Abstract
Treatment of mitral regurgitation (MR) with the MitraClip (MC), a percutaneous, transseptal edge-to-edge reconstruction of the mitral valve, has become an interesting treatment option in most patients not eligible for surgery. Lately a variety of studies have been published analyzing the treatment of MR with the MC in degenerative as well as functional MR. The results for both entities of MR show negligible intraprocedural mortality, low periprocedural complications rates and a beneficiary outcome in terms of reduction in MR as well as an improvement in functional capacity and quality of life. Here we summarize the latest results focusing on safety and efficacy of MC treatment.
Keywords: MitraClip (MC), percutaneous mitral valve repair, safety outcome
Introduction
The incidence of mitral regurgitation (MR) is exponentially increasing with age (1). Demographical changes toward an aging population challenges health care systems world-wide to develop adequate treatment options for the elderly. The MitraClip (MC) system has evolved as such a new option for the therapy of severe MR in a selective collective of the elderly but also of patients with multiple comorbidities and reduced ejection fraction (EF).
Despite the absence of randomized trials state of the art therapy of severe MR is surgical mitral valve repair (MVR) or if the latter is not suitable MV replacement (MVRx) (2). In all young and low surgical risk patients results are excellent and mortality rate for MVR is low with 1.4% (MVRx respectively 1.6%). However, in elderly patients (age >80 years) and patients with high surgical risk 30-day mortality has been reported to be substantially higher with 11.0% for MVR and 18.9% for MVRx (3).
Especially for these patients there is great need for a less invasive interventional therapy. The MC device (Abbott, Abbott Park, Illinois) is a transvenous, transseptal, edge-to-edge repair system for the treatment of severe functional MR (FMR) and degenerative MR (DMR) eligible for patients with high surgical risk (Figure 1). First clinical application of the MC was performed in the Endovascular Valve Edge-to-Edge Repair Study I (EVEREST I) trial (4). By now 18,500 patients (as of 11/2014, according to the manufacturer Abbott Vascular) have been treated worldwide (Table 1). Indications for treatment of FMR as well as DMR have been incorporated into European guidelines (5). Until now there are approximately 10 years of clinical experience with percutaneous MVR. This review focuses on results of recent registries and clinical studies published in 2013 and 2014 emphasizing procedural safety as well as therapeutically results, such as ACCESS-EU (6,7), EVEREST II (8-12), transcatheter mitral valve interventions (TRAMI) (13-15), Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation (GRASP) Registry (16,17) and one meta-analysis (18).
Figure 1.
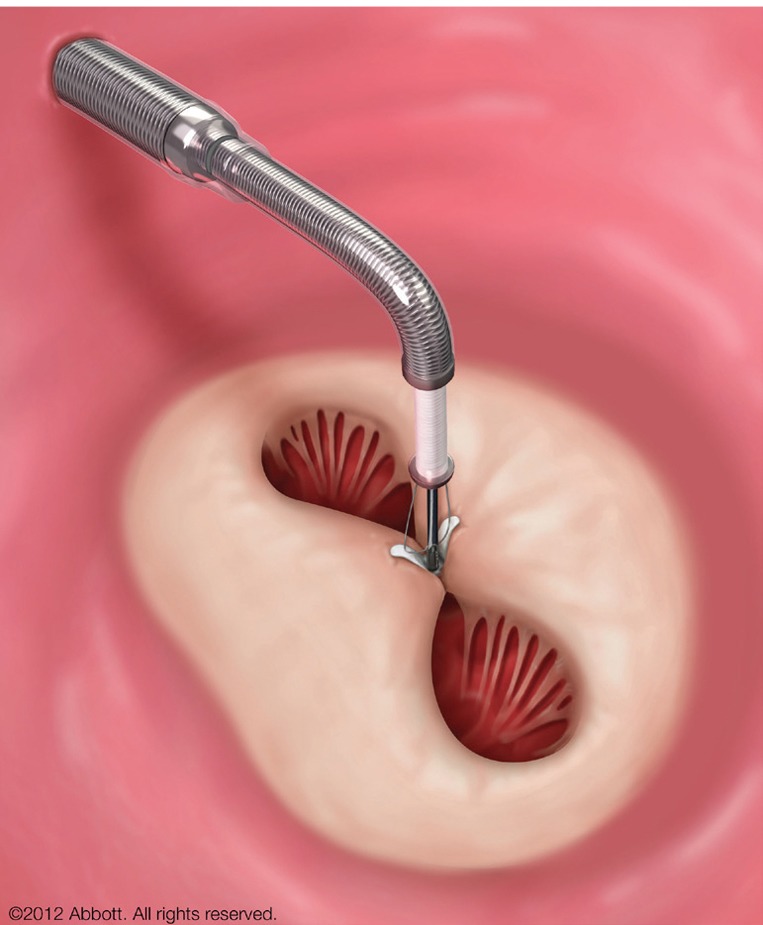
MitraClip in position, grasping the anterior and posterior mitral leaflet while still attached to the clip delivery system (kindly provided by Abbott Vascular).
Table 1. MitraClip clinical trials and commercial use with corresponding number of patients (Data as of 11/30/2014, Abbott Vascular).
| Study | Population | N* (n=18,505+95 surgery) |
|---|---|---|
| EVEREST I (feasibility) | Feasibility patients | 55 |
| EVEREST II (pivotal) | Pre-randomized patients | 60 |
| EVEREST II (pivotal) | Non-randomized patients (high risk study) | 78 |
| EVEREST II (pivotal) | Randomized patients (2:1 clip to surgery) | 279: 184 Clip; 95 surgery |
| REALISM (continued access) | Non-randomized patients | 899 |
| Compassionate/emergency use | Non-randomized patients | 66 |
| ACCESS Europe phase I | Non-randomized patients | 567 |
| ACCESS Europe phase II | Non-randomized patients | 286 |
| Commercial use | Commercial patients | 16,310 |
EVEREST II, Endovascular Valve Edge-to-Edge Repair Study II trial; TRAMI, transcatheter mitral valve interventions.
Mitral valve surgery (MVS) vs. MitraClip (MC)
A recently published Meta-analysis of 21 studies with 6,463 high-risk patients (logistic EuroSCORE >18 or STS score >10) compared the outcome of MVS (n=3,265) and MC (n=3,198) demonstrating similar high rates of procedural success (MVS 98% vs. MC 96%) (19). However, while 30-day technical failure rate was higher with MC compared to MVS (3.2% vs. 0.6%; P=0.002) the pooled key safety analysis at 30 days revealed a much better outcome for MC [mortality: 3.3% (95% CI, 2.6-4.2) vs. 16.2% (95% CI, 13.0-20.0); stroke: 1.1% (95% CI, 0.6-1.6) vs. 4.5% (95% CI, 3.6-5.3); bleeding: 4.2% (95% CI, 3.0-7.0) vs. 59.0% (95% CI, 50.0-67.0); prolonged mechanical ventilation: 1.7% (95% CI, 1.1-2.2) vs. 36.3% (95% CI, 33.1-40.0)]. These results were shown despite a higher surgical risk profile in the patient group treated with MC [e.g., mean left ventricular ejection fraction (LVEF) of 38% vs. 52%] (19). MC patients showed a 1-year mortality of 13%. Long-term data were not available for MVS and thus results were not comparable. By contrast, patients under best medical therapy without intervention or surgery with severe MR and heart failure showed a 1-year mortality of 20%, 5-year mortality of 50% and a high rate of recurrent hospitalization for heart failure (20).
EVEREST II
So far the EVEREST II trial is the only randomized controlled trial comparing MVS and MC (12). For comparison with European registries it is of importance to emphasize the difference in the study population of the EVEREST trial with mainly degenerative etiology of MR (73.3%) and a relatively good general state of health (inclusion criterion was eligibility for surgery), whereas European registries mainly include patients with FMR and a poor general state of health.
The study shows an inferiority of MC regarding acute efficacy in MR reduction as well as a clear inferiority of MC for the composite endpoint of primary efficacy (freedom of death, MVS or reoperation and MR grade 3+ or 4+) at 1-year follow-up in the intent to treat analysis (73% for MVS vs. 55% for MC, P=0.007). This significant difference was mainly driven by the requirement of surgery for mitral-valve dysfunction in 20% following MC therapy compared to 2.2% reoperation in surgical patients (4-year: 24.8% and 5.5% respectively). Although more patients had MR grade 2+ after MC therapy, patients had less symptomatic heart failure according to NYHA class when compared to those who underwent MVS. Interestingly, at 4-year follow-up the rate of the composite endpoint of freedom from death, surgery or MR ≥ grade 3+ was 39.8% in the MC group and 53.4% after MVS, a difference that was not statistically significant (P=0.07). Sub group analysis revealed an advantage in the efficacy endpoint at 4 years (as well as after 12 months) for patient ≥70 years and patients with FMR. Furthermore mortality rates after 4 years were similar in both groups. In addition, the interventional and surgical results proved to be stable at 4 years with 25% vs. 5.5% (MC vs. MVS) requiring surgery for mitral valve dysfunction. Thus, if a good result after 6 months was found with the MC, the likelihood for recurrent MR was low and there was no evidence of late device related complications.
Current indication for MitraClip (MC)
European guidelines recommend interventional mitral device therapy for severe symptomatic FMR as well as DMR in all patients with high surgical risk (recommendation class IIb, level of evidence C). Life expectancy has to exceed 1 year and a heart team consisting of cardiologist and cardiac surgeon should mutually agree that patients are ineligible for surgery (5). Furthermore, MR pathology has to be eligible for intervention by defined echocardiographic parameters (8,21).
By contrast, the Northern American 2014 guidelines for the management of valvular heart disease (22) recommend interventional mitral device therapy only for DMR based on the results of the EVEREST I and II trials, the only randomized and controlled trial comparing MVR/MVRx with the MC device so far (8,9,12).
Mortality and safety of MitraClip (MC)
In a recent meta-analysis of 2,980 patients from 16 studies (12 European and 4 Northern American) procedural mortality was extremely low with only 0.1%. Nevertheless, 30-day mortality was 4.2% and all-cause mortality during a mean follow up of 310 days was 15.8% (18). The 30-day mortality ranged between 0.9% (17), 4.2% (18) and 4.7% (7) in most clinical trials. A recent analysis of 1-year mortality was comparatively high ranging between 12% and 18.2% in all recent trials (7,17,18,23). The reason for the high mortality rate during the first year is most likely related to significant co-morbidities of these patient populations with a mean logistic EuroSCORE I of 23.4%±1.5% (18). In the GRASP registry 55% of deaths during the first year were attributed to non-cardiac reasons (17). Therefore it can be speculated that in the beginning of MC therapy the majority of MC cases were attempted as rescue therapy in terminally sick patients, whose clinical course, despite intervention, could eventually not be altered.
Complications of MitraClip (MC)
MC is accompanied by a low rate of procedural complications. In the previously mentioned meta-analysis the most relevant procedure associated complication was major bleeding (requiring transfusion) with 9.7%. Other major complications are stroke/transient ischemic attack (TIA) (1.3%), chordal rupture (0.8%), pericardial tamponade (0.7%), myocardial infarction (0.4%) (18), and transseptal complications 1.2-3% (24). The ACCESS-EU registry reports even lower rates of stroke (0.7%) and bleeding complications (3.8%) (7). Overall, MC complication rates are relatively low (Table 2), particularly when compared with complication rates of transcatheter aortic valve replacement. The later requires an arterial puncture and calcification of vessels and the aortic annulus increases the risk for both stroke/TIA, ranging from 4.0% (25) to 6.7% (26) and for major vascular complications, ranging from 8.2% (25) to 16.2% (26).
Table 2. Complication rates of immediate and long term safety outcomes of percutaneous mitral valve repair are listed according to a recent meta-analysis (18), two registries (7,13) and the EVEREST II trial (9).
| Complication rates | Meta-analysis [Vakil et al. (18)] (%) | ACCESS-EU [Maisano et al. (7)] (%) | TRAMI [Wiebe et al. (13) (Euo score ≥20%/EuroSCORE <20%)] (%) | EVEREST II [Whitlow et al. (9)] (%) |
|---|---|---|---|---|
| Procedural death | 0.1 | 0 | – | 0 |
| 30-day mortality | 4.2 | 3.4 | 4.3/1.1* (in hospital mortality) | 7.7 |
| All-cause mortality during follow-up | 15.8 (mean follow-up 310 days) | 17.3% (12–month follow-up) | 13.4/9.6 (mean follow-up of 72 days) | 24.4 (12–month follow-up) |
| Vascular complications needing intervention | 1.0§ | – | – | – |
| Major bleeding requiring transfusion | 9.7§ | – | 13.7/8.7* | 17.9¶ |
| Bleeding complications | – | 3.9¶ | – | – |
| Tamponade or significant pericardial effusion | 0.7§ | 1.1¶ | 1.1/1.6* | – |
| Emergent cardiac surgery | 0.7§ | 0.4¶ | – | 0.0¶ |
| Non-fatal myocardial infarction | 0.4§ | 0.7¶ | 0.0/0.2* | 2.6¶ |
| Chordal rupture | 0.8§ | – | – | – |
| Single leaflet clip detachement | 2.3§ | 4.8 (diagnosed within 6 months) | – | – |
| Clip embolism | 0.04§ | 0.0¶ | – | – |
| Hemorrhagic or ischemic stroke/TIA | 1.3§ | 0.7¶ | 0.7/0.0* | 2.6¶ |
| Acute renal failure | 4.2§ | 4.8¶ | 1.8/0.2* (dialysis at discharge) | 3.8¶ |
| Need for repeat MitraClip | 1.6§ | 3.4¶ | 1.8/1.6* | 0.0¶ |
§, immediate safety outcomes; ¶, data for 30-day follow up; *, data for in-hospital events. EVEREST II, Endovascular Valve Edge-to-Edge Repair Study II trial; TRAMI, transcatheter mitral valve interventions.
The MC device is rather complex in appliance. Trials disclosing procedural length (time after transseptal puncture until removal of the steerable system) show a decrease in procedural times by the gain of experience. The GRASP registry documented a drop in median implantation time from 71-58 min between the first to the third part of the study period (17). Fluoroscopy duration ranges around 25 min. Contrast agent is not needed and rarely used (7). Echocardiography as guiding modality is essential for all steps of the procedure especially during clip positioning whereas fluoroscopy is only essential for wiring, transseptal puncture and control of coaxial alignment of the clip to the line of coaptation during transvalvular maneuvering. Further use of fluoroscopy as a second tool of visualization is optional and highly operator dependent.
Procedural success and long-term outcome
A recent meta-analysis showed an acute procedural success in 91.4% of the patients, defined as a reduction of MR to ≤ grade 2+. MR was found to be reduced (MR ≤ grade 2+) in 85.3% of the patients at 30-day follow up and in 86.9% at a mean follow up of 310 days (range, 80 days to 4 years) (18). In only 3% of the patients no clip could be implanted. Single center experiences show even better results. A study in 108 patients with predominantly FMR and LV dysfunction (mean LVEF 28%±11% with 88% having a LVEF <40%) demonstrated a procedural success rate of 99% (23). In line with reduction of MR, functional capacity according to NHYA class showed a relief of symptoms (86% in NYHA class I and II) 1 year after treatment (23). The meta-analysis showed an improved functional capacity according to NHYA (class I and II) in 76.6% of the patients (18).
Moreover, the analysis demonstrated an improvement in LVEF, 6-minute walk distance and quality of life (18). Indeed, the gain in 6-minute walk distance [(260.6±13.6 m at baseline) vs. (359.8±24.9 m at follow up)] was larger than seen as result of cardiac resynchronization therapy (27). Similar data was reported in a cohort of FMR patients with severe LV dysfunction (328.7±80.1 m; mean improvement 108 m). Most interestingly, there were clear signs of LV reverse remodeling with an increase in LVEF (27%±9.8% to 34.7%±10.4%; P=0.02 at 1 year follow up) and decreases in left ventricular end-diastolic volume (LVEDV) as well as left ventricular end-systolic volume (LVESV) (23). It can be speculated that the MC is able to restrict further mitral annular plane expansion leading to reversed remodeling.
So far a mortality benefit with MC over medical treatment in high-risk surgical patients and those with advanced congestive heart failure and FMR is not yet proven. However, the currently enrolling studies COAPT (Clinical Outcomes Assessment of the MitraClip Therapy Percutaneous Therapy for High Surgical Risk Patients; ClinicalTrials.gov registration number: NCT01626079) and RESHAPE-HF (A Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Mitral Regurgitation; NCT01772108) will address this important question.
Advanced LV dysfunction and MitraClip (MC)
Congestive heart failure in conjunction with MR has a very poor prognosis [Figure 2 (28)] (20,28). Although several studies have reported reverse LV remodeling and improvements in symptoms after MVS in patients with advanced LV dysfunction in FMR (29,30), 30-day mortality ranges between 8-9% (29,31). In this regard, the MC is a promising, minimally invasive percutaneous treatment technique (32). In a study 108 patients with predominantly FMR and LV dysfunction (mean LVEF 28%±11% with LVEF <40% in 88% of patients) demonstrated an impressively low 30-day mortality of only 1.8% (23). Most importantly, there is accumulating data that the MC appears not only to be safe but also efficacious in patients with FMR (23). In this regard the access EU registry showed a similar clinical improvement in patients with an LVEF ≤30% vs. >30% (Figure 3). In addition, there is some evidence for reverse remodeling after successful treatment with the MC (33).
Figure 2.
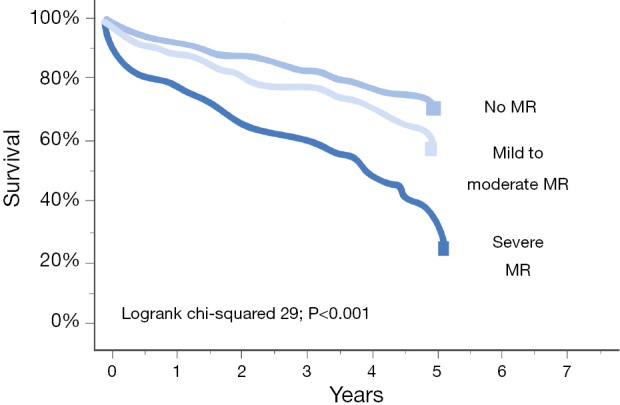
Kaplan-Meier plot showing time to all-cause mortality according to the severity of functional mitral regurgitation (FMR) in 424 un-operated patients with non-ischemic FMR. Severe FMR defined as effective orifice area >0.2 cm2 or regurgitation volume >30 mL or vena contracta diameter >0.4 cm. Figure adapted from Rossi et al. (28).
Figure 3.
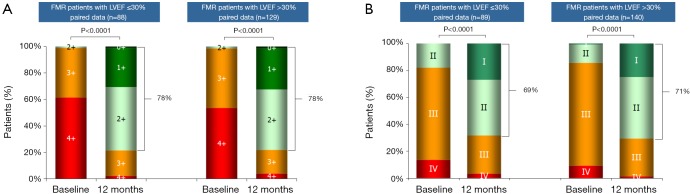
ACCESS-EUROPE phase I study: analysis of outcomes at 1 year in functional mitral regurgitation patients with reduced left ventricular ejection fraction. At 1 year, patients treated with MC show comparable improvements in MR grade and NYHA functional class, over baseline in the 2 subgroups LVEF >30% vs. ≤30%. (A) Mitral regurgitation grade; (B) NYHA Functional Class. Data unpublished, publication authorized by Abbott vascular. MC, MitraClip; MR, mitral regurgitation; LVEF, left ventricular ejection fraction.
Nevertheless there is an ongoing discussion whether a severely depressed LV function might be susceptible to further acute reduction of LVEF induced by correction of MR. Interestingly, the occurrence of a so called afterload mismatch was frequently found (26%) in a study comprising 73 patients with FMR and severely reduced LVEF (27%±9%) (34). Comparison of patients with to those without after load mismatch after MC revealed that left ventricular end diastolic diameter (LVEDD) [(71±8) vs. (67±7) mm; P=0.02] and left ventricular end systolic diameter (LVESD) [(57±9) vs. (53±7) mm; P=0.04] was larger, pulmonary pressure was higher [(49±10) vs. (40±10) mmHg; P=0.04] and right ventricular (RV) dysfunction was more prevalent (68% vs. 31%; P=0.049). The authors suggested that afterload mismatch was the consequence of an abrupt increase in LV end systolic wall stress after MR correction on a preexisting status of absent or reduced contractile reserve. Fortunately, the observed hemodynamic deterioration in patients was a transient phenomenon and did not translate into an adverse outcome at 12 months (1-year survival: 81.2% vs. 75.2%; P=0.44). This study observed no difference in the need for inotropes between patients with and without afterload mismatch in the early postoperative time period (34).
The previously mentioned study comprising only patients with FMR and reduced LVEF reported that 57.7% of patients required inotropic support on the intensive care unit and 13% of patients were transiently bridged with intra-aortic balloon counter pulsation (IABP) underlining the incidence of a transient window of aggravated heart failure immediately after intervention (23).
Predictors of adverse outcome of MitraClip (MC)
While mortality of the MC procedure is already low, it is of great importance to better define predictors of adverse clinical outcome. So far only few studies have addressed this topic. In a study of selected FMR patients with severe LV dysfunction univariate analysis demonstrated adverse outcome for pre-interventional logistic EuroSCORE I ≥20% (HR =4.4; 95% CI, 1.8-9.5; P=0.01), pre-interventional pro-brain natriuretic peptide (BNP) >1,600 pg/mL (HR =21.2; 95% CI, 2.5-38; P=0.01), need for post-interventional IABP-treatment (HR =3.8; 95% CI, 1.2-13.5; P=0.02) and peri-interventional occurrence of acute kidney injury (HR =4.1; 95% CI, 2-16; P=0.01) (23). These findings are in line with results of a single center study (65% FMR, 35% DMR) analyzing predictors of mid-term clinical and survival outcome (all-cause mortality or hospitalization): NYHA IV at baseline (HR =2.4; 95% CI, 1.4-4.3; P=0.002) and GFR <60 mL/min/1.73 m (HR =2.05; 95% CI, 1.1-4.0; P=0.03), STS score >12% (HR =2.20; 95% CI, 1.3-3.8; P=0.004) and failure of procedural success (HR =2.66; 95% CI, 1.4-5.0; P=0.002) (35).
In a study of 300 MC patients (68% FMR, 32% DMR) regurgitant orifice area ≥70.8 mm2, trans-mitral pressure gradient ≥4 mmHg in combination with a mitral valve orifice area ≤3.0 cm2 (assessed by echocardiography) were defined as predictors of increased risk for procedural failure (36).
Applicability of MitraClip (MC)
Apart from standard procedures in FMR or DMR patients there are an increasing number of case reports and smaller series where the MC device was used as a bail-out strategy, disregarding the current guideline recommendations.
In a case of severe coaptation failure, two MCs were implanted via a double guide approach with two simultaneous introduced clip delivery systems. After one clip was used to improve the coaptation between the posterior and anterior leaflet, the second clip was positioned for principle MR treatment (Figure 4). Once a successful grasp was performed with the second clip, the first clip was reopened and optimized (“mitral titration technique”) (37);
The MC was used after MVR to reduce residual severe MR (38,39);
The MC was implanted in a series of patients with left outflow tract obstruction with subsequent MR due to systolic anterior movement of the anterior leaflet in hypertrophic obstructive cardiomyopathy (HOCM) patients. Not only MR but also subvalvular left ventricular outflow tract (LVOT) gradient were successfully reduced (40,41);
The MC was used as primary rescue therapy for patients with severe MR in cardiogenic shock and/or critically ill (42-44);
The MC was implanted in a trileaflet MV (45).
Figure 4.
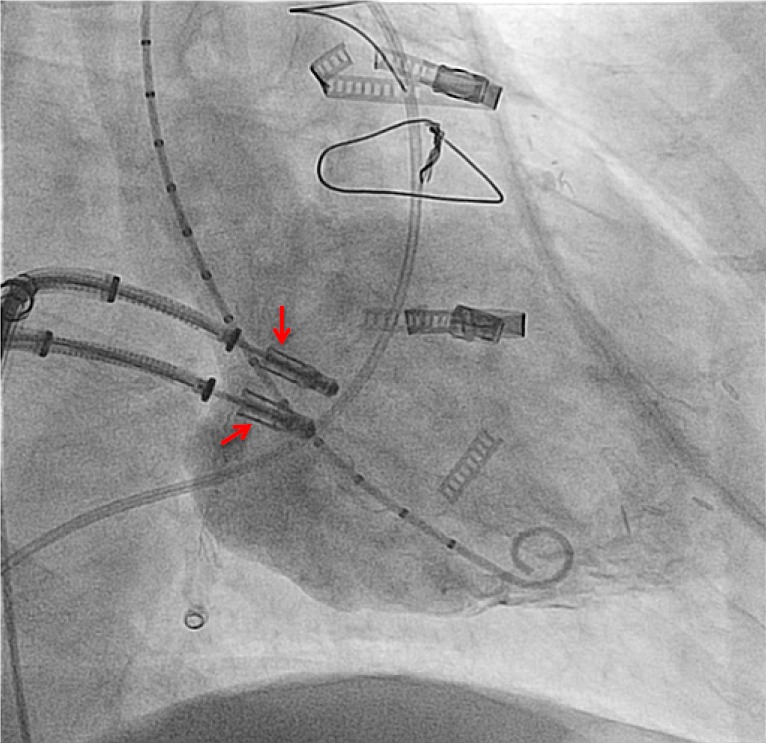
Simultaneous use of two clip delivery systems in a patient with severe mitral regurgitation. Two MCs are closely aligned at the line of coaptation and still attached to the delivery system. A marker pigtail is positioned in the left ventricle through the aortic valve. MCs, MitraClips.
These reports demonstrate that anatomical criteria for applicability of MC implantation are evolving with increasing experience. Current guideline based indications might therefore underestimate the chances of MC therapy. These cases demonstrate the potential of the device and might be an inspiration for responsibly reconsidering the use of the MC in further trials. Due to the remarkably low peri-interventional risk, the MC might be considered as bail-out therapy for treatment of severe MR even in critically ill patients.
The future development of MC therapy will be influenced by new imaging modalities, e.g., 3D real-time imaging, fusion imaging incorporating computed tomography and echocardiography in combination with fluoroscopy as overlay or subtraction. Better visualization will eventually enable an even better understanding of complex anatomical structures. Last but not least, newer generations of clips with varying length and angulation of clip-arms, as well as other interventional devices for different pathologies might change the clinical perspective in the near future.
Summary
During the last 5 years interventional MC device therapy has revolutionized the therapy for patients with severe MR and high surgical risk. For these patients with MR the treatment has meanwhile evolved as the therapy of choice in Europe (FMR and DMR) and Northern America (DMR). Peri-interventional mortality is astonishingly low and short, as well as long-term results are satisfactory.
However, in the future it is of great importance to not only define and extend the group of patients that may benefit, but also to get a better understanding of those patients that do not improve after MC treatment.
Acknowledgements
None.
Footnotes
Conflicts of Interest: F Deuschl received speakers honoraria and traveling support. U Schaefer is a faculty member at Crossroads Abbott Vascular in Brussels, besides that he received research funding, speakers honoraria and traveling support.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661. [DOI] [PubMed] [Google Scholar]
- 3.Chikwe J, Goldstone AB, Passage J, et al. A propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenarians. Eur Heart J 2011;32:618-26. [DOI] [PubMed] [Google Scholar]
- 4.Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005;46:2134-40. [DOI] [PubMed] [Google Scholar]
- 5.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) , European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [DOI] [PubMed] [Google Scholar]
- 6.Reichenspurner H, Schillinger W, Baldus S, et al. Clinical outcomes through 12 months in patients with degenerative mitral regurgitation treated with the MitraClip® device in the ACCESS-EUrope Phase I trial. Eur J Cardiothorac Surg 2013;44:e280-8. [DOI] [PubMed] [Google Scholar]
- 7.Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [DOI] [PubMed] [Google Scholar]
- 8.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [DOI] [PubMed] [Google Scholar]
- 9.Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130-9. [DOI] [PubMed] [Google Scholar]
- 10.Lim DS, Reynolds MR, Feldman T, et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol 2014;64:182-92. [DOI] [PubMed] [Google Scholar]
- 11.Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [DOI] [PubMed] [Google Scholar]
- 12.Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317-28. [DOI] [PubMed] [Google Scholar]
- 13.Wiebe J, Franke J, Lubos E, et al. Percutaneous mitral valve repair with the MitraClip system according to the predicted risk by the logistic EuroSCORE: preliminary results from the German Transcatheter Mitral Valve Interventions (TRAMI) Registry. Catheter Cardiovasc Interv 2014;84:591-8. [DOI] [PubMed] [Google Scholar]
- 14.Schillinger W, Hünlich M, Baldus S, et al. Acute outcomes after MitraClip therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) Registry. EuroIntervention 2013;9:84-90. [DOI] [PubMed] [Google Scholar]
- 15.Baldus S, Schillinger W, Franzen O, et al. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050-5. [DOI] [PubMed] [Google Scholar]
- 16.Attizzani GF, Ohno Y, Capodanno D, et al. Gender-related clinical and echocardiographic outcomes at 30-day and 12-month follow up after MitraClip implantation in the GRASP registry. Catheter Cardiovasc Interv 2015;85:889-97. [DOI] [PubMed] [Google Scholar]
- 17.Grasso C, Capodanno D, Scandura S, et al. One- and twelve-month safety and efficacy outcomes of patients undergoing edge-to-edge percutaneous mitral valve repair (from the GRASP Registry). Am J Cardiol 2013;111:1482-7. [DOI] [PubMed] [Google Scholar]
- 18.Vakil K, Roukoz H, Sarraf M, et al. Safety and efficacy of the MitraClip® system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv 2014;84:129-36. [DOI] [PubMed] [Google Scholar]
- 19.Philip F, Athappan G, Tuzcu EM, et al. MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic review. Catheter Cardiovasc Interv 2014;84:581-90. [DOI] [PubMed] [Google Scholar]
- 20.Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong EJ, Rogers JH, Swan CH, et al. Echocardiographic predictors of single versus dual MitraClip device implantation and long-term reduction of mitral regurgitation after percutaneous repair. Catheter Cardiovasc Interv 2013;82:673-9. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [DOI] [PubMed] [Google Scholar]
- 23.Taramasso M, Maisano F, Latib A, et al. Clinical outcomes of MitraClip for the treatment of functional mitral regurgitation. EuroIntervention 2014;10:746-52. [DOI] [PubMed] [Google Scholar]
- 24.Munkholm-Larsen S, Wan B, Tian DH, et al. A systematic review on the safety and efficacy of percutaneous edge-to-edge mitral valve repair with the MitraClip system for high surgical risk candidates. Heart 2014;100:473-8. [DOI] [PubMed] [Google Scholar]
- 25.Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [DOI] [PubMed] [Google Scholar]
- 26.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [DOI] [PubMed] [Google Scholar]
- 27.Linde C, Gold MR, Abraham WT, et al. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J 2013;34:2592-9. [DOI] [PubMed] [Google Scholar]
- 28.Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80. [DOI] [PubMed] [Google Scholar]
- 29.De Bonis M, Taramasso M, Grimaldi A, et al. The GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathy. Eur J Cardiothorac Surg 2011;40:488-95. [DOI] [PubMed] [Google Scholar]
- 30.De Bonis M, Lapenna E, Verzini A, et al. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg 2008;85:932-9. [DOI] [PubMed] [Google Scholar]
- 31.Braun J, Bax JJ, Versteegh MI, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 2005;27:847-53. [DOI] [PubMed] [Google Scholar]
- 32.De Bonis M, Lapenna E, La Canna G, et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the "edge-to-edge" technique. Circulation 2005;112:I402-8. [DOI] [PubMed] [Google Scholar]
- 33.Grayburn PA, Foster E, Sangli C, et al. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation 2013;128:1667-74. [DOI] [PubMed] [Google Scholar]
- 34.Melisurgo G, Ajello S, Pappalardo F, et al. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol 2014;113:1844-50. [DOI] [PubMed] [Google Scholar]
- 35.Puls M, Tichelbäcker T, Bleckmann A, et al. Failure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClip. EuroIntervention 2014;9:1407-17. [DOI] [PubMed] [Google Scholar]
- 36.Lubos E, Schlüter M, Vettorazzi E, et al. MitraClip therapy in surgical high-risk patients: identification of echocardiographic variables affecting acute procedural outcome. JACC Cardiovasc Interv 2014;7:394-402. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer U, Frerker C, Kreidel F. Simultaneous double clipping delivery guide strategy for treatment of severe coaptation failure in functional mitral regurgitation. Heart Lung Circ 2015;24:98-102. [DOI] [PubMed] [Google Scholar]
- 38.Grasso C, Attizzani GF, Ohno Y, et al. Catheter-based edge-to-edge mitral valve repair after percutaneous mitral valve annuloplasty failure. JACC Cardiovasc Interv 2014;7:e85-6. [DOI] [PubMed] [Google Scholar]
- 39.Grasso C, Ohno Y, Attizzani GF, et al. Percutaneous mitral valve repair with the MitraClip system for severe mitral regurgitation in patients with surgical mitral valve repair failure. J Am Coll Cardiol 2014;63:836-8. [DOI] [PubMed] [Google Scholar]
- 40.Schäfer U, Kreidel F, Frerker C. MitraClip implantation as a new treatment strategy against systolic anterior motion-induced outflow tract obstruction in hypertrophic obstructive cardiomyopathy. Heart Lung Circ 2014;23:e131-5. [DOI] [PubMed] [Google Scholar]
- 41.Schäfer U, Frerker C, Thielsen T, et al. Targeting systolic anterior motion and left ventricular outflow tract obstruction in hypertrophic obstructed cardiomyopathy with a MitraClip. EuroIntervention 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Pleger ST, Chorianopoulos E, Krumsdorf U, et al. Percutaneous edge-to-edge repair of mitral regurgitation as a bail-out strategy in critically ill patients. J Invasive Cardiol 2013;25:69-72. [PubMed] [Google Scholar]
- 43.Zuern CS, Schreieck J, Weig HJ, et al. Percutaneous mitral valve repair using the MitraClip in acute cardiogenic shock. Clin Res Cardiol 2011;100:719-21. [DOI] [PubMed] [Google Scholar]
- 44.Couture P, Cloutier-Gill LA, Ducharme A, et al. MitraClip intervention as rescue therapy in cardiogenic shock: one-year follow-up. Can J Cardiol 2014;30:1108.e15-6. [DOI] [PubMed]
- 45.Freixa X, Hayami D, Basmadjian A, et al. MitraClip repair of a "trileaflet" regurgitant mitral valve. EuroIntervention 2015;11:355. [DOI] [PubMed] [Google Scholar]


