Abstract
Epilepsy has 2-3% incidence worldwide. However, present antiepileptic drugs provide only partial control of seizures. Calcium ion accumulation in hippocampal neurons has long been known as a major contributor to the etiology of epilepsy. TRPV1 is a calcium-permeable channel and mediator of epilepsy in the hippocampus. TRPV1 is expressed in epileptic brain areas such as CA1 area and dentate gyrus of the hippocampus. Here the author reviews the patent literature on novel molecules targeting TRPV1 that are currently being investigated in the laboratory and are candidates for future clinical evaluation in the management of epilepsy.
A limited number of recent reports have implicated TRPV1 in the induction or treatment of epilepsy suggesting that this may be new area for potential drugs targeting this debilitating disease. Thus activation of TRPV1 by oxidative stress, resiniferatoxin, cannabinoid receptor (CB1) activators (i.e. anandamide) or capsaicin induced epileptic effects, and these effects could be reduced by appropriate inhibitors, including capsazepine (CPZ), 5'-iodoresiniferatoxin (IRTX), resolvins, and CB1 antagonists. It has been also reported that CPZ and IRTX reduced spontaneous excitatory synaptic transmission through modulation of glutaminergic systems and desensitization of TRPV1 channels in the hippocampus of rats. Immunocytochemical studies indicated that TRPV1 channel expression increased in the hippocampus of mice and patients with temporal lobe epilepsy
Taken together, findings in the current literature support a role for calcium ion accumulation through TRPV1 channels in the etiology of epileptic seizures, indicating that inhibition of TRPV1 in the hippocampus may possibly be a novel target for prevention of epileptic seizures.
Keywords: Anadamide, Calcium ion, Epilepsy, Hippocampus, Seizures, TRPV1 channels
INTRODUCTION
Epilepsy is a common neurological disorder with approximately 50 million individuals (>2-3% of the population) affected worldwide. Increased excitability of neurons in various brain regions plays an important role in the etiology of epilepsy. The disease is considered an acute transient complex neurobehavioral disease [1, 2]. Although numerous studies have been conducted on the etiology of epilepsy, clear cellular and molecular mechanisms involved in the etiology of epilepsy are still unclear [3-5]. Treatment of epilepsy is possible although currently available antiepileptic drugs provide only partial control of seizures [3, 6, 7]. Molecular targets of most of these antiepileptic drugs are ion channels such as glutamate receptor channels and transient receptor potential (TRP) channels that are thought to be partially responsible for epileptic seizures and peripheral pain [7-9]. For these reasons, the discovery of novel drugs to treat epilepsy would be highly desirable.
Calcium ion (Ca2+) is an important second messenger and plays a role in numerous signal transduction pathways including neuronal excitability, neurotransmitter release, cell proliferation, and cell death [10,11]. It has long been known that Ca2+ is involved in the etiology of epilepsy. Different types of Ca2+ channels, such as voltage gated calcium channels (VGCC) and chemically gated calcium channels, likely play an important role in the etiology of epilepsy. Apart from the VGCC and chemically gated channels, one family of Ca2+ channels comprises transient receptor potential (TRP) cation channels. The TRP channels were firstly expressed in photoreceptors carrying trp gene induced a transient voltage changes to continuous light mutations of Drosophila flyers [12, 13]. One subfamily of TRP channels is the vanilloid group containing 8 members, including TRP vanilloid type 1 (TRPV1) cation channels. TRPV1 channels are non-selective cation channels. The polymodal transducer TRPV1 channel was first reported in sensory neurons such as dorsal root ganglion (DRG) and trigeminal ganglia neurons because the channels respond to various stimuli including oxidative stress, noxious heat (> 43 oC), protons and vanilloids (i.e. capsaicin) [14]. Na+ and Ca2+ entry result from activation of TRPV1 channels and neuronal excitability ensues [15-17]. In addition to expression of TRPV1 in the peripheral neurons [14], more recent studies have suggested that TRPV1 channels may also be a novel potential antiepileptic target [18, 19]. Indeed, the expression of TRPV1 protein was increased in epileptic brain areas such as the dentate gyrus of temporal lobe epilepsy-induced mice [20]. Recently, it was reported that epileptic activity was increased in hippocampal slices of rats by the TRPV1 channel agonist capsaicin, and this activity was blocked by a selective TRPV1 channel antagonist iodoresiniferatoxin (IRTX) [2]. Other recent papers [18, 19, 21-23] have also reported antiepileptic actions of the TRPV1 channel antagonist, capsazepine (CPZ).
Current knowledge regarding the functional importance of TRPV1 channels in the hippocampus and epilepsy is still relatively sparse. Studies utilizing pharmacological manipulation of TRPV1 indicate that this channel is not only an important element of hippocampal functions but may also play a role in epilepsy. In the review, I have analyzed the most recent findings about the expression and function of TRPV1 in the hippocampus and epilepsy, and discussed the possibility of these channels as a potential target for the treatment of epilepsy.
EPILEPSY AND Ca2+
About 50 million (2-3%) of the population worldwide are suffer from the chronic neurological disorder of epilepsy [24]. Epilepsy has been divided into three forms, specifically idiopathic, symptomatic, and cryptogenic forms. Some of the factors that are thought to contribute to the etiology of these epileptic forms include overload of Ca2+, genetic defects and oxidative stress [4, 24-26].
Numerous functions of neurons such as action potentials, synaptic transmission, plasticity, and cell survival are affected by the cytosolic Ca2+ concentration [1,10,27]. Cation channels play a major role in regulating cytosolic Ca2+ concentrations in all cells, including neurons, because Ca2+ crosses the cell membranes to enter the cytosol by way of these channels.
It has long been known that Ca2+ entering through neuronal VGCC regulates activity-dependent processes such as neurotransmitter release, gene transcription, and cytosolic signaling processes. In healthy neurons, calcium channels regulate and activate homeostatic signaling processes [28]. In presynaptic neurons, VGCCs are opened by action potential-induced depolarization and neurotransmitter release is dependent upon calcium entry that creates local domains of high Ca2+ concentration. In post synaptic neurons, many signaling processes are regulated by changes in cytosolic Ca2+ concentration following Ca2+entry through receptor operated channels and L-type VGDC. Neurons, synapses, and circuits in the nervous systems have very sensitive but powerful homeostatic set points of activity, and small changes in calcium channel activities can fine tune many synaptic outputs in a variety of ways [10, 28].
Epileptic seizure-induced brain injury involves many neuronal cell death inducing factors, including genetic changes, glutamate-mediated excitoxicity leading to changes in cytosolic Ca2+ metabolism, mitochondrial membrane abnormalities, induction of oxidative stress, and increased cytokine production [1]. At the cellular level, an enormous influx of Ca2+ via VGCC and N-methyl-D-aspartate (NMDA)-dependent calcium channels results in massive seizure activities [3, 7]. Following epilepsy, activation of VGCC and NMDA receptors causes an elevation in cytosolic Ca2+ that in turn initiates biochemical cascades resulting in acute neuronal cell death [7]. In addition to VGCC and NMDA receptors, recent reports indicated the importance of massive Ca2+ entry through TRP channels, including TRPV1 channels, in the etiology of epilepsy [19-23]. This latter aspect with be the focus of the remaining sections of this review.
TRP CHANNELS
The transient receptor potential (TRP) family has transmembrane domains with hydrophobic 4 pores. The pores are located between the fifth and sixth transmembrane domains, and for most TRP channels Ca2+ passes the cell membrane through these pores non-selectively. There are 30 mammalian TRP channels and new members of the family continue to be discovered. Based on their structural homology, The TRP channels are divided into 7 subfamilies namely canonical (TRPC1-7), ankyrin (TRPA1-3), melastatin (TRPM1-8), mucolipin (TRPML1-3), vanilloid (TRPV1-6), polycystin (TRPP1-3), and no-mechano-potential (NOMCP or TRPN). The activation and inhibition mechanisms are very different within the subfamilies. For example, TRPM8 is activated by menthol and environmental noxious cold (<15 oC) while TRPV1 channels are activated by different stimulants, including environmental high temperature (≥43 oC), oxidative stress, capsaicin and products of inflammation. TRPA1 is activated by cinnamaldehyde of cinnamon oil, Wasabi, garlic and environmental vehicle exhaust gas. Expressions of the channels are also differing in different tissues of body. For example, TRPV1 and TRPM2 channels are mostly expressed in brain and neurons while TRPC1 channels are mostly expressed in the cardiovascular system. Hence, TRP channels have different cellular polymodal integrators that are sensitive to environment factors [8, 9, 11, 13, 16, 29].
An Overview of TRPV1
The TRPV1 channel was discovered in sensory neurons in 1997 [17]. Subsequently it was found that the channels are also expressed in non-neuronal cells [31, 32]. In the last decade, the first exogenous expression studies were performed and this led to screens for antagonists of TRPV1 channels for clinical studies. As it was mentioned above, TRPV1 is activated by painful physical stimuli such as high temperature (≥43 oC), voltage changes, N-arachidonoylethanolamide (anandamide) and protons (low pH). It can also be activated by capsaicin, the pungent ingredient of hot chili peppers, and by anandamide, oxidative stress, nitric oxide, hydrogen peroxide, and oxidized linoleic acid [29, 30, 33-35]. In primary DRG neurons, TRPV1 has an essential role in the induction of inflammatory hyperalgesia [17, 36]. TRPV1 channels are also activated in the central nervous system by fatty acid amide hydrolase (FAAH, the catabolic enzyme of anandamide) and anandamide [37]. In addition to these activators, TRPV1 levels in DRG neurons were increased by nerve growth factor and the activation of p38 mitogen-activated protein kinase in inflamed skin [38]. It has also been suggested that the TRPV1 channel is activated by phosphatidylinositol 3-kinase-induced heat hyperalgesia [39, 40]. In addition, the TRPV1 channel is activated by low pH [41, 42]. Thus, in addition to their role as thermo-receptors, the current reports indicated that TRPV1 channels are also modulated by various molecules and distinct molecular pathways.
Studies indicate that TRPV1 is expressed in the brain area. For example, the CA1 area and dentate gyrus of the hippocampus have important roles in the induction of epilepsy and recent immunocytochemical studies indicated the presence of TRPV1 channels in the CA1 area and dentate gyrus of the hippocampus. Thus TRPV1 may play a role in the central nervous system in addition to its role as a polymodal sensory signal detector of peripheral pain 15, 31,43-45.
AGONISTS AND ANTAGONISTS OF TRPV1 CHANNEL
Cannabinoids: Anandamide
An endogenous agonist of CB1 receptors is anandamide and TRPV1 channels are also activated by high concentrations of anandamide [33]. Glutamate release due to TRPV1 channel activation in the brain is well-known [46]. In addition, VGCC are blocked by CB1 activation. Hyperpolarization through potassium ion efflux and a decrease of neuro-transmitter release are also induced by CBI activation [47]. Low and high dose (biphasic) anandamide-induced effects on behavioral models correlate with CB1 receptors and TRPV1 activation, respectively [48]. In addition to the dose dependent effects of anandamide on TRPV1 channels, anandamide mediated TRPV1 responses are affected by many factors, including phosphorylation, voltage, temperature changes, pH, and CB1 receptor activation [33].
Activation of TRPV1 Channels Through Physical and Chemical Stimulus
The TRPV1 channel was first discovered and expressed as a receptor for capsaicin. Subsequently, the role of TRPV1 on different cellular processes was reported, for example the detection of noxious physical and chemical stimuli in peripheral neurons. The presence of TRPV1 channels in nociceptive Aä and C-fibers of small and medium diameter sensory neurons (which are responsible for peripheral nociceptive pain) was also reported [49]. Recently we also observed a role for TRPV1 channels in the etiology of an inflammatory disease [50]. It seems that TRPV1 functions as a multi-detector against physical and chemical stimuli. The hippocampus is a main area in the etiology of epilepsy and recent evidence suggests functional TRPV1 channels are expressed in the hippocampus.
Activation of TRPV1 Channels Through Capsaicin and Resiniferatoxin
Capsaicin is a pungent ingredient in red hot peppers of the Capsicum plants [51] and TRPV1 was originally discovered as a receptor of capsaicin. Although capsaicin induces pain it has been used as a treatment of pain due to its ability to desensitize TRPV1 channels in neurons [52]. In addition to the agonist effect of capsaicin, resiniferatoxin is also a potent TRPV1 channel agonist at low concentrations [32, 53].
Activation of TRPV1 Channels via Oxidative Stress
Oxidative stress occurs due to over production of reactive oxygen species (ROS). ROS are produced through electron transport mechanisms during physiological functions such as mitochondrial substrate oxidation and phagocytic activity. They are also produced in response to different environmental factors and metabolic diseases including, traumatic brain injury, diabetes, and electromagnetic radiation [54, 55]. If the production of ROS is controlled by antioxidants their ability to cause damage DNA, lipids, proteins and carbohydrates is lessened [8, 9, 11, 55]. There is considerable interest in the relationship between these reactive species and the etiology of a variety of diseases, including epilepsy [1]. There are two major types of free radical species: ROS and reactive nitrogen species (RNS). The ROS and RNS involve changes in specific amino acid residues, especially cysteine sulfhydryls, which may be located on signaling proteins [56]. TRPV1 is activated directly by RNS and oxidants through modification of cysteine free sulfhydryl groups [57] although cysteine redox system antioxidants, such as selenium, glutathione and N-acetyl cysteine, inhibited capsaicin and oxidative stress-induced activation of TRPV1 channels [15, 30, 34, 50]. It has been shown that Cys553 and Cys558 are present between the fifth and sixth transmembrane of TRPV1 channels and these amino acids have an essential role for the TRPV1 activation in response to nitric oxide stimulation [29, 57, 58]. TRPV1 channels are also activated by nitric oxide directly, while a TRPV1 mutant with substitutions at these conserved cysteines gives significantly suppressed responses to nitric oxide.
TRPV1 Blockers and Potential Therapeutic Use
There are two main TRPV1 channel blockers, namely CPZ and resiniferatoxin. CPZ is the first reported blocker of TRPV1 channels and it has been used in pharmacological studies [60]. CPZ binds in the channel pore of TRPV1 and blocks all four monomers of the tetrameric channel through interacting with residues of the monomers [45]. 5'-Iodoresiniferatoxin (IRTX) is also potential blocker for TRPV1 channel although some TRPV1 agonist effects were reported for this drub. IRTX has been also proposed as a potentially useful analgesic agent through modulation of TRPV1 channels [32,53]. It appears that TRPV1 receptor antagonists cannot treat the pain totally but in fact provide complementary approaches to management of pain.
The TRPV1 channel is of great interest in the pharmaceutical industry because the TRPV1 channel, as the molecular target for capsaicin, was first discovered in peripheral neurons [36]. Other TRP channels such as TRPM8 and TRPA1 are found in DRG and also have important roles in the etiology of peripheral pain [59, 61]. In patients with acute and chronic pain, the threshold of pain sensitivity increases due to cation channel-mediated cytosolic Ca2+ accumulation. As a result, searches for potential pain alleviating drugs have focused on this Ca2+ modulating cation channels. Specifically, drug companies have sought to develop TRPV1 antagonists for the management of acute and chronic pain [52]. In addition to the TRPV1 channel agonist effects of capsaicin, this agent has also been used as an analgesic given as a low-dose cream or as a single application of a high-dose patch [52,62]. However, its ability to alleviate analgesia is temporary due to adaptation and desensitization of neurons (graphical abstract). At the beginning of the treatment, application of capsaicin induces pain until down regulation of TRPV1 channels through desensitization occurs. Hence, a local anesthetic may be useful during the first treatment phase with capsaicin [59]. Several studies have reported that neuropathic and peripheral pain was reduced by TRPV1 antagonists. It was reported that TRPV1 channel inhibition by antagonists reduced neuropathic pain [36]. Recently, a number of TRPV1 antagonists, including ABT-102, SB-705498, AMG-517, MK2295 and GRC-6211 have been used as analgesic agents for treatment of patients with chronic pain. However, some TRPV1 antagonists (e.g. AMG517) have adverse health effects by disrupting the effect of TRPV1 in body-temperature regulation (hyperthermia). Nonetheless, TRPV1 antagonists and agonists remain promising phar-macological targets [63-65].
EPILEPSY AND THE TRPV1 CHANNEL
The discovery of activators of TRP channels provided useful tools for unraveling the molecular pathways of epilepsy in experimental animal and human studies. A understanding of the molecular pathways developed, this fueled interest in developing drug therapies for the treatment of patients with epilepsy.
Glutamate neurotransmitters are known to play an important role in the etiology of epilepsy. It was reported that glutamate release and glutamate-induced signaling in humans and experimental animals are enhanced by TRPV1 channel activity [46, 66, 67]. Thus, the duration of postsynaptic stimulation is increased through activation of TRPV1 channels at glutamatergic synapses [68]. Therefore, targeting the TRPV1 channel activity may provide a promising strategy for the regulation of neuronal activity in epilepsy [2].
It’s well known that influxes of Na+ and Ca2+ cations induce neurons to depolarize and fire action potentials, which in turn can further increase the influx of Na+ and Ca2+ cations [69]. In addition to interaction between glutamate release and TRPV1 channel activation, the activation of TRPV1 channels caused a decrease in the release of GABA and catecholamines [19, 68, 69], neurotransmitters that have been shown to modulate activity-dependent synaptic efficacy and epilepsy [70, 71].
Studies have demonstrated that CB1 receptor activation by endocannabinoids like anandamide or 2-arachidonylglycerol induced neuroprotection in the hippocampus. Endo-cannabinoids were found effective in inhibiting epileptic seizures in epileptic models [70, 71]. In contrast, other studies indicated that seizure threshold was reduced thus facilitating epileptogenesis [72]. Sun et al. [73] reported increased TRPV1 expression in the hippocampus of patients with temporal lobe epilepsy. Following on this observation, von Rüden et al. [19] investigated the role of the endocannabinoid and endovanilloid systems through TRPV1 channel activation on the etiology of epilepsy in temporal lobe epilepsy-induced mice. They observed an increase in hippocampal CB1 receptor expression and seizure rate in the mice although TRPV1 channel expression did not correlate with the repetitive seizures. They suggested that TRPV1 does not play a role in etiology of epilepsy induction although the endovanilloid system may be strongly involved in ictogenesis or seizure induction. The endocannabinoid signaling pathways are blocked in severe chronic epilepsy and induction of absence epilepsy is reduced by down regulation of CB1 receptors in the endocannabinoid system although there are also conflicting views on the role of TRPV1 channels in the induction of epilepsy [71].
In contrary to results of von Rüden et al. [19], results of recent studies have reported that TRPV1 may be a novel anti-epileptogenic drug target [21-23, 38]. This hypothesis is supported by results of several experimental studies. For example, induction of temporal lobe epilepsy is associated with an increase in the expression of TRPV1 channels [20]. In addition, temporal lobe epilepsy is associated with increased excitatory circuit activity and TRPV1 channel activation in the dentate gyrus of mice, implicating TRPV1 channels in the etiology of epilepsy [20, 38].
The biphasic effects of anandamide and TRPV1 (CPZ) or CB1 (AM251) receptor antagonists on marble-burying behavior (MBB) as a behavioral model was investigated in mice [48]. MBB is reduced by a low dose (10 ìg/mouse) of CPZ but not by high doses (20 and 40 ìg/mouse) of CPZ. In addition, blockade of the CB1 receptor induced an anti-compulsive effect in the mice. Similarly, Shirazi et al. [74] investigated the role of TRPV1 receptors on the development of pentylenetetrazole (PTZ) and amygdala-induced kindling in rats. They observed that the pro-convulsant effects of OLDA (a TRPV1 receptor agonist) were reversed by pre-treatment of rats with AMG-9810, a (TRPV1 antagonist).
TRPV1 channels are activated by high environmental temperature. Recently, Kong et al. [75] investigated the role of TRPV1 on the threshold to PTZ-induced epileptic seizures in wild type and TRPV1 gene knock-out C57/BL6 mice. Their results indicated that the threshold to PTZ-induced seizures temporally correlated with hyperthermia-induced TRPV1 channel activation in the mice. According to these results, hyperthermia-induced TRPV1 might be an important candidate therapeutic target in neurological disorders with heat-induced hyper-excitation.
Piperine is important active principal of black pepper. Black pepper has been used as a traditional medicine analgesic in the treatment of epilepsy [76]. A recent study reported an anti-epileptic effect of piperine through TRPV1 channels in the PTZ and maximal electroshock seizure mouse models [18].
It’s well known that 4-aminopyridine (4-AP) induces epileptiform electrical depolarization by blocking voltage gated K+ channels in rat hippocampus. An antiepileptic activity of CPZ was tested using 4-AP as a convulsant agent by Gonzalez-Reyes et al. [5]. CPZ administration suppressed ongoing ictal activity in the rat hippocampus. In addition, they concluded that peripherally injected CPZ passed the blood-brain barrier easily to reach anti-convulsant concentrations in the brain.
Overload of cytosolic Ca2+ induces the generation of ROS, through uncoupling of mitochondria and activation of many cytosolic catabolic enzymes [30, 34]. Exposure of mitochondria to an overload of cytosolic free Ca2+ has been shown to increase formation of ROS [23, 34]. Production of NADPH and ATP are also impaired by increased mitochondrial membrane depolarization and enhanced ROS formation [11]. Currently, ROS are known to be both a contributor to the cause, as well as a consequence of epileptic seizures [1].
Prior to 2014, publications on epilepsy and TRPV1 channels dealt with channel expression and seizure activity but not with Ca2+ influx (by patch-clamp or optical techniques). More recently, we have performed three molecular level studies on TRPV1 channels with Ca2+ influx analyses in PTZ-induced epileptic rats. In two studies, hippocampal and DRG neurons from epileptic rats were isolated and they were pre-incubated with CPZ before capsaicin stimulation. We found that hippocampal apoptosis, caspase-3, caspase-9, ROS, mitochondrial depolarization and Ca2+i concentration were increased by epilepsy induction. Hence, PTZ administration to the rats is characterized by increased oxidative stress, Ca2+ influx and apoptosis. Administration of the TRPV1 channel blocker, CPZ, caused a decrease in Ca2+i concentration. To the best of our knowledge, these two studies were the first to investigate epilepsy with particular reference to its effects on oxidative stress, Ca2+ signaling and the apoptosis-redox system in PTZ-induced hippocampal injury in rats [22, 23]. In a third study [77], we demonstrated that cytosolic calcium elevation through activation of TRPV1 channels by the agonist capsaicin, causes apoptosis in DRG and hippocampus of rats. These results indicate that calcium accumulation through TRPV1 channels plays a physiologically relevant role in the regulation of epileptic seizures. In addition, the TRPV1 channel blockers, IRTX and CPZ, induced protective effects against epilepsy, capsaicin-induced Ca2+ entry through TRPV1 and apoptosis in the neurons. Hence, the results of the three studies taken together indicate that a decrease in calcium accumulation, through inhibition of TRPV1 channels, can play a neuronal protective role against epilepsy-induced Ca2+ entry in hippocampal and DRG neurons. This interaction may play an important role in epilepsy and peripheral pain diseases associated with activation of TRPV1 channels.
CONCLUSIONS AND FUTURE DIRECTIONS
TRPV1 is a non-selective channel with high Ca2+ permeability [8], and its activation typically promotes glutamate release by increasing the excitability of neurons and synaptic terminals [37]. The hippocampus is highly sensitive to environmental stimuli including Ca2+ entry, especially given its inability to regenerate. Also, it has been known for some time that glutamate receptors and their agonists such as NMDA and kainic acid, are thought to play an important role in the etiology of epilepsy. Glutamate-induced spontaneous excitatory synaptic transmission is increased in the hippocampus by the TRPV1 channel agonist capsaicin [20, 66] while it was reduced in the hippocampus slices of rats by both CPZ and IRTX, presumably acting through through modulation of glutaminergic systems [37]. Moreover, pretreatment with CPZ in hippocampal neurons exerts a protective effect on the capsaicin-induced increase in caspase activity and apoptosis [22]. Current findings in the literature suggest that the antiepileptic effects of TRPV1 channel antagonists may be result from modulation of glutamate release. Therefore, in this review, on the basis of recent findings in the literature, we may conclude that Ca2+ accumulation through TRPV1 channels has a regulatory role in the diseases of hippocampal and sensory neurons.
Anandamide is an endogenous agonist at CB1 and also activates TRPV1. Results of recent reports indicate that activation of CB1 receptors and TRPV1 channels are affected in epileptic animals by anandamide and CPZ in a dose dependent manner [48]. Although the role of anandamide in induction and treatment of epilepsy is clear, the role of TRPV1 channels in the etiology of epilepsy is less clear. In addition, there are few reports on anandamide-dependent activation of TRPV1 channels and induction of epilepsy in the literature. Hence, additional studies on this subject are needed.
Oxidative stress plays an important role in the induction of epilepsy, and TRPV1 channels are activated by oxidative stress. The results of recent studies indicate that critical cysteine groups are present in the structure of TRPV1 channels [57]. Glutathione, selenium and N-acetyl cysteine have potent effects on the cysteine redox system. Recently we observed a modulatory role of glutathione and N-acetyl cysteine on inhibition of TRPV1 channels in DRG and hippocampus of rats [15, 30]. To my knowledge, there is no study on the roles of thiol- and cysteine- containing antioxidants in epileptic animals. Hence, the role of TRPV1 channels should be investigated in epileptic animals with respect to possible modulation by glutathione and N-acetyl cysteine.
As yet, the TRPV1 channel has not been fully recognizes as a potentially novel drug target by the drug industry. In the future, there is a need to investigate TRPV1 channel inhibitors as possible new antiepileptic drugs.
Table 1.
Role of TRPV1 channels on molecular pathways in human and animals with epilepsy.
| Material | Drugs | Effects | References |
|---|---|---|---|
| Mice | Anandamide Capsaicin Capsazepine | CPZ and low doses of anandamide anticonvulsant but capsaicin and high doses of anandamide pro-convulsant. | Manna and Umathe [21] |
| Mice | Anandamide Capsaicin CPZ | CPZ and low doses of anandamide inhibit marble-burying behavior effect but capsaicin and high doses of anandamide induce marble-burying behavior. | Umathe et al. [48] |
| Rat | Piperine | Anti-seizure property | Chen et al. [18] |
| Human | - | Increased TRPV1 expression in the hippocampus of patients with temporal lobe epilepsy | Sun et al. [73] |
| Mice | - | TRPV1 expression is unaltered in the hippocampus of mice with seizure history | von Rüden et al. [19] |
| Mice | CPZ | TRPV1 channel is expressed in the hippocampus of epileptic mice. CPZ suppressed ongoing ictal activity and propagation of seizure activity. | Gonzalez-Reyes et al. [5] |
| Rat | OLDA and AMG-9810 | OLDA (TRPV1 receptor agonist)- induced pro-convulsant effect were reversed by the pre-treatment of rat with AMG-9810 (TRPV1) antagonist. | Shirazi et al. [74] |
| Trpv1 deficient mice | Occurrence of febrile seizures temporally correlated with hyperthermia evoked TRPV1 activation | Kong et al. [75] | |
| Rat | Capsaicin and CPZ | Capsaicin-induced TRPV1 channel activity is increased in hippocampus of rats by epilepsy. | Ghazizadeh and Nazıroğlu [22] Nazıroğlu et al. [23] |
| Rat | Capsaicin, CPZ and IRTX | Anti-seizure and anti-apoptotic properties | Nazıroğlu and Ovey [77] |
CPZ, capsazepine; IRTX, 5'-iodoresiniferatoxin; OLDA, N-oleoyldopamine
ACKNOWLEDGEMENTS
There is no financial support for the manuscript.
LIST OF ABBREVIATIONS
- 4-AP =
4-aminopyridine
- CB1 =
cannabinoid receptors type 1
- CB2 =
cannabinoid receptors type 2
- CNS =
central nervous system
- CPZ =
capsazepine
- DRG =
dorsal root ganglion
- FAAH =
fatty acid amide hydrolase
- IRTX =
5'-iodoresiniferatoxin
- LTD =
long-term depression
- LTP =
long-term potentiation
- NMDA =
N-methyl-D-aspartate
- PTZ =
pentylenetetrazole
- ROS =
reactive oxygen species
- TRP =
transient receptor potential
- TRP =
transient receptor potential
- TRPC =
transient receptor cononcial
- TRPM =
transient receptor melastatin
- TRPV1 =
transient receptor potential vanilloid type 1
- VGCC =
voltage gated calcium channels
AUTHORS’ ROLES
MN formulated the present hypothesis and was responsible for writing the report. The author wishes thanks to Prof. Dr. James W. Putney (NC, USA) for the critical revision of the manuscript and Bilal Çiğ (Ph.D. Student, Department of Neurosceince, Medical Faculty, Suleyman Demirel University) for preparation of Fig. 1.
Fig. (1).
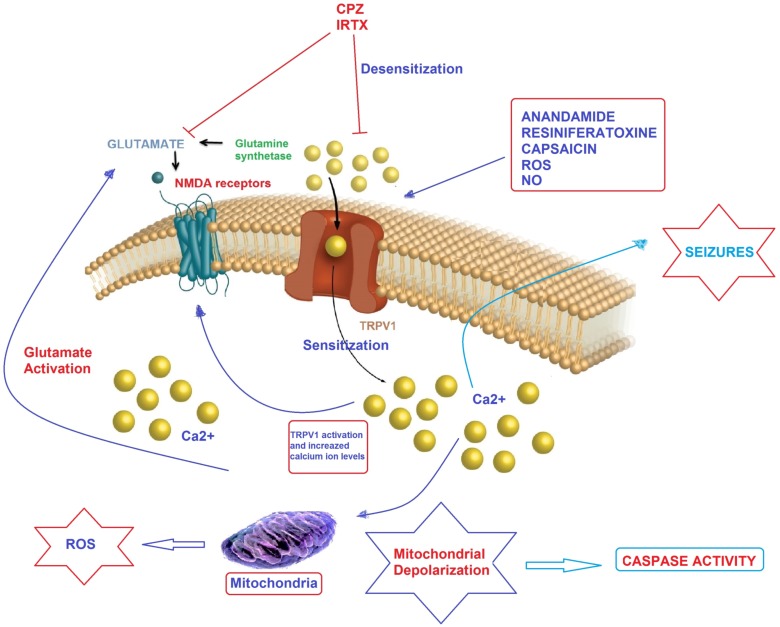
Possible molecular pathways of TRPV1 channel activation on epilepsy in hippocampal neurons. Convulsions in epilepsy can results in augmented glutamate release, leading to Ca2+ uptake through NMDA receptor and TRP channels. Mitochondria were reported to accumulate Ca2+ provided cytosolic Ca2+ rises, thereby leading to depolarization of mitochondrial membranes. At the extreme, Ca2+ entry causes severe mitochondrial permeability transition or even the rupture of the mitochondrial membrane, substantial swelling of the mitochondria with rupture of the outer membrane and release of apoptosis-inducing factors such as caspase 3 and 9. Anandamide, capsaicin, resiniferatoxin, nitric oxide (NO) and reactive oxygen species (ROS) induce Ca2+ accumulation through desensitization of TRPV1 channels although pharmacological desensitization of TRPV1 channels through antagonists such as capsazepine (CPZ) and 5'-iodoresiniferatoxin (IRTX) contributes to an immediate reduction on neuronal excitability [78]. ROS enhance also spontaneous release of glutamate from presynaptic terminals onto neurons through TRPV1 channel activation. The molecular pathway may be a cause of epileptic seizures and the subject should urgently investigate.
DISCLOSER
There is no conflict to disclose in the current study.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Nazıroglu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009;34(12):2181–2191. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- 2.Iannotti F.A., Hill C.L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., Russo E., Whalley B.J., Di Marzo V., Stephens G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014;5(11):1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 3.Yürekli V.A., Nazıroğlu M. Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: a clinical pilot study. Biol. Trace Elem. Res. 2013;152(2):180–186. doi: 10.1007/s12011-013-9616-9. [DOI] [PubMed] [Google Scholar]
- 4.Nazıroğlu M., Akay M.B., Çelik Ö., Yıldırım M.İ., Balcı E., Yürekli V.A. Capparis ovata modulates brain oxidative toxicity and epileptic seizures in pentylentetrazol-induced epileptic rats. Neurochem. Res. 2013;38(4):780–788. doi: 10.1007/s11064-013-0978-3. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Reyes L.E., Ladas T.P., Chiang C.C., Durand D.M. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013;250:321–332. doi: 10.1016/j.expneurol.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demirci S., Kutluhan S., Naziroğlu M., Uğuz A.C., Yürekli V.A., Demirci K. Effects of selenium and topiramate on cytosolic Ca(2+) influx and oxidative stress in neuronal PC12 cells. Neurochem. Res. 2013;38(1):90–97. doi: 10.1007/s11064-012-0893-z. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz M., Naziroğlu M., Kutluhan S., Yilmaz N., Yürekli V.A., Vural H. Topiramate modulates hippocampus NMDA receptors via brain Ca(2+) homeostasis in pentylentetrazol-induced epilepsy of rats. J. Recept. Signal Transduct. Res. 2011;31(2):173–179. doi: 10.3109/10799893.2011.555914. [DOI] [PubMed] [Google Scholar]
- 8.Nazıroğlu M., Dikici D.M., Dursun S. Role of oxidative stress and Ca²⁺ signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem. Res. 2012;37(10):2065–2075. doi: 10.1007/s11064-012-0850-x. b. [DOI] [PubMed] [Google Scholar]
- 9.Nazıroğlu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012;32(3):134–141. doi: 10.3109/10799893.2012.672994. a. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V.S., Gopalakrishnan A., Naziroğlu M., Rajanikant G.K. Calcium ion--the key player in cerebral ischemia. Curr. Med. Chem. 2014;21(18):2065–2075. doi: 10.2174/0929867321666131228204246. [DOI] [PubMed] [Google Scholar]
- 11.Naziroğlu M. New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem. Res. 2007;32(11):1990–2001. doi: 10.1007/s11064-007-9386-x. [DOI] [PubMed] [Google Scholar]
- 12.Montell C., Rubin G.M. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2(4):1313–1323. doi: 10.1016/0896-6273(89)90069-X. [DOI] [PubMed] [Google Scholar]
- 13.Nazıroğlu M. TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem. Res. 2011;36(3):355–366. doi: 10.1007/s11064-010-0347-4. [DOI] [PubMed] [Google Scholar]
- 14.Nagy I., Friston D., Valente J.S., Torres Perez J.V., Andreou A.P. Pharmacology of the capsaicin receptor, transient receptor potential vanilloid type-1 ion channel. Prog. Drug Res. 2014;68:39–76. doi: 10.1007/978-3-0348-0828-6_2. [DOI] [PubMed] [Google Scholar]
- 15.Nazıroğlu M., Ciğ B., Ozgül C. Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience. 2013;242:151–160. doi: 10.1016/j.neuroscience.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 17.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.Y., Li W., Qu K.P., Chen C.R. Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur. J. Pharmacol. 2013;714(1-3):288–294. doi: 10.1016/j.ejphar.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 19.von Rüden E.L., Jafari M., Bogdanovic R.M., Wotjak C.T., Potschka H. Analysis in conditional cannabinoid 1 receptor-knockout mice reveals neuronal subpopulation-specific effects on epileptogenesis in the kindling paradigm. Neurobiol. Dis. 2015;73:334–347. doi: 10.1016/j.nbd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskaran M.D., Smith B.N. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp. Neurol. 2010;223(2):529–536. doi: 10.1016/j.expneurol.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manna S.S., Umathe S.N. A possible participation of transient receptor potential vanilloid type 1 channels in the antidepressant effect of fluoxetine. Eur. J. Pharmacol. 2012;685(1-3):81–90. doi: 10.1016/j.ejphar.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Ghazizadeh V., Nazıroğlu M. Electromagnetic radiation (Wi-Fi) and epilepsy induce calcium entry and apoptosis through activation of TRPV1 channel in hippocampus and dorsal root ganglion of rats. Metab. Brain Dis. 2014;29(3):787–799. doi: 10.1007/s11011-014-9549-9. [DOI] [PubMed] [Google Scholar]
- 23.Nazıroğlu M., Ozkan F.F., Hapil S.R., Ghazizadeh V., Ciğ B. Epilepsy but not mobile phone frequency (900 MHz) induces apoptosis and calcium entry in hippocampus of epileptic rat: Involvement of TRPV1 channels. J. Membr. Biol. 2014 doi: 10.1007/s00232-014-9744-y. [DOI] [PubMed] [Google Scholar]
- 24.Mula M. Emerging drugs for focal epilepsy. Expert Opin. Emerg. Drugs. 2013;18(1):87–95. doi: 10.1517/14728214.2013.750294. [DOI] [PubMed] [Google Scholar]
- 25.Kutluhan S., Naziroğlu M., Celik O., Yilmaz M. Effects of selenium and topiramate on lipid peroxidation and antioxidant vitamin levels in blood of pentylentetrazol-induced epileptic rats. Biol. Trace Elem. Res. 2009;129(1-3):181–189. doi: 10.1007/s12011-008-8287-4. [DOI] [PubMed] [Google Scholar]
- 26.Nazıroğlu M., Yürekli V.A. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell. Mol. Neurobiol. 2013;33(5):589–599. doi: 10.1007/s10571-013-9936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naziroğlu M., Kutluhan S., Yilmaz M. Selenium and topiramate modulates brain microsomal oxidative stress values, Ca2+-ATPase activity, and EEG records in pentylentetrazol-induced seizures in rats. J. Membr. Biol. 2008;225(1-3):39–49. doi: 10.1007/s00232-008-9132-6. [DOI] [PubMed] [Google Scholar]
- 28.Frank C.A. How voltage-gated calcium channels gate forms of homeostatic synaptic plasticity. Front. Cell. Neurosci. 2014;8:40. doi: 10.3389/fncel.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N., Kozai D., Mori Y. TRP channels: sensors and transducers of gasotransmitter signals. Front. Physiol. 2012;3:324. doi: 10.3389/fphys.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Övey I.S., Naziroğlu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience. 2015;284:225–233. doi: 10.1016/j.neuroscience.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 31.Tóth A., Boczán J., Kedei N., Lizanecz E., Bagi Z., Papp Z., Edes I., Csiba L., Blumberg P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005;135((1-2)):162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Jeffry J.A., Yu S.Q., Sikand P., Parihar A., Evans M.S., Premkumar L.S. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4((9)):e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140(5):790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazıroğlu M., Senol N., Ghazizadeh V., Yürüker V. Neuroprotection induced by N-acetylcysteine and selenium against traumatic brain injury-induced apoptosis and calcium entry in hippocampus of rat. Cell. Mol. Neurobiol. 2014;34(6):895–903. doi: 10.1007/s10571-014-0069-2. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi S., Motta C., Musella A., Centonze D. The interplay between inflammatory cytokines and the endocannabinoid system in the regulation of synaptic transmission. Neuropharmacology. 2014:S0028-3908(14)00329-3. doi: 10.1016/j.neuropharm.2014.09.022. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 37.Edwards J.G., Gibson H.E., Jensen T., Nugent F., Walther C., Blickenstaff J., Kauer J.A. A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons. Hippocampus. 2012;22(2):209–221. doi: 10.1002/hipo.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Geis C., Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J. Neurosci. 2008;28(22):5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnerer J., Liebmann I. The pain pathway in the rat following noxious thermal stimulation: effect of morphine on pERK1/2 and TRPV1 at the dorsal horn level, and on hyperalgesia. Pharmacology. 2013;92(1-2):32–38. doi: 10.1159/000353141. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen T.L., Kwon S.H., Hong S.I., Ma S.X., Jung Y.H., Hwang J.Y., Kim H.C., Lee S.Y., Jang C.G. Transient receptor potential vanilloid type 1 channel may modulate opioid reward. Neuropsychopharmacology. 2014;39(10):2414–2422. doi: 10.1038/npp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khairatkar-Joshi N., Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol. Med. 2009;15(1):14–22. doi: 10.1016/j.molmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Poblete H., Oyarzún I., Olivero P., Comer J., Zuñiga M., Sepulveda R.V., Báez-Nieto D., González L. C., González-Nilo F., Latorre R. Molecular Determinants of Phosphatidylinositol 4,5bisphosphate (PI(4,5)P2) binding to transient receptor potential V1 (TRPV1) channels. J. Biol. Chem. 2014. [DOI] [PMC free article] [PubMed]
- 43.Roberts J.C., Davis J.B., Benham C.D. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995(2):176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J., Ma L. Structure and function of the thermoTRP channel pore. Curr. Top. Membr. 2014 doi: 10.1016/B978-0-12-800181-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 45.Hellmich U.A., Gaudet R. Structural biology of TRP channels. Handb. Exp. Pharmacol. 2014 doi: 10.1007/978-3-319-05161-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fawley J.A., Hofmann M.E., Andresen M.C. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J. Neurosci. 2014;34(24):8324–8332. doi: 10.1523/JNEUROSCI.0315-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerdeman G., Lovinger D.M. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J. Neurophysiol. 2001;85(1):468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- 48.Umathe S.N., Manna S.S., Jain N.S. Endocannabinoid analogues exacerbate marble-burying behavior in mice via TRPV1 receptor. Neuropharmacology. 2012;62(5-6):2024–2033. doi: 10.1016/j.neuropharm.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 49.Tominaga M., Caterina M.J., Malmberg A.B., Rosen T.A., Gilbert H., Skinner K., Raumann B.E., Basbaum A.I., Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 50.Köse S.A., Nazıroğlu M. Selenium reduces oxidative stress and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Biol. Trace Elem. Res. 2014;158(2):136–142. doi: 10.1007/s12011-014-9929-3. [DOI] [PubMed] [Google Scholar]
- 51.Szallasi A., Blumberg P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 52.Smith H., Brooks J.R. Capsaicin-based therapies for pain control. Prog. Drug Res. 2014;68:129–146. doi: 10.1007/978-3-0348-0828-6_5. [DOI] [PubMed] [Google Scholar]
- 53.Abdelhamid R.E., Kovács K.J., Honda C.N., Nunez M.G., Larson A.A. Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors. J. Pain. 2013;14(12):1629–1641. doi: 10.1016/j.jpain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazıroğlu M., Yüksel M., Köse S.A., Özkaya M.O. Recent reports of Wi-Fi and mobile phone-induced radiation on oxidative stress and reproductive signaling pathways in females and males. J. Membr. Biol. 2013;246(12):869–875. doi: 10.1007/s00232-013-9597-9. [DOI] [PubMed] [Google Scholar]
- 55.Nazıroğlu M., Tokat S., Demirci S. Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J. Recept. Signal Transduct. Res. 2012;32(6):290–297. doi: 10.3109/10799893.2012.737002. [DOI] [PubMed] [Google Scholar]
- 56.Lipton S.A., Choi Y.B., Takahashi H., Zhang D., Li W., Godzik A., Bankston L.A. Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25(9):474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006;2(11):596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 58.Susankova K., Tousova K., Vyklicky L., Teisinger J., Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol. Pharmacol. 2006;70(1):383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 59.Vay L., Gu C., McNaughton P.A. The thermo-TRP ion channel family: properties and therapeutic implications. Br. J. Pharmacol. 2012;165:787–801.. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walpole C.S., Bevan S., Bovermann G., Boelsterli J.J., Breckenridge R., Davies J.W., Hughes G.A., James I., Oberer L., Winter J., et al. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 1994;37(13):1942–1954. doi: 10.1021/jm00039a006. [DOI] [PubMed] [Google Scholar]
- 61.Naziroğlu M., Ozgül C. Effects of antagonists and heat on TRPM8 channel currents in dorsal root ganglion neuron activated by nociceptive cold stress and menthol. Neurochem. Res. 2012;37(2):314–320. doi: 10.1007/s11064-011-0614-z. [DOI] [PubMed] [Google Scholar]
- 62.Mathie A. Ion channels as novel therapeutic targets in the treatment of pain. J. Pharm. Pharmacol. 2010;62(9):1089–1095. doi: 10.1111/j.2042-7158.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- 63.Steiner A.A., Turek V.F., Almeida M.C., Burmeister J.J., Oliveira D.L., Roberts J.L., Bannon A.W., Norman M.H., Louis J.C., Treanor J.J., Gavva N.R., Romanovsky A.A. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J. Neurosci. 2007;27(28):7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kissin I., Szallasi A. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr. Top. Med. Chem. 2011;11(17):2159–2170. doi: 10.2174/156802611796904924. [DOI] [PubMed] [Google Scholar]
- 65.Yuen N.Y., Vincent S.G., Foo B., Fisher J.T. Interaction of hypoxia and core temperature: potential role of TRPV1. Adv. Exp. Med. Biol. 2012;758:173–178. doi: 10.1007/978-94-007-4584-1_24. [DOI] [PubMed] [Google Scholar]
- 66.Starowicz K., Maione S., Cristino L., Palazzo E., Marabese I., Rossi F., de Novellis V., Di Marzo V. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J. Neurosci. 2007;27(50):13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.G., Yon J.M., Lin C., Jung A.Y., Jung K.Y., Nam S.Y. Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem. Toxicol. 2012;50(11):3877–3885. doi: 10.1016/j.fct.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 68.Shoudai K., Peters J.H., McDougall S.J., Fawley J.A., Andresen M.C. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J. Neurosci. 2010;30(43):14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.González-Aparicio R., Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson´s disease. Neurobiol. Dis. 2014;62:416–425. doi: 10.1016/j.nbd.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Romigi A., Bari M., Placidi F., Marciani M.G., Malaponti M., Torelli F., Izzi F., Prosperetti C., Zannino S., Corte F., Chiaramonte C., Maccarrone M. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51(5):768–772. doi: 10.1111/j.1528-1167.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 71.Citraro R., Russo E., Scicchitano F., van Rijn C.M., Cosco D., Avagliano C., Russo R., D’Agostino G., Petrosino S., Guida F., Gatta L., van Luijtelaar G., Maione S., Di Marzo V., Calignano A., De Sarro G. Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-α receptor activation in a genetic model of absence epilepsy. Neuropharmacology. 2013;69:115–126. doi: 10.1016/j.neuropharm.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 72.Wallace M.J., Martin B.R., DeLorenzo R.J. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur. J. Pharmacol. 2002;452(3):295–301. doi: 10.1016/S0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- 73.Sun F.J., Guo W., Zheng D.H., Zhang C.Q., Li S., Liu S.Y., Yin Q., Yang H., Shu H.F. Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J. Mol. Neurosci. 2013;49(1):182–193. doi: 10.1007/s12031-012-9878-2. [DOI] [PubMed] [Google Scholar]
- 74.Shirazi M., Izadi M., Amin M., Rezvani M.E., Roohbakhsh A., Shamsizadeh A. Involvement of central TRPV1 receptors in pentylenetetrazole and amygdala-induced kindling in male rats. Neurol. Sci. 2014;35(8):1235–1241. doi: 10.1007/s10072-014-1689-5. [DOI] [PubMed] [Google Scholar]
- 75.Kong W.L., Min J.W., Liu Y.L., Li J.X., He X.H., Peng B.W. Role of TRPV1 in susceptibility to PTZ-induced seizure following repeated hyperthermia challenges in neonatal mice. Epilepsy Behav. 2014;31:276–280. doi: 10.1016/j.yebeh.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 76.Bukhari I.A., Pivac N., Alhumayyd M.S., Mahesar A.L., Gilani A.H. The analgesic and anticonvulsant effects of piperine in mice. J. Physiol. Pharmacol. 2013;64(6):789–794. [PubMed] [Google Scholar]
- 77.Nazıroğlu M., Övey I.S. Involvement of apoptosis and calcium accumulation through TRPV1 channels in neurobiology of epilepsy. Neuroscience. 2015;293:55–66. doi: 10.1016/j.neuroscience.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 78.Novakova-Tousova K., Vyklicky L., Susankova K., Benedikt J., Samad A., Teisinger J., Vlachova V. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149(1):144–154. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]