mouse. Dpi, day postinfection; n.s., not significant; *, P < 0.05; ***, P < 0.001.
Inflammatory monocytes kill H. pylori in an iNOS-dependent manner.
We showed that the efficacy of the vaccine-induced reduction of H. felis infection was decreased by the injection of anti-CCR2 treatment (Fig. 3A). These data suggest that inflammatory monocytes participate in the killing of Helicobacter. However, we cannot exclude the possibility that another CCR2+ (Fig. 1F; see also Fig. 2SC in the supplemental material) and/or CCR2− cell population might be depleted and/or expanded in anti-CCR2-treated mice, leading to the inhibition of the vaccine-induced reduction of bacterial infection. We previously published data showing that CD11b+ Gr1+ cells, among which were inflammatory monocytes, infiltrated the stomach mucosa of vaccinated and infected mice (15). These inflammatory monocytes are in close contact with stomach epithelial cells and could potentially have direct access to Helicobacter and thus kill the pathogen. Hence, in order to confirm that inflammatory monocytes play a role in the vaccine-induced reduction of Helicobacter infection, we studied their antimicrobial functions. It is well described that the antimicrobial activities of monocytes rely on the expression of TNF-α and iNOS (24). Therefore, we performed intracellular staining to probe for the expression of TNF-α and iNOS within the monocytes infiltrating the stomach mucosa during the vaccine-induced reduction of H. felis infection. We indeed observed that at least half of the stomach Ly6Chigh and Ly6Clow inflammatory monocytes produced both TNF-α and iNOS (Fig. 5A), while inflammatory monocytes from control mice produced very low levels (Fig. 5A). To confirm this potential antimicrobial activity, on day 5 postchallenge we performed an H. pylori in vitro killing assay with different myeloid cells sorted from the stomach mucosa of vaccinated mice (Fig. 5B). Compared to unsorted lymph node cells, stomach CD11b+ cells directly mixed with H. pylori killed the bacteria with up to 100% efficacy. Similarly, both Ly6Chigh and Ly6Clow inflammatory monocytes efficiently killed H. pylori (Fig. 5B). In comparison, stomach neutrophils exhibited poor killing activity. The low efficacy of H. pylori killing by sorted neutrophils might come from the procedures used to isolate and sort the neutrophils from the stomach mucosa; indeed, most of the sorted neutrophils used in this in vitro killing assay were already degranulated (Fig. 1E). In order to evaluate the respective role of phagocytosis and NO production in the killing of H. pylori by inflammatory monocytes, we performed experiments using transwell filter plates in the presence of 1400W, a specific inhibitor of iNOS (38). Inflammatory monocytes were placed on the transwell filter (pore size, 0.4 μm), and H. pylori cells were placed in the bottom of the well. In the absence of phagocytosis, Ly6Chigh and Ly6Clow inflammatory monocytes killed H. pylori (Fig. 5C) with 50% efficacy. Notably, the H. pylori killing was completely abrogated by inhibition of iNOS (Fig. 5C). We next performed additional experiments to test whether human monocytes could also kill H. pylori through an iNOS-dependent mechanism. Peripheral blood-derived human monocytes were incubated either directly or on transwell filters in the presence of 1400W. Resting human blood monocytes did not efficiently kill H. pylori. However, following overnight incubation with IFN-γ, a cytokine which is highly expressed in the stomach mucosa during vaccine-induced Helicobacter clearance (15), the activated monocytes killed H. pylori (Fig. 5D). Inhibition of iNOS abolished 50% of the monocyte-induced H. pylori killing. The killing of H. pylori by human monocytes was not dependent on phagocytosis; indeed, the H. pylori killing activity by monocytes was not inhibited by the transwell filter (Fig. 5D). Collectively, we showed that murine Ly6Chigh and Ly6Clow inflammatory monocytes and activated human monocytes potently kill H. pylori in an iNOS-dependent manner.
FIG 5.
Inflammatory monocytes kill H. pylori in an iNOS-dependent manner. (A) CD45+ CD11b+ MHC-II+ Ly6Chigh and CD45+ CD11b+ MHC-II+ Ly6Clow inflammatory monocytes recovered from the stomach mucosa of vaccinated or control mice on day 5 after H. felis infection were intracellularly labeled to detect TNF-α and iNOS production. The results are representative of those from 3 independent experiments performed on 8 vaccinated mice and 4 control mice. MFI, mean fluorescence intensity. (B) H. pylori killing activities of inflammatory monocytes. CD11b+ cells were isolated by the MACS procedure, and neutrophils and CD45+ CD11b+ MHC-II+ Ly6Chigh and CD45+ CD11b+ MHC-II+ Ly6Clow inflammatory monocytes were sorted by FACS analysis from a pool of immune cells extracted from the stomach mucosa of 8 vaccinated and H. felis-infected mice (day 5 after bacterial infection). These cells were incubated with H. pylori P49 for 4 h. Medium was recovered and plated to determine the numbers of CFU. The results are expressed as percent killing. Lymph node cells showed no bacterial killing, whereas 100% bacterial killing was shown for CD11b+ cells. We show the results of 2 independent experiments performed on a total of 16 vaccinated mice. (C) Sorted CD45+ CD11b+ MHC-II+ Ly6C− cells, CD45+ CD11b+ MHC-II− Ly6Clow Ly6G+ neutrophils, and CD45+ CD11b+ MHC-II+ Ly6Chigh and CD45+ CD11b+ MHC-II+ Ly6Clow inflammatory monocytes from the stomach mucosa of vaccinated and H. felis-infected (on day 5 after bacterial infection) mice were introduced onto transwell filters (pore size, 0.4 μm) and incubated for 4 h with H. pylori P49 cells seeded into the bottom of a 24-well plate. Medium was recovered and plated to determine the numbers of CFU. A chemical inhibitor of iNOS, 1400W, was added to CD45+ CD11b+ MHC-II+ Ly6Clow inflammatory monocytes during the coincubation with H. pylori. The results are expressed as percent killing. The results are representative of those from 2 independent experiments performed on 16 vaccinated mice. (D) Sorted CD14+ human monocytes were activated or not overnight with γ-IFN. Human monocytes were either introduced into transwells or added directly into contact with H. pylori P49 in the presence or not of the iNOS inhibitor (1400W). After 4 h of coculture, medium was recovered and plated to determine the numbers of CFU. The results are expressed as percent killing. The results are representative of those from 3 independent experiments performed with cells from 5 different donors. n.s., not significant; *, P < 0.05.
DISCUSSION
In this study, we evaluated the role of inflammatory monocytes in the vaccine-induced reduction of Helicobacter infection. This hypothesis was based on previous results showing that during the vaccine-induced reduction of Helicobacter infection, CD11b+ Gr1+ cells infiltrate the stomach mucosa (15) and that inflammatory monocytes are well-known to be critical in several defense mechanisms against mucosal pathogens (24, 42). We first showed that Ly6Clow Ly6G− MHC-II+ CCR2+ CD64+ inflammatory monocytes accumulate in the stomach mucosa during the vaccine-induced reduction of H. felis infection (Fig. 1F and G). To determine whether inflammatory monocytes play a role in the vaccine-induced reduction of Helicobacter infection, these cells were depleted with anti-CCR2 antibodies. Depletion of inflammatory monocytes was associated with an impaired vaccine-induced reduction of H. felis infection (Fig. 3B). To determine whether inflammatory monocytes have a direct or indirect role in the vaccine-induced reduction of Helicobacter infection, we studied their antimicrobial activities. We first showed that Ly6Chigh and Ly6Clow inflammatory monocytes produced TNF-α and iNOS, two major antimicrobial factors (Fig. 4A) (24). Then, by using a Helicobacter in vitro killing assay, we showed that Ly6Chigh and Ly6Clow inflammatory monocytes and activated human monocytes potently kill H. pylori in an iNOS-dependent manner (Fig. 4B). Taken together, our results show that the Ly6Chigh and Ly6Clow inflammatory monocytes play a direct role in the vaccine-induced reduction of H. felis infection.
The paradigm of inflammatory monocyte-dependent protective antimicrobial activity was first established in listeriosis, on the basis of the observation that inflammatory monocyte-derived population such as Tip-DCs (24) play a major role in innate immune defense. Subsequent studies confirmed the key roles of inflammatory monocytes in antimicrobial responses and demonstrated the direct killing of Salmonella (43), Candida albicans (44), and Toxoplasma gondii (45) by isolated inflammatory monocytes. From the findings of this study, we may add H. pylori to the list of pathogens sensitive to the antimicrobial activities of inflammatory monocytes. Indeed, we provide evidence that inflammatory monocytes recovered from the stomach mucosa of vaccinated mice kill H. pylori in vitro (Fig. 5B). Our study and previous studies demonstrate that H. pylori can be killed by macrophages and human and mouse monocytes, even when they are physically separated, in an NO-dependent manner (Fig. 5C) (46). Since NO is a water- and lipid-soluble gas, it is conceivable that it exerts antimicrobial effects by diffusing though the epithelial cell monolayer (24). Hence, iNOS-positive (iNOS+) inflammatory monocytes recruited to the stomach mucosa might not need direct contact with the bacteria to kill Helicobacter cells located in close proximity to the apical surface of stomach epithelial cells.
We observed that inflammatory monocytes recovered from the stomach mucosa of nonimmune mice expressed very low levels of iNOS and TNF-α on day 5 after Helicobacter infection (Fig. 4B). The low number of Ly6Clow inflammatory monocytes infiltrating the stomach mucosa of nonimmune mice (Fig. 1G) associated with the production by H. pylori of arginase, an enzyme that competes for the iNOS substrate l-arginine (47), might prevent the iNOS-dependent killing of the bacterium in nonvaccinated hosts.
The urease-specific memory Th17 CD4+ T cells primed during the vaccination protocol and reactivated by the infection might play a role in the recruitment and/or the increased expression of iNOS and TNF-α by inflammatory monocytes. Indeed, it has previously been shown that depletion of CD4+ T cells or neutralization of IL-17 prevents inflammatory monocyte recruitment during a secondary immune response directed against pneumococcal lung colonization (48). Moreover, in a second study, memory CD4+ Th17 or Th1 cells were shown to promote, in a pathogen-associated molecular pattern (PAMP)-independent manner, innate immune responses (49). More importantly, it has been demonstrated that during Salmonella intestinal infection, IFN-γ and IL-12 p40 are critical for the expression of iNOS by inflammatory monocytes (43). Hence, since Helicobacter is minimally invasive, therefore delivering low levels of antigenic stimuli and evading PAMP recognition (46, 49, 50), it can be speculated that during Helicobacter infection of vaccinated mice, the reactivation of urease-specific memory Th1 and/or Th17 cells promoted inflammatory monocyte recruitment and differentiation and licensed these cells to kill Helicobacter. Our observation that only low numbers of inflammatory monocytes infiltrate the stomach of nonvaccinated mice (Fig. 1G) and that they express very low levels of iNOS (Fig. 5A) is in accordance with this hypothesis.
We observed that Ly6Chigh and Ly6Clow inflammatory monocytes infiltrate the stomach mucosa during the vaccine-induced reduction of H. felis infection. It is well established that in an inflamed tissue, inflammatory monocytes are activated and/or differentiate into macrophages and dendritic cells (24, 40–42, 51). In our model, it can be speculated that Ly6Chigh cells differentiate into Ly6Clow inflammatory monocytes. These Ly6Clow inflammatory monocytes are CD11b+ TNF-α positive (TNF-α+) iNOS+ MHC-II+ CCR2+ CD64+ and are very similar to the Tip-DCs that produce prodigious amounts of TNF-α and iNOS in the spleens of Listeria-infected mice (24).
Although we clearly observed that Ly6Chigh and Ly6Clow inflammatory monocytes play a critical role in the vaccine-induced reduction of H. felis infection on day 5 postinfection, we observed on day 7 that H. felis infection had been cleared from the stomach mucosa of vaccinated mice injected with anti-CCR2 antibodies (data not shown). One can interpret these data in two different ways. In the first hypothesis, it might be possible that Ly6Chigh and Ly6Clow inflammatory monocytes play a role only at early time points. In a second hypothesis, it might also be possible that without Ly6Chigh and Ly6Clow inflammatory monocytes, neutrophils that are recruited to the stomach mucosa play a prominent role in the vaccine-induced reduction of H. felis infection. Indeed, it has recently been demonstrated that in the absence of prostaglandin E2-producing inflammatory monocytes, Toxoplasma gondii oral infection leads to a massive influx of activated neutrophils (52). In our experimental setting, it can then be hypothesized that neutrophils are the main players in the vaccine-induced reduction of H. felis infection in anti-CCR2-injected mice. The critical role for neutrophils in the reduction of Helicobacter infection has already been reported in IL-10-deficient mice (17), which displayed major defects in T cells and myeloid cells.
One unanswered question from this study is which chemokines are involved in the recruitment of inflammatory monocytes to the stomach mucosa of vaccinated and infected mice? We did not observe any upregulation of CCL2 in vaccinated mice during the vaccine-induced reduction of H. felis infection (Fig. 4A). Moreover, vaccinated CCR2-deficient mice still cleared H. felis on day 5 after bacterial infection (data not shown). Taken together, these data argue that inflammatory monocyte recruitment is not dependent on CCR2 signaling. CCR6-CCL20 has been shown to be essential for the homing of inflammatory monocytes to the inflamed dermis (53). Since CCL20 is upregulated during the vaccine-induced reduction of H. felis infection (Fig. 4C), one could postulate that inflammatory monocyte recruitment is dependent on CCR6 signaling. Moreover, we observed an increased expression of CXCL2 (macrophage inflammatory protein 1α) during the vaccine-induced reduction of H. felis infection (Fig. 4D); this chemokine has recently been shown to be upregulated in the stomach mucosa of H. pylori-infected patients and responsible for the recruitment of CXCL2R+ myeloid suppressive cells in their stomach mucosa (54). We are currently evaluating whether the recruitment of inflammatory monocytes in the vaccine-induced reduction of Helicobacter infection is CXCL2R dependent.
In summary, to our knowledge, our study is the first to describe the protective antimicrobial activity of inflammatory monocytes recruited to the stomach mucosa and that the antimicrobial activity of inflammatory monocytes increases dramatically in the vaccinated host. From the perspective of the clinical development of a H. pylori vaccine, these data suggest that an efficient vaccine has to elicit H. pylori-specific Th1 and/or Th17 cells, allowing the maturation of inflammatory monocytes into iNOS+ TNF-α+ cells with Helicobacter killing activities.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Swiss National Foundation grant 310030_141145 (to D. Velin).
We thank Aimable Nahimana for access to the Cytomics FC 500 flow cytometer, Matthieu Perreau and Line Leuenberger for FACS analysis, and B. J. Marsland for critical reading of the manuscript.
We have no conflicts to disclose.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01026-15.
REFERENCES
- 1.Frenck RW Jr, Clemens J. 2003. Helicobacter in the developing world. Microbes Infect 5:705–713. doi: 10.1016/S1286-4579(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 2.Cover TL, Blaser MJ. 2009. Helicobacter pylori in health and disease. Gastroenterology 136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farinha P, Gascoyne RD. 2005. Helicobacter pylori and MALT lymphoma. Gastroenterology 128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M, Japan Gast Study Group. 2008. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Ernst PB, Peura DA, Crowe SE. 2006. The translation of Helicobacter pylori basic research to patient care. Gastroenterology 130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Wolle K, Malfertheiner P. 2007. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol 21:315–324. doi: 10.1016/j.bpg.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Kreiss C, Buclin T, Cosma M, Corthesy-Theulaz I, Michetti P. 1996. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet 347:1630–1631. doi: 10.1016/S0140-6736(96)91119-8. [DOI] [PubMed] [Google Scholar]
- 8.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthesy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath TP, Blum AL. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804–812. doi: 10.1016/S0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Medina-Fatimi A, Nichols R, Tendler D, Michetti M, Simon J, Kelly CP, Monath TP, Michetti P. 2002. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut 51:634–640. doi: 10.1136/gut.51.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton P. 2011. Vaccinating against Helicobacter pylori: dissecting the mechanism. Gastroenterology 141:1149–1151. doi: 10.1053/j.gastro.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Ermak TH, Giannasca PJ, Nichols R, Myers GA, Nedrud J, Weltzin R, Lee CK, Kleanthous H, Monath TP. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med 188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michetti M, Kelly CP, Kraehenbuhl JP, Bouzourene H, Michetti P. 2000. Gastric mucosal alpha(4)beta(7)-integrin-positive CD4 T lymphocytes and immune protection against Helicobacter infection in mice. Gastroenterology 119:109–118. doi: 10.1053/gast.2000.8548. [DOI] [PubMed] [Google Scholar]
- 13.Aebischer T, Laforsch S, Hurwitz R, Brombacher F, Meyer TF. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect Immun 69:556–558. doi: 10.1128/IAI.69.1.556-558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, Iwakura Y, Imanishi J. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun 67:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. 2009. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 136:2237–2246.e1. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 16.Velin D, Bachmann D, Bouzourene H, Michetti P. 2005. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology 129:142–155. doi: 10.1053/j.gastro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. 2003. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol 170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 18.Velin D, Bachmann D, Bouzourene H, Michetti P. 2008. Reduction of Helicobacter infection in IL-10−/− mice is dependent on CD4+ T cells but not on mast cells. Helicobacter 13:361–369. doi: 10.1111/j.1523-5378.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 19.DeLyria ES, Redline RW, Blanchard TG. 2009. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136:247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. 2011. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity 35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. 2007. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59–70. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 25.Robben PM, LaRegina M, Kuziel WA, Sibley LD. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med 201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. 2009. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. 2007. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serbina NV, Pamer EG. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. 2011. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity 34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindran R, Rusch L, Itano A, Jenkins MK, McSorley SJ. 2007. CCR6-dependent recruitment of blood phagocytes is necessary for rapid CD4 T cell responses to local bacterial infection. Proc Natl Acad Sci U S A 104:12075–12080. doi: 10.1073/pnas.0701363104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rottman JB, Slavin AJ, Silva R, Weiner HL, Gerard CG, Hancock WW. 2000. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur J Immunol 30:2372–2377. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. 2009. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med 206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. 2007. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Renia L, Pol S, Mallet V, Gilgenkrantz H. 2009. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174:1766–1775. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol 166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 36.Velin D, Narayan S, Bernasconi E, Busso N, Ramelli G, Maillard MH, Bachmann D, Pythoud C, Bouzourene H, Michetti P, So A. 2011. PAR2 promotes vaccine-induced protection against Helicobacter infection in mice. Gastroenterology 141:1273–1282.e1. doi: 10.1053/j.gastro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Quah BJ, Warren HS, Parish CR. 2007. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc 2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki C, Aoki-Yoshida A, Kimoto-Nira H, Kobayashi M, Sasaki K, Mizumachi K. 2014. Effects of strains of Lactococcus lactis on the production of nitric oxide and cytokines in murine macrophages. Inflammation 37:1728–1737. doi: 10.1007/s10753-014-9901-6. [DOI] [PubMed] [Google Scholar]
- 39.D'Angelo F, Bernasconi E, Schafer M, Moyat M, Michetti P, Maillard MH, Velin D. 2013. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin Exp Immunol 174:60–72. doi: 10.1111/cei.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. 2012. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 42.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. 2008. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rydstrom A, Wick MJ. 2007. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol 178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 44.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. 2014. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis 209:109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mordue DG, Sibley LD. 2003. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol 74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 46.Wilson KT, Crabtree JE. 2007. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci U S A 98:13844–13849. doi: 10.1073/pnas.241443798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salama NR, Hartung ML, Muller A. 2013. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moyat M, Velin D. 2014. Immune responses to Helicobacter pylori infection. World J Gastroenterol 20:5583–5593. doi: 10.3748/wjg.v20.i19.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. 2012. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. 2013. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, Dubois B. 2006. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang Y, Cheng P, Liu XF, Peng LS, Li BS, Wang TT, Chen N, Li WH, Shi Y, Chen W, Pang KC, Zeng M, Mao XH, Yang SM, Guo H, Guo G, Liu T, Zuo QF, Yang HJ, Yang LY, Mao FY, Lv YP, Zou QM. 2014. A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis. Gut 64:1368–1378. doi: 10.1136/gutjnl-2014-307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.