Abstract
There is rapidly growing evidence indicating multiple and important roles of Ca2+-permeable cation TRP channels in the airways, both under normal and disease conditions. The aim of this review was to summarize the current knowledge of TRP channels in sensing oxidative, chemical irritant and temperature stimuli by discussing expression and function of several TRP channels in relevant cell types within the respiratory tract, ranging from sensory neurons to airway smooth muscle and epithelial cells. Several of these channels, such as TRPM2, TRPM8, TRPA1 and TRPV1, are discussed in much detail to show that they perform diverse, and often overlapping or contributory, roles in airway hyperreactivity, inflammation, asthma, chronic obstructive pulmonary disease and other respiratory disorders. These include TRPM2 involvement in the disruption of the bronchial epithelial tight junctions during oxidative stress, important roles of TRPA1 and TRPV1 channels in airway inflammation, hyperresponsiveness, chronic cough, and hyperplasia of airway smooth muscles, as well as TRPM8 role in COPD and mucus hypersecretion. Thus, there is increasing evidence that TRP channels not only function as an integral part of the important endogenous protective mechanisms of the respiratory tract capable of detecting and ensuring proper physiological responses to various oxidative, chemical irritant and temperature stimuli, but that altered expression, activation and regulation of these channels may also contribute to the pathogenesis of respiratory diseases.
Keywords: Airway disease, air pollution, calcium signaling, oxidative stress, TRP channel
1. INTRODUCTION
Air pollution, both ambient (outdoor) and household (indoor), is defined by World Health Organization as a major environmental risk to health, causing over 7 million premature deaths worldwide [1]. The problem of inhaling respiratory irritants and toxic chemicals is especially common in the workplace. These chemicals evoke many adverse affects, which can range from specific allergic responses limited to only susceptible individuals to more commonly occurring inflammation of the airways and even to systemic toxic effects. Such harmful substances for the respiratory system, their sources and physical and biochemical properties are comprehensively described in the ILO Encyclopedia of Occupational Health and Safety, including the review of mechanisms of cell injury and respiratory diseases they cause [2].
While the adverse effects induced by inhaled air pollutants are generally considered to be non-specific, it should be noted that some of the best characterised irritants commonly induce oxidative stress and injury as they either act as oxidants themselves or engage the oxidative pathway of cell damage via related derivatives, such as free radicals. Other pathological mechanisms, which can be at least in part understood in terms of the specific molecular and cellular events involved, include alteration of pH via formation of acidic or alkaline substances, protein folding and binding, and direct stimulation of nerve endings. The latter causes immediate respiratory symptoms and, if persistent, together with epithelial injury can lead to long-lasting neurogenic inflammation, which is a key component of respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), as well as the sensitized cough response in chronic cough [3].
In this context of as yet poorly understood molecular and cell signaling specificity leading to respiratory diseases, in recent years there has been a continuously growing interest to various members of the mammalian superfamily of Transient Receptor Potential (TRP) channels, many of which can rather specifically detect a diverse array of physical and chemical stimuli, such as mechanical forces, temperature changes, pH, osmolarity, noxious compounds, and reactive oxygen and nitrogen species [4-8]. Indeed, various TRP subtypes are widely present in chemo-, thermo- and mechanosensory nerves (e.g. thinly myelinated Aδ-type and polymodal unmyelinated C-fibres), which innervate the airways and which can sense various noxious physical and chemical stimuli, as well as adverse conditions, such as cold air, hypoxia, pressure and mechanical stress, and chemical irritants [9-12]. Moreover, as will be discussed in this review, TRP channels are present not only in neurons, but also in other cell types, which are important for respiratory pathophysiology including airway epithelial cells, mast cells, macrophages, lymphocytes, neutrophils, eosinophils, and airway smooth muscle cells.
There is also accumulating evidence implicating TRP channels in coordinated parasympathetic reflex responses, such as cough and bronchoconstriction, which efficiently protect the airways against respiratory irritants and toxic chemicals. However, either abnormal sensitization or suppression of these reflexes (e.g. in neurogenic inflammation or chronic exposure to harmful agents) may become a clinical problem in its own right [9-9]. When exaggerated, these same mechanisms give rise to neurogenic inflammation and airways hyperactivity – hallmarks of respiratory diseases, such as chronic cough, COPD, and asthma exacerbation.
The importance of TRP channels in the respiratory system health and disease is most definitely and convincingly illuminated by several studies that used the gene knock-out (KO) strategy, as summarized in Table 1.
Table 1.
TRP knock-out (KO) and respiratory system phenotype
| Subtype | KO Phenotype |
|---|---|
| TRPC6 | Impaired lung allergic inflammatory responses to allergen; reduction in airways eosinophilia and blood IgE levels and decreased levels of T-helper type 2 (Th2) cytokines IL-5, IL-13 in bronchalveolar lavage fluid [20]. |
| TRPA1 | Target of environmental irritants [21]; reduced chemically and allergen-induced neuropeptide release, leukocyte infiltration, cytokine and mucus production, as well as inflammation in the airways; airway hyperreactivity to contractile stimuli is abolished [22]. |
| TRPV1 | Enhanced airway inflammation and bronchial hyperreactivity during endotoxin-induced airway inflammation [23]. |
| TRPV2 | Impaired macrophage migration, binding, and phagocytosis and increased susceptibility and mortality following bacterial infection [24]. |
| TRPV4 | Impaired osmotic and pressure sensations, abnormal osmotic regulation [25-27], decreased lung edema induced by high tidal volume ventilation [28]. TRPV4 contributes to macrophage activation and increase in vascular permeability after ventilator-induced lung injury [28]. |
| TRPM2 | Reduced H2O2-induced Ca2+ influx and production of the macrophage inflammatory protein-2 (CXCL2) in monocytes [ 40], increased endotoxin-induced lung inflammation and injury and decreased survival [30]. |
Thus, considering the growing evidence for multiple key functions of TRP channels as cellular sensors in respiratory function and dysfunction, the purpose of this review was to discuss this evidence with focus on several TRP subtypes that commonly mediate signaling induced by oxidative, temperature and chemical irritant stimuli. There are several excellent recent reviews discussing in detail the important roles of these and other TRPs in specific respiratory diseases, which is outside the scope of this paper [11, 12, 17-19, 31-36].
2. OXIDATIVE STRESS AND TRP CHANNELS
As already noted, the action of some of the best understood chemical irritants that cause respiratory symptoms, including ozone (O3) and particulate matter (PM) as the two most common air pollutants, involves oxidative stress and cell injury [2]. While the link between ozone and other substances (e.g. nitrogen oxides, sulphur dioxide, chlorine, some metals and their oxides) and oxidative injury is clear, there is also evidence that reactive oxygen species (ROS) and oxidative stress are the main components of PM-induced cell injury [37, 38]. Inhalation of such substances, which can trigger a number of the redox-sensitive signaling cascades, can cause cough, wheezing, and the exacerbation of airway inflammation, especially in susceptible people with asthma and COPD, children and the elderly. ROS can also be produced endogenously under hypoxic and ischemia/reperfusion conditions. Lipid peroxidation through oxidative stress-induced damage the protective epithelial lining of the respiratory tract, eventually resulting in an inflammatory response. Oxidative stress thus plays a major role in many diseases, including asthma and COPD.
Important cell types, which are targets of PM and oxidative stress in the lung, are macrophages and bronchial epithelial cells [39, 40]. Their exposure to PM induces generation of ROS and oxidative stress that in turn causes increased production and release of cytokines, e.g. TNF-α and IL-6 in macrophages and IL-8 in bronchial epithelial cells [38, 41]. Furthermore, these and other pro-inflammatory cytokines can sensitize the thermo- and chemo-sensitive TRP channels (e.g. TRPA1, TRPM8, TRPV1) expressed in sensory nerves, thus contributing to neurogenic inflammation, as will be discussed in the next section.
Although ROS and reactive nitrogen species (RNS) are important messengers for normal cell function, redox imbalance when ROS and RNS overwhelm the protective cell defence capabilities contributes to pathological states whereby lipid peroxidation, DNA and protein oxidation, and disruption of cell signaling pathways occur leading to cell death. Calcium signaling, such as enhanced Ca2+ influx and Ca2+ release from the endoplasmic reticulum followed by mitochondrial Ca2+ uptake and overload, as well as abnormal Ca2+-dependent gene transcription, is largely responsible for the switch from normal ROS/RNS messenger function to cell damage and death [42, 43]. For example, in human leukocytes intracellular calcium overload induced ROS production, caspase activation and DNA fragmentation [44, 45]. Calcium overload-induced leukocyte apoptosis was largely inhibited by the cytosolic calcium chelator, dimethyl BAPTA, as well as by blocking calcium uptake into mitochondria.
Notably, several members of the TRP superfamily of non-selective Ca2+-permeable cation channels have been recently implicated in sensing ROS and RNS [46] and likely in abnormal Ca2+ signaling during oxidative stress. In addition, activation of TRP channels can stimulate production of ROS and RNS, likely via the increased calcium entry. For example, activation of TRPV4 in alveolar macrophages significantly increased [Ca2+]i, superoxide and NO production in WT, but not in cells from TRPV4-/- mice [28]. Interestingly, a negative feedback mechanism can also exist as was shown in case of TRPM2, whereby activation of TRPM2 inhibits ROS production in phagocytic cells [30].
2.1. TRPM2
TRPM2 is activated by ROS such as H2O2 either via the production of nicotinamide adenine dinucleotide (NAD+) or, more likely, the product of its hydrolysis - ADP-ribose. The activation occurs in synergy with Ca2+ rise [47-52]. TRPM2 is widely expressed in neurones, in the vasculature and cells of the immune system [53].
Activation of TRPM2 by ROS and RNS strongly suggests its role in oxidative stress, inflammation and cell death. Thus, although several studies have mainly linked TRPM2 activity with pathophysiological events of ischemic stroke and neuronal death [54, 55], it is possible that similar pathophysiological events triggered by TRPM2 activation, such as enhanced cytokine release and inflammation, are also relevant to other diseases, including respiratory diseases. Indeed, TRPM2-mediated Ca2+ influx induces chemokine production, such as IL-8, in monocytes and this aggravates inflammatory neutrophil infiltration. In monocytes from TRPM2-/- mouse both H2O2-evoked Ca2+ influx and production of the macrophage inflammatory protein-2 (CXCL2) were impaired [29]. The link between ROS and TRPM2 activation was suggested to contribute to endothelial hyperpermeability, injury and apoptotic cell death [56-59], but, surprisingly, TRPM2 was not found to be involved in inflammatory responses in mouse models of COPD when comparing wild type (WT) and TRPM2-/- mice [60]. Moreover, increased endotoxin-induced lung inflammation and injury and decreased survival were found in TRPM2-/- mice compared to WT controls in another study [30]. The cellular mechanisms are very complex and relate to membrane depolarisation, rather than intracellular free calcium concentration ([Ca2+]i) elevation, produced by TRPM2 activation in phagocytes. In turn, inhibition of the membrane potential-sensitive nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase) inactivates ROS production in a negative feedback manner. As a result, TRPM2 activation decreased production of the inflammatory mediators and oxidative lung damage, thus playing a protective role. Furthermore, similar inhibitory role of TRPM2 in ROS production was also found in polymorphonuclear leukocytes and macrophages [30]. No compensation for Ca2+ entry in TRPM2-/- cells, such as upregulation of TRPM4 and TRPM5, was evident. Inhibition of ROS production by TRPM2 may be a wide-spread cellular mechanism as similar findings have been recently reported in cardiac myocytes. By investigating ischemia-reperfusion in WT and TRPM2-/- cardiac myocytes it was found that ROS levels were significantly higher in TRPM2-/- cells, a combined effect of decreased ROS production and enhanced scavenging of ROS by superoxide dismutases (SODs) SOD1 and SOD2 mediated by TRPM2 activation [61].
Another study using TRPM2-/- model also did not find any TRPM2 role in airway inflammation, at least in ovalbumin (OVA)-induced severe allergic asthma in mice. By inspecting lung pathology, airway resistance, mucus production, cytokine response, airway inflammation, and production of allergen-specific antibodies no obvious differences were wound in TRPM2-/- mice compared to WT controls [62]. The authors conclude that further studies are needed to fully address the role of TRPM2 in lung inflammation, perhaps with the use of other, less severe asthma models. Such additional studies are indeed warranted considering much more clear role of TRPM2 in other diseases with a strong inflammatory component, such as inflammatory and neuropathic pain and myocardial ischaemia/reperfusion injury [63, 64].
TRPM2 channels may also be important for the maintenance of the respiratory epithelial barrier, which provides defence against allergens, microorganisms and PM. Thus, oxidative stress disrupts the bronchial epithelial tight junctions via activation of TRPM2 with the involvement of phospholipase Cγ1 (PLCγ1) and PKCα signaling cascades, while TRPM2 silencing caused increased resistance to hyperpermeability induced by H2O2 [65].
2.2. Other TRP Channels Involved in Oxidative Stress Phenomena in the Respiratory System
Multiple other TRP subtypes are also sensitive and regulated by oxidative stress, although unlike TRPM2 their primary mechanisms of activation do not involve sensing oxidative stress per se. Among them, TRPA1 and TRPV1 have received considerable attention in the context of the respiratory system health and disorders. Function of these channels in the respiratory system will be discussed in more detail in the next section. However, it is important to note now that the cold- and environmental irritant-sensing TRPA1 channel has also been recently shown to be directly activated by H2O2 via modification of cysteine residues in its N-terminus [66]. Apart from H2O2 several other inflammatory mediators, such as (15-deoxy-Δ12,14-prostaglandin J2, nitric oxide (NO) and protons, also activated human TRPA1 heterologously expressed in HEK cells, suggesting importance of this mechanism under inflammatory conditions, e.g. sensation of pain. The effects of H2O2 on TRPA1 were also mimicked by both ROS and RNS [67]. Indeed, TRPA1 was shown to be the major oxidant sensor in airway sensory neurones [68]. Trpa1–/– mice showed profound deficiencies in hypochlorite- and H2O2–induced respiratory depression.
The heat and capsaicin-activated TRPV1, which can generally function as a focal point for detection of multiple harmful stimuli, is also regulated by oxidative stress. Thus, oxidative stress was found to markedly sensitize TRPV1 to other stimuli, e.g. oxidative modulation of the channel potentiates heat-induced activation of TRPV1 [69] and could even recover agonist-induced activation of TRPV1 following prolonged exposure to capsaicin [70]. In addition, it acted in synergy with kinase or proton-mediated upregulation of channel activity. Similarly to TRPA1, the effects were mediated by modification of multiple intracellular cysteine residues and could be reversed by reducing agents. It should be noted, that reducing agents by modification of extracellular Cys621 also potentiated heat- and voltage-activated TRPV1 currents [69, 71], thus suggesting that TRPV1 depends on an optimal redox state as long as both reduced and oxidised states of TRPV1 demonstrate an enhanced thermal sensitivity [69]. These mechanisms likely maintain thermal hyperalgesia during oxidative damage, inflammation or tissue injury. By analogy with DRG sensory neurones similar scenarios can be envisaged for the role of TRPV1 in some respiratory symptoms, such as exacerbated cough reflexes.
Another interesting parallel can be made based on the findings that increased expression of TRPV1 is seen both in large DRG neurons in diabetic sensory neuropathy [72] and in bronchial epithelial cells in patients with severe asthma [73]. While DRG neurons from diabetes rats showed increased oxidative stress and activation of cell injury markers compared with healthy controls, thus implicating TRPV1 in diabetic sensory neuropathy [72], further studies are needed to evaluate such possibilities in asthma.
Several members of the canonical subfamily of TRP channels are also mediators of oxidative stress (see [46, 74, 75] for detailed reviews). For example, TRPC3 and TRPC4 associate endogenously and form a redox-sensitive cation channel, which mediates oxidative stress-activated membrane cation conductance in porcine aortic endothelial cells [76].
Most TRPC subtypes are expressed in airway smooth muscle cells [77-79], implicating these channels in the Ca2+ influx pathways required for airway smooth muscle contraction, which controls airway calibre and plays a central role in the pathogenesis of asthma. In addition, TRPC-mediated increase in [Ca2+]i in airway smooth muscle cells may be responsible for smooth muscle proliferation and hypertrophy, as well as for secretion of inflammatory mediators in asthma [80-83]. Indeed, there is growing evidence for the roles of TRPC channels expressed in airway myocytes in these pathogenic events, although implications of oxidative stress-induced phenomena mediated by TRPC channels remains to be evaluated. Among them, TRPC3 not only shows constitutive activity and thus plays an important role in the regulation of the resting membrane potential and [Ca2+]i in airway smooth muscle cells, but it also mediates methacholine-evoked increase in [Ca2+]i [84]. In OVA-sensitized mouse, a model for asthma, TRPC3 both protein expression and gating were enhanced, accompanied by increased contractile responses to the muscarinic agonist [84].
TRPC3 may also play important role in inflammatory airway diseases, such as asthma and COPD since the proinflammatory cytokine TNF-α contributes to airway hyper-responsiveness by altering airway smooth muscle calcium signaling [85] and since TRPC3 gene silencing by siRNA treatment significantly inhibits both TNF-α- and acetylcholine-induced Ca2+ influx in airway myocytes [79]. These findings are physiologically relevant as the airways are innervated by cholinergic nerve fibres.
TRPC6 is another redox-regulated ion channel, although primarily it is activated in a receptor-dependent manner with the involvement of diacylglycerol (DAG). The effects of H2O2 on TRPC6 expressed in HEK293 cells are multiple, including direct activation by cysteine oxidation, sensitization to DAG and enhanced plasma membrane expression [86]. TRPC6 plays important role in hypoxic pulmonary vasoconstriction, which is the physiological basis of ventilation/perfusion matching during acute local alveolar hypoxia [87-89]. Oxidative stress is likely to occur during intermittent or chronic hypoxia and hypoxia is involved in many pathological states, including hypertension, heart and respiratory diseases. Moreover, TRPC6 is also involved in lung allergic inflammatory responses to allergens [20], migration of neutrophils [90] and in mucus hypersecretion in chronic bronchitis [91], which suggests TRPC6 as a suitable drug target in asthma and COPD [85]. Such hypothesis is also supported by examination of TRPC expression in human lung macrophages, which play key roles in the pathophysiology of COPD. TRPC6 expression was found to be significantly elevated in alveolar macrophages, and especially in small macrophages, in patients with COPD when compared with healthy subjects, while the expression levels of TRPC3 and TRPC7 were not altered [92].
3. CHEMICAL IRRITANT AND THERMOSENSITIVE TRP CHANNELS
Apart from the oxidative stress, which commonly mediates the action of various chemical irritants, further molecular and cell signaling specificity underlying respiratory diseases can be sought within the next widely studied in this context group of TRP channels - thermosensitive, or thermoTRPs. An important operational criterion for defining thermoTRPs is that their temperature coefficient of activation Q10, which is equal to fold current increase per 10OC change in temperature, should be higher than 5, and 11 TRP subtypes confirm to such definition [93]. Among them, the cold-activated TRPA1 and TRPM8 and the heat-activated TRPV1 channels have received much interest in the studies of the respiratory system and its disease states [12, 17, 18, 31, 34, 35].
Such large interest to these TRP subtypes relates to the fact that they are commonly activated not only by temperature stimuli, but also, and in the context of respiratory disease probably even more importantly, by various chemicals, such as acrolein, crotonaldehyde (noxious aldehyde in photochemical smog and cigarette smoke) and formaldehyde (potent industrial and environmental irritant), which activate TRPA1, or capsaicin (pungent ingredient in chili peppers and potent respiratory irritant), which activates TRPV1.
Thus, these channels can integrate the action of thermal irritants, such as inhalation of cold air that can provoke coughing, especially in respiratory virus-induced cough hypersensitivity [94, 95], with the action of chemical irritants, such as the TRPV1 agonist capsaicin, which is useful for the assessment of cough response during the inhalation cough challenge [96]. In addition and as already discussed above, TRPA1 and TRPV1 channels are regulated by oxidative stress, often in synergy with their main activators – temperature changes and agonists. There is recent evidence that oxidative stress induced by H2O2 caused upregulation of TRPM8-mediated calcium signaling in the urothelium and enhanced urinary bladder afferent nerve activity [97]. Thus, it is important to understand such signal integration in its full complexity.
3.1. TRPA1
TRPA1 is a widely expressed neuronal sensory channel, that can detect noxious cold (<17OC) and various chemicals, including allyl isothiocyanate, acrolein and crotonaldehyde, formaldehyde and ROS to name some of the most important ones for the purposes of this review. Reactive chemicals affect many proteins, but in case of TRPA1 the electrophilic nature of many of its activators suggests covalent modification of the protein, therefore TRPA1 can be considered as an intrinsically chemosensitive channel and likely the major airway irritant receptor [12, 98].
TRPA1 is mostly expressed in a sub-population of TRPV1-expressing neurons and in non-neuronal cells, such as epithelial cells (including in the airways), mast cells, fibroblasts and pancreatic β-cells. Activation of TRPA1 via influx of Na+ and Ca2+ causes membrane depolarisation, which triggers an action potential discharge, as well as induces the release of sensory neuropeptides, such as calcitonin gene-related peptide (CGRP), substance P and neurokinin (NKA). In addition to its major role in nociception, TRPA1 has been implicated in visceral reflexes and responses to tissue injury [98].
Thus, depending on the physiological system various functional and pathophysiological responses can occur subsequent to TRPA1 activation. In the respiratory tract, activation of sensory pathways can cause cough, sneezing, bronchospasm, mucus secretion and, in extreme cases, inflammation. Indeed, TRPA1 has been tested, including knock-out studies (Table 1), and shown to participate in various respiratory physiological and pathological processes, as well as defensive reflex control [12, 99]. Pharmacological studies showed that chemical irritants activate TRPA1 in the airways and produce asthma-like symptoms, as well as heightened responses to chemical and physical stimuli [12, 22, 32, 100-102]. Pharmacological and knockout studies revealed, that TRPA1 mediates the inflammatory effects of chemical irritants [21].
TRPA1 has also been implicated in the progression of COPD. Crotonaldehyde, α,β-unsaturated aldehydes and acrolein contained in cigarette smoke induced an early inflammatory response in rodent airways via activation of TRPA1, which is co-expressed with TRPV1 channels in the same sensory neurones [103]. These compounds also evoked contraction of isolated guinea pig bronchi. The effects of cigarette smoke extract and aldehydes on Ca2+ entry in DRG neurones were absent in TRPA1-deficient mice. Acetaminophen, the common antipyretic/analgesic medicine, also activates TRPA1 and causes airway neurogenic inflammation by releasing proinflammatory neuropeptides (substance P and CGRP) – responses which are absent in TRPA1-deficient mice [104]. Furthermore, airway inflammation can also be promoted by activation of non-neuronal TRPA1 present in fibroblasts, epithelial and smooth muscle cells through the release of IL-8 from these cells. This effect was attenuated by TRPA1 antagonists or in TRPA1-deficient mice [105]. These findings are very interesting in light of similar roles of TRPV1, as will be discussed below.
3.2. TRPV1
TRPV1, or capsaicin receptor, is one of the well-characterised TRP channels. Starting from the initial discovery of TRPV1 expression in a subset of DRG sensory neurones involved in the transduction of painful stimuli [106] there has been much interest in its role in health and disease [107]. TRPV1 is a polymodal receptor that can be activated by heat (>42°C), protons, and some pungent chemicals, such as capsaicin, resiniferatoxin, endocannabinoid lipids and eicosanoids. Moreover, TRPV1 is sensitised by activation of protein kinase A (PKA), protein kinase C (PKC), Ca2+/calmodulin-dependent kinase II (CaMKII), phophatidylinositol 3-kinase (PI3K), as well as by pro-inflammatory mediators, such as bradykinin, prostaglandins, histamine, purines, proteases, NGF and chemokines [6, 8, 107-112].
TRPV1 is widely expressed in sensory neurones and in some non-neuronal cell types, including vascular myocytes and epithelial cells. TRPV1-positive afferent fibers are present throughout the entire respiratory tract and their number increases in allergic inflammation [113, 114]. Thus, a number of studies have addressed function of TRPV1 in sensory nerves, such as its role in airway inflammation and sensitization of cough reflex by inflammatory mediators and other endogenous ligands [12, 85, 94, 115-119].
Inhalation of the TRPV1 agonist capsaicin readily induces cough response in cough challenge test [96], which is enhanced in asthma and COPD. Conversely, TRPV1 antagonists have shown efficacy in suppressing this response [120, 121]. Thus, TRPV1 is currently considered as an attractive antitussive drug target [12, 94, 118]. Interestingly, one genetic variant (SNP) of TRPV1 shows decreased channel activity in response to capsaicin and heat, and this loss-of-function is associated with a reduced risk of childhood asthma [122].
Similarly to the above discussed TRPA1 channel, non-neuronal TRPV1 channels can also play important roles in airway diseases. We found functional expression of TRPV1 in human bronchial epithelial cells and showed its enhanced expression in patients with severe asthma [73]. Activation of TRPV1 in these cells by capsaicin induced IL-8 release, which could be inhibited by the TRPV1 antagonist capsazepine. Thus, a rather consistent picture arises from the studies of TRPA1 and TRPV1 channels (and possibly TRPM8 as discussed below) in sensory neurons and non-neuronal cell types of the airways, suggesting that these channels can contribute to airway hypersensitivity with the involvement of pro-inflammatory mediators, as illustrated in Fig. 1. According to this model, activation of non-neuronal thermoTRPs by thermal stimuli and harmful irritants causes the release of pro-inflammatory mediators from airway epithelial cells, thus inducing or aggravating neurogenic inflammation and relevant respiratory symptoms, such as chronic cough. In this scenario, nerve growth factor (NGF), which does not normally induce a cough response, enhances citric acid-induced cough and airway obstruction via TrkA receptor and TRPV1 activation, likely downstream of PI3K and PLCγ activation [123], and PI3K is involved in TRPV1 sensitisation during inflammation [124]. In this sequence of events, accompanying oxidative stress by activating TRPM2 (and possibly other above discussed oxidative stress-regulated TRP channels) may disrupt tight junctions [65], thus facilitating access and aggravating the action of various harmful irritants and allergens (Fig. 1).
Fig. (1).
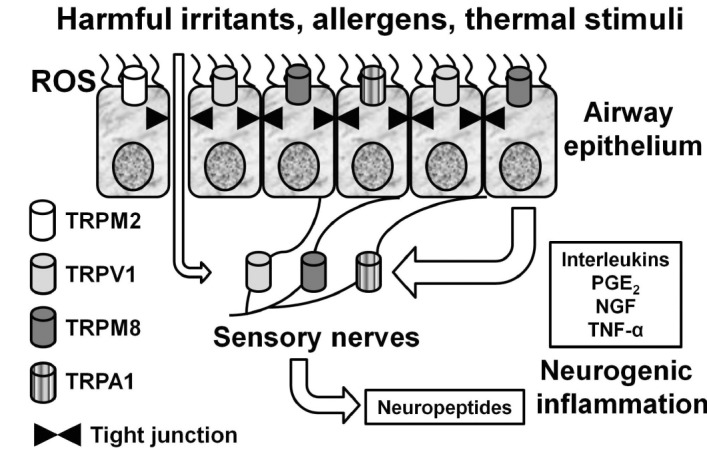
Schematic diagram depicting the signaling cross-talk between thermoTRPs expressed in sensory nerves and non-neuronal cells, such as airway epithelial cells, leading to neurogenic inflammation induced by neuropeptides and further enhanced by pro-inflammatory mediators released by epithelial cells, such as interleukins, prostaglandin E2 (PGE2), NGF and TNF-α. Furthermore, activation on TRPM2 by ROS disrupts the respiratory epithelial barrier, thus aggravating the action of chemical irritants.
It should be noted that persistent nerve activation also induces neurogenic inflammation mediated by the release of neuropeptides, such as tachykinins and CGRP, which in turn can induce a further release of inflammatory mediators [125-127]. Thus, TRPV1 can function at multiple points, from sensory nerves to the secretion of pro-inflammatory cytokines by airway epithelial cells [73, 127, 128]. Similar mechanisms involving upregulation of neuronal TRPV1, as well as TRPA1 and TRPM8, are also involved in respiratory virus-induced cough hypersensitivity [95]. TRPV1 knockdown by siRNA treatment attenuated airway hyperresponsiveness, airway inflammation, goblet cell metaplasia and subepithelial fibrosis induced by IL-13, while IL-13 enhanced TRPV1 expression in bronchial epithelial cells [129]. TRPV1 has also been implicated in another, formaldehyde-induced model of asthma [130]. Sepsis-evoked acute lung injury induced by hydrogen sulphide (H2S) has also been shown to enhance neurogenic inflammation through TRPV1 activation [131]. In addition, endogenous agonists of TRPV1 such as endovanilloids are also involved in lung injury during inflammation [132]. Interestingly, TRPV1 activation can, to some extent, counteract inflammation via somatostatin release [23].
Similarly to TRPA1, TRPV1 expression was increased in lung tissue samples from patients with COPD and its activation cigarette smoke compounds cause ATP release from primary bronchial epithelial cells [133].
Activation of TRPV1 can cause endoplasmic reticulum stress and lung cell death [134]. PM, such as coal fly ash, is a selective and potent TRPV1 activator [135]. PM evoked apoptosis of human airway and epithelial cells and mouse sensory neurons, which was associated with sustained Ca2+ influx via TRPV1 channels and which was completely prevented by the TRPV1 antagonist capsazepine or in sensory neurons from TRPV1-/- mice [136]. Sulfur dioxide (SO2) exposure also causes TRPV1 upregulation associated with sensitization of the cough response to capsaicin [137].
Hyperplasia of airway smooth muscles is an important feature of airway remodelling in asthma. Expression of TRPV1 is markedly increased in asthmatic airway smooth muscle and TRPV1 channel is involved in the regulation of proliferation and apoptosis in asthmatic myocytes [138], although its role in contractility of airway smooth muscles remains unclear [139]. TRPV1 sensitisation in inflammation, which is mediated by the PI3K and PKC signaling pathways, also causes mucus hypersecretion [124].
Taking together, there is growing evidence indicating TRPV1 role in airway responses to various chemical irritants, cough, asthma, and COPD, and both neuronal and non-neuronal TRPV1 channels may be involved in airway disorders.
3.3. TRPM8
TRPM8 was originally cloned from human prostate as gene, expression of which was upregulated in prostate cancer [140]. Afterwards, TRPM8 expression was found in a subpopulation of small diameter cold-sensitive sensory neurones [141, 142] and later several TRPM8 knockout studies have firmly established its crucial role in cold sensation [143-145]. Similarly to TRPA1 and TRPV1 there exist a large number of diverse chemicals that either activate or inhibit TRPM8, which led to its characterisation as truly a “pharmacophore receptor” [146, 147]. Interestingly, TRPM8 can also be activated at physiological temperature via a biochemical pathway that involves PLA2 and its products, lysophospholipids [148, 149]. In addition to cold- and agonist-induced activation, TRPM8 is regulated by phosphorylation, various lipids and directly by G-proteins [147, 150].
As already noted, upper airways can be exposed to marked changes in temperature and inhalation of cold air can provoke cough, airway constriction, mucus secretion and trigger asthma attacks. Hence the expression and function of the cold- and menthol receptor TRPM8 in the airways has been addressed in several studies, but the data still remain somewhat controversial (see [12, 151] for recent reviews). For example, the available pharmacological tools make it difficult to distinguish between the effects of TRPA1 or TRPM8 activation. It is however clear that rhinovirus upregulates expression of neuronal TRPM8 in a manner distinct from TRPV1 and TRPA1, and this implies TRPM8 contribution to virus-induced cough hypersensitivity [95].
TRPM8 immunoreactive nerve fibres are abundant in the sub-epithelium, and especially around blood vessels in deeper regions. Thus, TRPM8 may mediate neurovascular reflexes [152]. However, no differences were found between control subjects and patients with allergic rhinitis. Notably, TRPA1, but not TRPM8 expression was found in vagal afferent nerves innervating mouse lungs [153].
There is also some controversy surrounding the possible involvement of TRPM8 activation in inflammatory responses. One study reported that activation of a shorter splice variant of TRPM8 in bronchial epithelial cells by cold and menthol can produce the inflammatory cytokines IL-6 and IL-8 [154]. However, in mouse model of chemically induced colitis activation of TRPM8 by icilin attenuated the inflammatory response, and this effect was absent in TRPM8-/- mice confirming its specificity [155]. Furthermore, icilin inhibited production of inflammatory cytokines and chemokines in colonic inflammation, in part due to an inhibition of neuropeptide release.
Upregulation of TRPM8 expression was found to be significantly increased in bronchial epithelial cells in patients with COPD compared with TRPM8 expression in healthy subjects. Activation of TRPM8 in these cells by cold caused [Ca2+]i rises and mucus hypersecretion through the Ca2+-PLC-PIP2-MARCKS signaling pathway [156]. In human nasal epithelial cells maintained in short-term culture, we found TRPM8 expression at the mRNA and protein levels [157]. Treatment of these cells with menthol induced membrane current responses and [Ca2+]i rises, which could be blocked by the TRPM8 antagonist BCTC [158].
TRPM8 is also expressed in mast cells, where it has been implicated in cold- and menthol-induced histamine release [159]. Since histamine is associated with the pathology of allergy and inflammation of the airways these results may explain the role of TRPM8 channels in the menthol- and cold-induced allergic responses.
4. CONCLUSIONS
As summarised in this review, even within the limits of the present focus on oxidative, chemical irritant and temperature stimuli, multiple TRP subtypes play important roles in airway function and disease. Many findings in this area of research are generally consistent with the present extensive knowledge of properties and regulation of TRP channels in heterologous expression systems, as well as TRP roles under related conditions in other tissues and cell types. Thus, the role of TRPA1 and TRPV1 in airway inflammation and hyperreactivity of the airways can be well paralleled with their involvement in chronic inflammatory and neuropathic pain. There are also some surprising findings. Although TRPM2 is activated under conditions of oxidative stress thus contributing to tissue inflammation, injury and apoptotic cell death, surprisingly TRPM2 seems to be “protective”, rather than “aggravating” channel under oxidative stress-induced airway disease conditions: it is not involved in inflammatory responses in mouse models of COPD and asthma, but TRPM2 is important for the maintenance of the respiratory epithelial barrier. Instead, TRPA1 was found to be the major oxidant sensor in airway sensory neurones. Although TRPM8 expression is upregulated in bronchial epithelial cells in patients with COPD, its role in airway inflammation also remains a controversial issue. Clearly, in order to fully realise the presently emerging potential of TRP channels as attractive novel targets for the treatment of airway diseases more research is needed to elucidate specific roles of individual TRP subtypes in different cell types, their involvement in cross-talk signaling and cell communication and, most importantly, their dual relevance to both normal airway defence mechanisms and pathophysiological changes.
ACKNOWLEDGEMENTS
Declared none
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ABBREVIATIONS
- [Ca2+]i =
intracellular free calcium concentration
- CaMKII =
Ca2+/calmodulin-dependent kinase II
- CGPR =
calcitonin gene-related peptide
- COPD =
chronic obstructive pulmonary disease
- CXCL2 =
macrophage inflammatory protein-2
- DAG =
diacylglycerol
- IL =
interleukin
- KO =
gene knock-out
- NAD+ =
nicotinamide adenine dinucleotide
- NADPH- =
nicotinamide adenine dinucleotide phosphate-oxidase oxidase
- NGF =
nerve growth factor
- NKA =
neurokinin
- NO =
nitric oxide
- OVA =
ovalbumin
- PGE2 =
prostaglandin E2
- PI3K =
phophatidylinositol 3-kinase
- PKA =
protein kinase A
- PKC =
protein kinase C
- PLA2 =
phospholipase A2
- PLC =
phospholipase C
- PM =
particulate matter
- ROS =
reactive oxygen species
- RNS =
reactive nitrogen species
- SOD =
superoxide dismutase
- Th2 =
T-helper type 2
- TNF-α =
tumor necrosis factor alpha
- TRP =
transient receptor potential
- WT =
wild type
REFERENCES
- 1.World Health Organisation. http://www.who.int . [(Assessed March 7, 2015)].
- 2.Rom W.N., Ryon D.L. Diseases caused by respiratory irritants and toxic chemicals. Encyclopedia of Occupational Health and Safety http://www.ilo.org/iloenc/part-i/respiratorysystem/item/411-diseases-caused-by-respiratory-irritants-and-toxicchemicals ; International Labor Organization 2011; Assessed March 7, 2015; Geneva. Chapter 10. [Google Scholar]
- 3.Patterson R.N., Johnston B.T., Ardill J.E., Heaney L.G., McGarvey L.P. Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax. 2007;62(6):491–495. doi: 10.1136/thx.2006.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 5.Dhaka A., Viswanath V., Patapoutian A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 6.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2(10):a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L.J., Sweet T.B., Clapham D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010;62(3):381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn. J. Physiol. 2000;50(1):3–14. doi: 10.2170/jjphysiol.50.3. [DOI] [PubMed] [Google Scholar]
- 10.Widdicombe J., Lee L.Y. Airway reflexes, autonomic function, and cardiovascular responses. Environ. Health Perspect. 2001;109(Suppl. 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undem B.J., Carr M.J. Targeting primary afferent nerves for novel antitussive therapy. Chest. 2010;137(1):177–184. doi: 10.1378/chest.09-1960. [DOI] [PubMed] [Google Scholar]
- 12.Grace M.S., Baxter M., Dubuis E., Birrell M.A., Belvisi M.G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 2014;171(10):2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzone S.B., McGovern A.E. Sensory neural targets for the treatment of cough. Clin. Exp. Pharmacol. Physiol. 2007;34(10):955–962. doi: 10.1111/j.1440-1681.2007.04702.x. [DOI] [PubMed] [Google Scholar]
- 14.Canning B.J., Spina D. Sensory nerves and airway irritability. Handb. Exp. Pharmacol . 2009;194:139–183. doi: 10.1007/978-3-540-79090-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasra J., Belvisi M.G. Modulation of sensory nerve function and the cough reflex: understanding disease pathogenesis. Pharmacol. Ther. 2009;124:354–375. doi: 10.1016/j.pharmthera.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Yu J. Airway receptors and their reflex function--invited article. Adv. Exp. Med. Biol. 2009;648:411–420. doi: 10.1007/978-90-481-2259-2_47. [DOI] [PubMed] [Google Scholar]
- 17.Bessac B.F., Jordt S.E. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc. Am. Thorac. Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geppetti P., Patacchini R., Nassini R. Transient receptor potential channels and occupational exposure. Curr. Opin. Allergy Clin. Immunol. 2014;14:77–83. doi: 10.1097/ACI.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 19.Benemei S., Patacchini R., Trevisani M., Geppetti P. TRP channels. Curr. Opin. Pharmacol. 2015;22C:18–23. doi: 10.1016/j.coph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Sel S., Rost B.R., Yildirim A.O., Sel B., Kalwa H., Fehrenbach H., Renz H., Gudermann T., Dietrich A. A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin. Exp. Allergy. 2008;38:1548–1558. doi: 10.1111/j.1365-2222.2008.03043.x. [DOI] [PubMed] [Google Scholar]
- 21.Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Clin. Exp. Allergy. 2008;38:1548–1558. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Caceres A.I., Brackmann M., Elia M.D., Bessac B.F., del Camino D., D’Amours M., Witek J.S., Fanger C.M., Chong J.A., Hayward N.J., Homer R.J., Cohn L., Huang X., Moran M.M., Jordt S.E. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma . Proc. Natl. Acad. Sci. USA. 2009;106(22):9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helyes Z., Elekes K., Németh J., Pozsgai G., Sándor K., Kereskai L., Börzsei R., Pintér E., Szabó A., Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292(5):L1173–L1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- 24.Link T.M., Park U., Vonakis B.M., Raben D.M., Soloski M.J., Caterina M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010;11(3):232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno A., Matsumoto N., Imai M., Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol. 2003;285(1):C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M., Mizuno A., Kodaira K., Imai M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 27.Liedtke W., Friedman J.M. Abnormal osmotic regulation in trpv4/- mice. PNAS 100. 2003. pp. 13698–13703. [DOI] [PMC free article] [PubMed]
- 28.Hamanaka K., Jian M.Y., Townsley M.I., King J.A., Liedtke W., Weber D.S., Eyal F.G., Clapp M.M., Parker J.C. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299(3):L353–L362. doi: 10.1152/ajplung.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., Kaneko S., Lange I., Fleig A., Penner R., Nishi M., Takeshima H., Mori Y. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008;14(7):738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di A., Gao X.P., Qian F., Kawamura T., Han J., Hecquet C., Ye R.D., Vogel S.M., Malik A.B. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation . Nat. Immunol. 2012;13:29–34. doi: 10.1038/ni0612-621e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banner K.H., Igney F., Poll C. TRP channels: emerging targets for respiratory disease. Pharmacol. Ther. 2011;130(3):371–384. doi: 10.1016/j.pharmthera.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Preti D., Szallasi A., Patacchini R. TRP channels as therapeutic targets in airway disorders: a patent review. Expert. Opin. Ther. Pat. 2012;22:663–695. doi: 10.1517/13543776.2012.696099. [DOI] [PubMed] [Google Scholar]
- 33.Smit L.A., Kogevinas M., Antó J.M., Bouzigon E., González J.R., Le Moual N., Kromhout H., Carsin A.E., Pin I., Jarvis D., Vermeulen R., Janson C., Heinrich J., Gut I., Lathrop M., Valverde M.A., Demenais F., Kauffmann F. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir. Res. 2012;13:26–13. doi: 10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott-Banner K., Poll C., Verkuyl J.M. Targeting TRP channels in airway disorders. Curr. Top. Med. Chem. 2013;13(3):310–321. doi: 10.2174/1568026611313030008. [DOI] [PubMed] [Google Scholar]
- 35.Büch T., Schäfer E., Steinritz D., Dietrich A., Gudermann T. Chemosensory TRP channels in the respiratory tract: role in toxic lung injury and potential as “sweet spots” for targeted therapies. Rev. Physiol. Biochem. Pharmacol. 2013;165:31–65. doi: 10.1007/112_2012_10. [DOI] [PubMed] [Google Scholar]
- 36.Chung K.F., McGarvey L., Mazzone S.B. Chronic cough as a neuropathic disorder. Lancet Respir. Med. 2013;1(5):414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 37.Xia T., Kovochich M., Nel A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin. Occup. Environ. Med. 2006;5(4):817–836. doi: 10.1016/j.coem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Li N., Xia T., Nel A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008;44(9):1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N., Wang M., Oberley T.D., Sempf J.M., Nel A.E. Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J. Immunol. 2002;169(8):4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- 40.Jung E.J., Avliyakulov N.K., Boontheung P., Loo J.A., Nel A.E. Pro-oxidative DEP chemicals induce heat shock proteins and an unfolding protein response in a bronchial epithelial cell line as determined by DIGE analysis. Proteomics. 2007;7(21):3906–3918. doi: 10.1002/pmic.200700377. [DOI] [PubMed] [Google Scholar]
- 41.Becker S., Mundandhara S., Devlin R.B., Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol. Appl. Pharmacol. 2005;207(2) Suppl.:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38(10):713–721. doi: 10.1016/S0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 43.González A., Granados M.P., Pariente J.A., Salido G.M. H2O2 mobilizes Ca2+ from agonist- and thapsigargin-sensitive and insensitive intracellular stores and stimulates glutamate secretion in rat hippocampal astrocytes. Neurochem. Res. 2006;31(6):741–750. doi: 10.1007/s11064-006-9078-y. [DOI] [PubMed] [Google Scholar]
- 44.Espino J., Bejarano I., Paredes S.D., González D., Barriga C., Reiter R.J., Pariente J.A., Rodríguez A.B. Melatonin counteracts alterations in oxidative metabolism and cell viability induced by intracellular calcium overload in human leucocytes: changes with age. Basic Clin. Pharmacol. Toxicol. 2010;107(1):590–597. doi: 10.1111/j.1742-7843.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 45.Espino J., Bejarano I., Paredes S.D., Barriga C., Rodríguez A.B., Pariente J.A. Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J. Pineal Res. 2011;51(2):195–206. doi: 10.1111/j.1600-079X.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu S., Takahashi N., Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handbook Exp. Pharmacol. 2014;223:767–794. doi: 10.1007/978-3-319-05161-1_3. [DOI] [PubMed] [Google Scholar]
- 47.Perraud A.L., Fleig A., Dunn C.A., Bagley L.A., Launay P., Schmitz C., Stokes A.J., Zhu Q., Bessman M.J., Penner R., Kinet J.P., Scharenberg A.M. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411(6837):595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 48.Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell. 2002;9(1):163–173. doi: 10.1016/S1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 49.Wehage E., Eisfeld J., Heiner I., Jungling E., Zitt C., Luckhoff A. 2002. [DOI] [PubMed]
- 50.Perraud A.L., Takanishi C.L., Shen B., Kang S., Smith M.K., Schmitz C., Knowles H.M., Ferraris D., Li W., Zhang J., Stoddard B.L., Scharenberg A.M. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005;280(7):6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 51.Naziroğlu M., Lückhoff A. Effects of antioxidants on calcium influx through TRPM2 channels in transfected cells activated by hydrogen peroxide. J. Neurol. Sci. 2008;270(1-2):152–158. doi: 10.1016/j.jns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Naziroğlu M., Lückhoff A. A calcium influx pathway regulated separately by oxidative stress and ADP-Ribose in TRPM2 channels: single channel events. Neurochem. Res. 2008;33(7):1256–1262. doi: 10.1007/s11064-007-9577-5. [DOI] [PubMed] [Google Scholar]
- 53.Faouzi M., Penner R. TRPM2. Handbook Exp. Pharmacol. 2014;222:403–426. doi: 10.1007/978-3-642-54215-2_16. [DOI] [PubMed] [Google Scholar]
- 54.Simard J.M., Tarasov K.V., Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. 2007. [DOI] [PMC free article] [PubMed]
- 55.Xie Y.F., Macdonald J.F., Jackson M.F. TRPM2, calcium and neurodegenerative diseases. Int. J. Physiol. Pathophysiol. Pharmacol. 2010;2(2):95–103. [PMC free article] [PubMed] [Google Scholar]
- 56.Hecquet C.M., Ahmmed G.U., Vogel S.M., Malik A.B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008;102(3):347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 57.Hecquet C.M., Malik A.B. Role of H(2)O(2)-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 2009;101(4):619–625. doi: 10.1160/th08-10-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hecquet C.M., Ahmmed G.U., Malik A.B. TRPM2 channel regulates endothelial barrier function. Adv. Exp. Med. Biol. 2010;661:155–167. doi: 10.1007/978-1-60761-500-2_10. [DOI] [PubMed] [Google Scholar]
- 59.Sun L., Yau H.Y., Wong W.Y., Li R.A., Huang Y., Yao X. Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. PLoS ONE. 2012;7:e43186. doi: 10.1371/journal.pone.0043186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardaker L., Bahra P., de Billy B.C., Freeman M., Kupfer N., Wyss D., Trifilieff A. The ion channel transient receptor potential melastatin-2 does not play a role in inflammatory mouse models of chronic obstructive pulmonary diseases. Respir. Res. 2012;13:30–13. doi: 10.1186/1465-9921-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller B.A., Wang J., Hirschler-Laszkiewicz I., Gao E., Song J., Zhang X.Q., Koch W.J., Madesh M., Mallilankaraman K., Gu T., Chen S.j., Keefer K., Conrad K., Feldman A.M., Cheung J.Y. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am. J. Physiol. 2013;304:H1010–H1022. doi: 10.1152/ajpheart.00906.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumoza-Toledo A., Fleig A., Penner R. TRPM2 channels are not required for acute airway inflammation in OVA-induced severe allergic asthma in mice. J. Inflamm. (Lond.) 2013;10(1):19. doi: 10.1186/1476-9255-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haraguchi K., Kawamoto A., Isami K., Maeda S., Kusano A., Asakura K., Shirakawa H., Mori Y., Nakagawa T., Kaneko S. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J. Neurosci. 2012;32(11):3931–3941. doi: 10.1523/JNEUROSCI.4703-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, Mori Y, Shimizu S. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013;97:271–281. doi: 10.1093/cvr/cvs332. [DOI] [PubMed] [Google Scholar]
- 65.Xu R., Li Q., Zhou X.D., Perelman J.M., Kolosov V.P. Oxidative stress mediates the disruption of airway epithelial tight junctions through a TRPM2-PLCγ1-PKCα signaling pathway. Int. J. Mol. Sci. 2013;14(5):9475–9486. doi: 10.3390/ijms14059475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi N., Mizuno Y., Kozai D., Yamamoto S., Kiyonaka S., Shibata T., Uchida K., Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2(4):287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 67.Sawada Y., Hosokawa H., Matsumura K., Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 2008;27(5):1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 68.Bessac B.F., Sivula M., von Hehn C.A., Escalera J., Cohn L., Jordt S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 2008;118(5):1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Susankova K., Tousova K., Vyklicky L., Teisinger J., Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol. Pharmacol. 2006;70(1):383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 70.Chuang H.H., Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc. Natl. Acad. Sci. USA. 2009;106(47):20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vyklicky L., Lyfenko A., Susankova K., Teisinger J., Vlachova V. Reducing agent dithiothreitol facilitates activity of the capsaicin receptor VR-1. Neurosci. 2002;111(47):435–441. doi: 10.1016/s0306-4522(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 72.Hong S., Agresta L., Guo C., Wiley J.W. The TRPV1 receptor is associated with preferential stress in large dorsal root ganglion neurons in early diabetic sensory neuropathy. J. Neurochem. 2008;105(4):1212–1222. doi: 10.1111/j.1471-4159.2008.05220.x. [DOI] [PubMed] [Google Scholar]
- 73.McGarvey L.P., Butler C.A., Stokesberry S., Polley L., McQuaid S., Abdullah H., Ashraf S., McGahon M.K., Curtis T.M., Arron J., Choy D., Warke T.J., Bradding P., Ennis M., Zholos A., Costello R.W., Heaney L.G. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 2014;133(3):704–12.e4. doi: 10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Miller B.A., Zhang W. TRP channels as mediators of oxidative stress. Adv. Exp. Med. Biol. 2011;704:531–544. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- 75.Nazıroğlu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012;32(3):134–141. doi: 10.3109/10799893.2012.672994. [DOI] [PubMed] [Google Scholar]
- 76.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281(19):13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 77.Ong H.L., Brereton H.M., Harland M.L., Barritt G.J. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology. 2003;8(1):23–32. doi: 10.1046/j.1440-1843.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 78.Corteling R.L., Li S., Giddings J., Westwick J., Poll C., Hall I.P. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am. J. Respir. Cell Mol. Biol. 2004;30(2):145–154. doi: 10.1165/rcmb.2003-0134OC. [DOI] [PubMed] [Google Scholar]
- 79.White T.A., Xue A., Chini E.N., Thompson M., Sieck G.C., Wylam M.E. Role of Transient Receptor Potential C3 in TNF-α-enhanced calcium influx in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amrani Y., Panettieri R.A., Jr Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr. Opin. Allergy Clin. Immunol. 2002;2(1):39–45. doi: 10.1097/00130832-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Janssen L.J., Killian K. Airway smooth muscle as a target of asthma therapy: history and new directions. Respir. Res. 2006;7:123. doi: 10.1186/1465-9921-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bara I., Ozier A., Tunon de Lara J.M., Marthan R., Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur. Respir. J. 2010;36:1174–1184. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 83.James A.L., Elliot J.G., Jones R.L., Carroll M.L., Mauad T., Bai T.R., Abramson M.J., McKay K.O., Green F.H. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am. J. Respir. Crit. Care Med. 2012;185(10):1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 84.Xiao J.H., Zheng Y.M., Liao B., Wang Y.X. Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2010;43(1):17–25. doi: 10.1165/rcmb.2009-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colsoul B., Nilius B., Vennekens R. On the putative role of transient receptor potential cation channels in asthma. Clin. Exp. Allergy. 2009;39(10):1456–1466. doi: 10.1111/j.1365-2222.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 86.Graham S., Ding M., Ding Y., Sours-Brothers S., Luchowski R., Gryczynski Z., Yorio T., Ma H., Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J. Biol. Chem. 2010;285(30):23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weissmann N., Dietrich A., Fuchs B., Kalwa H., Ay M., Dumitrascu R., Olschewski A., Storch U., Mederos y Schnitzler M., Ghofrani H.A., Schermuly R.T., Pinkenburg O., Seeger W., Grimminger F., Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc. Natl. Acad. Sci. USA. 2006;103(50):19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuchs B., Dietrich A., Gudermann T., Kalwa H., Grimminger F., Weissmann N. The role of classical transient receptor potential channels in the regulation of hypoxic pulmonary vasoconstriction. Adv. Exp. Med. Biol. 2010;661:187–200. doi: 10.1007/978-1-60761-500-2_12. [DOI] [PubMed] [Google Scholar]
- 89.Fuchs B., Rupp M., Ghofrani H.A., Schermuly R.T., Seeger W., Grimminger F., Gudermann T., Dietrich A., Weissmann N. Diacylglycerol regulates acute hypoxic pulmonary vasoconstriction via TRPC6. Respir. Res. 2011;12:20. doi: 10.1186/1465-9921-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Damann N., Owsianik G., Li S., Poll C., Nilius B. The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol. (Oxf.) 2009;195(1):3–11. doi: 10.1111/j.1748-1716.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 91.Li S., Westwick J., Poll C. Transient receptor potential (TRP) channels as potential drug targets in respiratory disease. Cell Calcium. 2003;33(5-6):551–558. doi: 10.1016/S0143-4160(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 92.Finney-Hayward T.K., Popa M.O., Bahra P., Li S., Poll C.T., Gosling M., Nicholson A.G., Russell R.E., Kon O.M., Jarai G., Westwick J., Barnes P.J., Donnelly L.E. Expression of transient receptor potential C6 channels in human lung macrophages. Am. J. Respir. Cell Mol. Biol. 2010;43:296–304. doi: 10.1165/rcmb.2008-0373OC. [DOI] [PubMed] [Google Scholar]
- 93.Voets T. TRP channels and thermosensation. In: Nilius B., Flockerzi V., editors. Mammalian Transient Receptor Potential (TRP) Cation Channels. Springer International Publishing; 2014. pp. 729–741. [DOI] [Google Scholar]
- 94.McGarvey L.P., Elder J. Future directions in treating cough. Otolaryngol. Clin. North Am. 2010;43(1):199–211, xii. doi: 10.1016/j.otc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Abdullah H., Heaney L.G., Cosby S.L., McGarvey L.P. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax. 2014;69(1):46–54. doi: 10.1136/thoraxjnl-2013-203894. [DOI] [PubMed] [Google Scholar]
- 96.Morice A.H., Kastelik J.A., Thompson R. Cough challenge in the assessment of cough reflex. Br. J. Clin. Pharmacol. 2001;52(4):365–375. doi: 10.1046/j.0306-5251.2001.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nocchi L., Daly D.M., Chapple C., Grundy D. Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell. 2014;13(3):540–550. doi: 10.1111/acel.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zygmunt P.M., Högestätt E.D. TRPA1. Handbook Exp. Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]
- 99.Belvisi M.G., Dubuis E., Birrell M.A. Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest. 2011;140(4):1040–1047. doi: 10.1378/chest.10-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrus M., Peier A.M., Bandell M., Hwang S.W., Huynh T., Olney N., Jegla T., Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Escalera J., von Hehn C.A., Bessac B.F., Sivula M., Jordt S.E. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J. Biol. Chem. 2008;283(35):24136–24144. doi: 10.1074/jbc.M710280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deering-Rice C.E., Romero E.G., Shapiro D., Hughen R.W., Light A.R., Yost G.S., Veranth J.M., Reilly C.A. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem. Res. Toxicol. 2011;24(6):950–959. doi: 10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrè E., Campi B., Materazzi S., Trevisani M., Amadesi S., Massi D., Creminon C., Vaksman N., Nassini R., Civelli M., Baraldi P.G., Poole D.P., Bunnett N.W., Geppetti P., Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 2008;118(7):2574–2582. doi: 10.1172/jci34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nassini R., Materazzi S., Andrè E., Sartiani L., Aldini G., Trevisani M., Carnini C., Massi D., Pedretti P., Carini M., Cerbai E., Preti D., Villetti G., Civelli M., Trevisan G., Azzari C., Stokesberry S., Sadofsky L., McGarvey L., Patacchini R., Geppetti P. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010;24(12):4904–4916. doi: 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- 105.Nassini R., Pedretti P., Moretto N., Fusi C., Carnini C., Facchinetti F., Viscomi A.R., Pisano A.R., Stokesberry S., Brunmark C., Svitacheva N., McGarvey L., Patacchini R., Damholt A.B., Geppetti P., Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7(8):e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 107.Bevan S., Quallo T., Andersson D.A. TRPV1. Handbook Exp. Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 108.Clapham D.E., Julius D., Montell C., Schultz G. International Union of Pharmacology.XLIX. Nomenclature and structurefunction relationships of Transient Receptor Potential channels. Pharmacol. Rev., 2005, 57, 427-450. 2005. [DOI] [PubMed]
- 109.Pedersen S.F., Owsianik G., Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 110.Ramsey I.S., Delling M., Clapham D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 111.Nilius B., Owsianik G., Voets T., Peters J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87(1):165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 112.Szallasi A., Blumberg P.M. Complex regulation of TRPV1 by vanilloids. In: Liedtke W.B., Heller S., editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- 113.Watanabe N., Horie S., Michael G.J., Keir S., Spina D., Page C.P., Priestley J.V. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neurosci. 2006;141:1533–1543. doi: 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 114.Watanabe N., Horie S., Spina D., Michael G.J., Page C.P., Priestley J.V. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized Guinea pigs. Int. Arch. Allergy Immunol. 2008;146(Suppl. 1):28–32. doi: 10.1159/000126057. [DOI] [PubMed] [Google Scholar]
- 115.Groneberg D.A., Niimi A., Dinh Q.T., Cosio B., Hew M., Fischer A., Chung K.F. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am. J. Respir. Crit. Care Med. 2004;170(12):1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 116.Morice A.H., Geppetti P. Cough. 5: The type 1 vanilloid receptor: a sensory receptor for cough. Thorax. 2004;59(3):257–258. doi: 10.1136/thx.2003.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang G., Lin R.L., Wiggers M., Snow D.M., Lee L.Y. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J. Physiol. 2008;586(Pt 23):5771–5786. doi: 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee L.Y., Ni D., Hayes D., Jr, Lin R.L. TRPV1 as a cough sensor and its temperature-sensitive properties. Pulm. Pharmacol. Ther. 2011;24(3):280–285. doi: 10.1016/j.pupt.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Maher S.A., Dubuis E.D., Belvisi M.G. G-protein coupled receptors regulating cough. Curr. Opin. Pharmacol. 2011;11(3):248–253. doi: 10.1016/j.coph.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Lalloo U.G., Fox A.J., Belvisi M.G., Chung K.F., Barnes P.J. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J. Appl. Physiol. 1995;79(4):1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 121.Bhattacharya A., Scott B.P., Nasser N., Ao H., Maher M.P., Dubin A.E., Swanson D.M., Shankley N.P., Wickenden A.D., Chaplan S.R. Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J. Pharmacol. Exp. Ther. 2007;323:665–674. doi: 10.1124/jpet.107.127258. [DOI] [PubMed] [Google Scholar]
- 122.Cantero-Recasens G., Gonzalez J.R., Fandos C., Duran-Tauleria E., Smit L.A., Kauffmann F., Antó J.M., Valverde M.A. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J. Biol. Chem. 2010;285(36):27532–27535. doi: 10.1074/jbc.C110.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Hashim A.Z., Jaffal S.M. Nerve growth factor enhances cough and airway obstruction via TrkA receptor- and TRPV1-dependent mechanisms. Thorax. 2009;64(9):791–797. doi: 10.1136/thx.2009.113183. [DOI] [PubMed] [Google Scholar]
- 124.Yang J., Yu H.M., Zhou X.D., Kolosov V.P., Perelman J.M. Study on TRPV1-mediated mechanism for the hypersecretion of mucus in respiratory inflammation. Mol. Immunol. 2013;53(1-2):161–171. doi: 10.1016/j.molimm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 125.Groneberg D.A., Quarcoo D., Frossard N., Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59(11):1139–1152. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 126.Chung K.F., Widdicombe J.G. Cough: setting the scene. Handbook Exp. Pharmacol. 2009;187(187):1–21. doi: 10.1007/978-3-540-79842-2_1. [DOI] [PubMed] [Google Scholar]
- 127.Couto M., de Diego A., Perpiñi M., Delgado L., Moreira A. Cough reflex testing with inhaled capsaicin and TRPV1 activation in asthma and comorbid conditions. J. Investig. Allergol. Clin. Immunol. 2013;23(5):289–301. [PubMed] [Google Scholar]
- 128.Reilly C.A., Johansen M.E., Lanza D.L., Lee J., Lim J.O., Yost G.S. Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J. Biochem. Mol. Toxicol. 2005;19(4):266–275. doi: 10.1002/jbt.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rehman R., Bhat Y.A., Panda L., Mabalirajan U. TRPV1 inhibition attenuates IL-13 mediated asthma features in mice by reducing airway epithelial injury. Int. Immunopharmacol. 2013;15(3):597–605. doi: 10.1016/j.intimp.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 130.Wu Y., You H., Ma P., Li L., Yuan Y., Li J., Ye X., Liu X., Yao H., Chen R., Lai K., Yang X. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J. Immunol. 2011;187(9):4778–4787. doi: 10.4049/jimmunol.1101559. [DOI] [PubMed] [Google Scholar]
- 131.Ang S.F., Sio S.W., Moochhala S.M., MacAry P.A., Bhatia M. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J. Immunol. 2011;187(9):4778–4787. doi: 10.4049/jimmunol.1101559. [DOI] [PubMed] [Google Scholar]
- 132.Thomas K.C., Roberts J.K. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am. J. Physiol. 2012;302:L111–L119. doi: 10.1152/ajplung.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baxter M., Eltom S., Dekkak B., Yew-Booth L., Dubuis E.D., Maher S.A., Belvisi M.G., Birrell M.A. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax. 2014;69(12):1080–1089. doi: 10.1136/thoraxjnl-2014-205467. [DOI] [PubMed] [Google Scholar]
- 134.Thomas K.C., Sabnis A.S., Johansen M.E., Lanza D.L., Moos P.J., Yost G.S., Reilly C.A. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J. Pharmacol. Exp. Ther. 2007;321(3):830–838. doi: 10.1124/jpet.107.119412. [DOI] [PubMed] [Google Scholar]
- 135.Deering-Rice C.E., Johansen M.E., Roberts J.K., Thomas K.C., Romero E.G., Lee J., Yost G.S., Veranth J.M., Reilly C.A. Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material. Mol. Pharmacol. 2012;81(3):411–419. doi: 10.1124/mol.111.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Agopyan N., Head J., Yu S., Simon S.A. TRPV1 receptors mediate particulate matter-induced apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286(3):L563–L572. doi: 10.1152/ajplung.00299.2003. [DOI] [PubMed] [Google Scholar]
- 137.McLeod R.L., Jia Y., McHugh N.A., Fernandez X., Mingo G.G., Wang X., Parra L.E., Chen J., Brown D., Bolser D.C., Kreutner W., Hey J.A. Sulfur-dioxide exposure increases TRPV1-mediated responses in nodose ganglia cells and augments cough in guinea pigs. Pulm. Pharmacol. Ther. 2007;20(6):750–757. doi: 10.1016/j.pupt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Zhao L., Zhang X., Kuang H., Wu J., Guo Y., Ma L. Effect of TRPV1 channel on the proliferation and apoptosis in asthmatic rat airway smooth muscle cells. Exp. Lung Res. 2013;39(7):283–294. doi: 10.3109/01902148.2013.813610. [DOI] [PubMed] [Google Scholar]
- 139.Mitchell J.E., Campbell A.P., New N.E., Sadofsky L.R., Kastelik J.A., Mulrennan S.A., Compton S.J., Morice A.H. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp. Lung Res. 2005;31(3):295–306. doi: 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- 140.Tsavaler L., Shapero M.H., Morkowski S., Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61(9):3760–3769. [PubMed] [Google Scholar]
- 141.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. 2002. [DOI] [PubMed]
- 142.Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M., Earley T.J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 143.Bautista D.M., Siemens J., Glazer J.M., Tsuruda P.R., Basbaum A.I., Stucky C.L., Jordt S.E., Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 144.Colburn R.W., Lubin M.L., Stone D.J., Jr, Wang Y., Lawrence D., D’Andrea M.R., Brandt M.R., Liu Y., Flores C.M., Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54(3):379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 145.Dhaka A., Murray A.N., Mathur J., Earley T.J., Petrus M.J., Patapoutian A. TRPM8 is required for cold sensation in mice. 2007. [DOI] [PubMed]
- 146.Bödding M., Wissenbach U., Flockerzi V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium. 2007;42(6):618–628. doi: 10.1016/j.ceca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 147.Almaraz L., Manenschijn J.A. de la, P.E.; Viana, F. TRPM8. Handbook Exp. Pharmacol. 2014;222:547–579. doi: 10.1007/978-3-642-54215-2_22. [DOI] [PubMed] [Google Scholar]
- 148.Vanden Abeele F., Zholos A., Bidaux G., Shuba Y., Thebault S., Beck B., Flourakis M., Panchin Y., Skryma R., Prevarskaya N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J. Biol. Chem. 2006;281(52):40174–40182. doi: 10.1074/jbc.M605779200. [DOI] [PubMed] [Google Scholar]
- 149.Andersson D.A., Nash M., Bevan S. Modulation of the coldactivated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci . 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bavencoffe A., Gkika D., Kondratskyi A., Beck B., Borowiec A.S., Bidaux G., Busserolles J., Eschalier A., Shuba Y., Skryma R., Prevarskaya N. The transient receptor potential channel TRPM8 is inhibited via the α 2A adrenoreceptor signaling pathway. J. Biol. Chem. 2010;285(13):9410–9419. doi: 10.1074/jbc.M109.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buday T., Brozmanova M., Biringerova Z., Gavliakova S., Poliacek I., Calkovsky V., Shetthalli M.V., Plevkova J. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8(1):11–18. doi: 10.1186/1745-9974-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keh S.M., Facer P., Yehia A., Sandhu G., Saleh H.A., Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology. 2011;49(4):453–457. doi: 10.4193/Rhino11.089. [DOI] [PubMed] [Google Scholar]
- 153.Nassenstein C., Kwong K., Taylor-Clark T., Kollarik M., Macglashan D.M., Braun A., Undem B.J. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. 2008;586(6):1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sabnis A.S., Shadid M., Yost G.S., Reilly C.A. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cell Mol. Biol. 2008;39(4):466–474. doi: 10.1165/rcmb.2007-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ramachandran R., Hyun E., Zhao L., Lapointe T.K., Chapman K., Hirota C.L., Ghosh S., McKemy D.D., Vergnolle N., Beck P.L., Altier C., Hollenberg M.D. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. USA. 2013;110(18):7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li M., Li Q., Yang G., Kolosov V.P., Perelman J.M., Zhou X.D. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. Allergy Clin. Immunol. 2011;128(18):626–634. doi: 10.1016/j.jaci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 157.de Courcey F., Zholos A.V., Atherton-Watson H., Williams M.T., Canning P., Danahay H.L., Elborn J.S., Ennis M. Development of primary human nasal epithelial cell cultures for the study of cystic fibrosis pathophysiology. Am. J. Physiol. Cell Physiol. 2012;303(11):C1173–C1179. doi: 10.1152/ajpcell.00384.2011. [DOI] [PubMed] [Google Scholar]
- 158.Hanrahan S., Stokesberry S., McGarvey L., Elborn J.S., Zholos A., Ennis M. Expression and functional role of TRPM8 in primary human nasal epithelial cells. Pulm. Pharmacol. Ther. 2011;24:e6. doi: 10.1016/j.pupt.2011.04.017. [DOI] [Google Scholar]
- 159.Cho Y., Jang Y., Yang Y.D., Lee C.H., Lee Y., Oh U. TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium. 2010;48:202–208. doi: 10.1016/j.ceca.2010.09.001. [DOI] [PubMed] [Google Scholar]


