Abstract
Background
To assess the effect of preoperative neoadjuvant therapy on resectability, downstaging, and the prognosis in patients with stage IIIA-N2 non-small cell lung cancer (NSCLC).
Methods
Eighty-four patients who underwent resections after induction therapy [76 with neoadjuvant chemotherapy (CTx) and 8 with induction chemoradiotherapy (CRTx)] for clinically evident [larger than 1 cm on computed tomography (CT)] and pathologically confirmed ipsilateral N2 positive NSCLC (stage IIIA) between January 2009 and July 2013 were reviewed retrospectively.
Results
Partial response (PR) was observed in 39 patients (46.4%). Standard lobectomy was performed in 63 cases (75.0%), and extensive resection was conducted in 21 cases (25.0%), including four pneumonectomies. Pathologic nodal downstaging (pN2 to pN0-1) was confirmed in 38 cases (45.2%). After induction therapy plus resection, 5-year progression-free survival (PFS) and overall survival (OS) in cases with radical resections were 37.9% and 34.2%, respectively. Patients who underwent lobectomy or pathologic nodal downstaging had better prognosis than those who had extensive resection or persistent N2 in PFS (P=0.036; P=0.025) and OS (P=0.023; P=0.024). On univariate analysis, lobectomy and pathological nodal downstaging were favourably predictive factors both in PFS and OS. Cox multivariate analyses identified only pathologic nodal downstaging to predict better PFS, and lobectomy to be significantly prognostic for OS.
Conclusions
These data suggest that neoadjuvant therapy was feasible, and helpful for tumor and pathologic nodal downstaging with promising rates of survival in patients with stage IIIA-N2 NSCLC. After induction therapy, patients with potentially radical lobectomy were more likely to benefit from operation. Pathological nodal downstaging of pN2 to pN0-1, rather than clinical response was predictive of a favorable outcome, and was correlated with a better chance of survival.
Keywords: Non-small cell lung cancer (NSCLC), neoadjuvant, surgery, downstaging, survival
Introduction
Patients with stage IIIA non-small cell lung cancer (NSCLC) with ipsilateral mediastinal nodal metastases (N2) have poor outcomes after resection alone (1,2). However, optimal management of this patient subset is controversial. Based on the latest guidelines set by the National Comprehensive Cancer Network for NSCLC, for management of stage IIIA-N2 NSCLC, definitive concurrent chemoradiotherapy (CRTx) or induction therapy followed by surgery is recommended (3). Whether such patients benefit more from a surgical approach rather than a non-surgical one is controversial (4-6). Results from studies in patients with stage IIIA N2 NSCLC have shown the feasibility of resection after induction chemotherapy (CTx) with a promising prevalence of survival being noted (7). However, some of these trials failed to show the benefit of surgery after induction therapy owing to a high prevalence of operative deaths (4), and included patients with stage IIB-IIIB (7), thereby making the specific effects of preoperative induction therapy on patients with stage IIIA N2 NSCLC difficult to discern. In recent studies, induction therapy plus surgery was preferred to definitive CRTx for stage IIIA N2 NSCLC (8-11).
We aimed to ascertain the effect of preoperative induction therapy on resection, pathologic response, and survival in patients with clinically evident [diameter >1 cm on computed tomography (CT) scan] and pathologically confirmed ipsilateral N2 positive stage IIIA NSCLC.
Patients and methods
Patients
Patients with pathologically confirmed ipsilateral mediastinal lymph node metastasis (diameter >1 cm on CT) stage IIIA N2 NSCLC between January 2009 and July 2013 in a single center were assessed retrospectively. All patients had preoperative biopsy-confirmed N2 disease by endobronchial ultrasound-guided transbronchial needle aspiration or mediastinoscopy.
Ninety-one patients were identified, and seven patients were lost to follow-up. Thus, 84 patients constituted the definite study population. All patients were staged with CT scans (n=84) or combined with positron emission tomography (PET) scans (n=56).
Therapy
All preoperative CTx regimens were platinum-based doublets. Resection with systematic dissection of mediastinal lymph nodes was undertaken 3-5 weeks after completion of induction therapy. Four cycles of CTx were used postoperatively. Additional radiotherapy was offered to patients with incomplete resection. Identical preoperative CTx regimens were used for the responders postoperatively, whereas non-responders were given other cisplatin-based/platinum-based regimens.
Radiologic response (clinical response) was divided into complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD) according to criteria set by the World Health Organization.
Outcomes of radiological response (clinical response), resection, pathologic downstaging, and survival were investigated.
Follow-up
Patients were followed up every 3 months for the first year, every 6 months for the second year, and then annually.
Statistical analyses
Overall survival (OS) and progression-free survival (PFS) was measured from the date of the diagnosis and were analyzed using the Kaplan-Meier and log-rank test. The Cox proportional hazards model was used for multivariate analyses. Variables found to be significant upon univariate analyses (defined as a probability value of less than 0.15) were subsequently entered into the multivariate analysis using the Cox proportional hazards model after backward stepwise Wald elimination. P<0.05 was considered significant. All statistical analyses were done using SPSS (version 19.0; IBM Armonk, NY, USA).
Results
Patient characteristics
Baseline clinical characteristics of patients are shown in Table 1. There were 64 men (76.2%) and 20 women (23.8%), with a mean age of 55.4 (median, 56; range, 30-72) years. Fifty-three patients (60.5%) had a smoking history, all of whom were male. All patients had performance status (PS) score [Eastern Cooperative Oncology Group (ECOG)] of 0 or 1. There was no operative mortality, and the prevalence of non-lethal postoperative complications was 17.9% (15/84), including persistent air leak (n=6), pneumonia/lobar atelectasis (n=4), respiratory failure (n=2), hemorrhage (n=1), recurrent laryngeal nerve palsy (n=1), and chylothorax (n=1). Mean duration of hospital stays after surgery in patients was 9.7 (range, 2-20) days. Further analyses showed an increase in duration of postoperative stays in patients who underwent preoperative CRTx, compared with those who underwent preoperative CTx (12.0 vs. 9.4 days, P=0.043).
Table 1. Baseline clinical characteristics (84 patients).
| Characteristics | N | Percent (%) |
|---|---|---|
| Age, mean (range) | 55.4 [30-72] | |
| Sex | ||
| Male | 64 | 76.2 |
| Female | 20 | 23.8 |
| Smoking history | ||
| Yes | 53 | 60.5 |
| No | 31 | 39.5 |
| PS score (ECOG) | ||
| 0 | 31 | 39.5 |
| 1 | 53 | 60.5 |
| Neoadjuvant strategy | ||
| CRTx | 8 | 9.5 |
| CTx | 76 | 90.5 |
| In-hospital complications | ||
| Mortality | 0 | 0.0 |
| Morbidity | 15 | 17.9 |
| Persistent air leak (≥7 days) | 6 | |
| Pneumonia/lobar atelectasis | 4 | |
| Respiratory failure | 2 | |
| Recurrent laryngeal nerve palsy | 1 | |
| Haemorrhage | 1 | |
| Chylothorax | 1 | |
| Postoperative stay, mean (range) | 9.7 [2-20] | |
| Histological types | ||
| Ad | 42 | 50.0 |
| SCC | 29 | 34.5 |
| Other | 12 | 14.3 |
| LCC | 5 | 6.0 |
| ASC | 7 | 8.3 |
| C-TNM stage | ||
| C-T1N2M0 | 4 | 4.8 |
| C-T2N2M0 | 55 | 65.5 |
| C-T3N2M0 | 25 | 29.8 |
| Clinical response | ||
| CR | 0 | 0.0 |
| PR | 39 | 46.4 |
| SD | 43 | 51.2 |
| PD | 2 | 2.4 |
PS, performance status; ECOG, Eastern Cooperative Oncology Group; CRTx, chemoradiotherapy; CTx, chemotherapy; Ad, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ASC, adenosquamous carcinoma; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The main histologic subtype was adenocarcinoma (Ad) (50.0%, 42/84), followed by squamous cell carcinoma (SCC) (34.5%, 29/84), adenosquamous carcinoma (ASC) (8.3%, 7/84) and large cell carcinoma (LCC) (6.0%, 5/84). Preoperative clinical staging (c-TNM) showed c-T1N2M0 (n=4), c-T2N2M0 (n=55), c-T3N2M0 (n=25). Upon radiologic evaluation for clinical response before and after induction therapy, PR was achieved in 39 patients (46.4%), SD in 43 patients (51.2%) and 2 patients (2.4%) showed PD. CR was not observed in our series. After induction therapy, all 84 cases were submitted to surgical intervention.
Tumor downstaging led to a high percentage of radical resections (89.3%, 75/84) (Table 2). Standard lobectomy was undertaken in 63 cases (75.0%), and extensive resection was conducted in 21 cases (25.0%). The latter comprised sleeve resection (n=3), bilobectomy (n=14), and pneumonectomy (n=4). Postoperative pathologic staging showed no residual tumor of the primary lesion (pT0) in four cases. PT1, pT2, and pT3 were demonstrated in 38, 40, and 2 cases respectively. Pathologic nodal downstaging (pN2 to pN0-1) was confirmed in 38 cases (45.2%). Persistent pN2 was identified in 46 cases (54.8%), which comprised 14 cases with single N2 and 32 cases with multiple N2. A precise correlation between clinical response and pathologic response was not found.
Table 2. Surgery and postoperatively pathological stage.
| Characteristics | N | Percent (%) |
|---|---|---|
| Standard resection | 63 | 75.0 |
| Lobectomy | 63 | 75.0 |
| Extensive resection | 21 | 25.0 |
| Sleeve lobectomy | 3 | 3.6 |
| Bilobectomy | 14 | 16.7 |
| Pneumonectomy | 4 | 4.7 |
| Margin status | ||
| R0 | 75 | 89.3 |
| R1 | 9 | 10.7 |
| P-T | ||
| T0* | 4 | 4.8 |
| T1 | 38 | 45.2 |
| T2 | 40 | 47.6 |
| T3 | 2 | 2.4 |
| P-N | ||
| Nodal downstaging | 38 | 45.2 |
| N2→0 | 21 | 25.0 |
| N2→1 | 17 | 20.2 |
| Persistent N2→2 | 46 | 54.8 |
| Single N2 | 14 | 16.7 |
| Multiple N2 | 32 | 30.1 |
| Total | 84 | 100.0 |
T0*, pathological complete response (no residual tumor in the initial site).
Recurrence and death
Mean duration of follow-up was 34.0 (mean 33.5; range, 5-73) months. Loco-regional recurrence was observed in 35 patients and distant metastasis in 37 subjects. The brain was the most common primary site of extrathoracic metastasis (15.5%), followed by bone (10.7%), liver (4.8%), and unknown (6.0%). Deaths occurred in 46 cases (54.8%) at final follow-up.
Survival analyses
OS of all stage IIIA-N2 NSCLC patients after induction plus resection was 57.7% at 3 years and 34.2% at 5 years. Three-year and 5-year PFS was 37.9% and 30.5%, respectively.
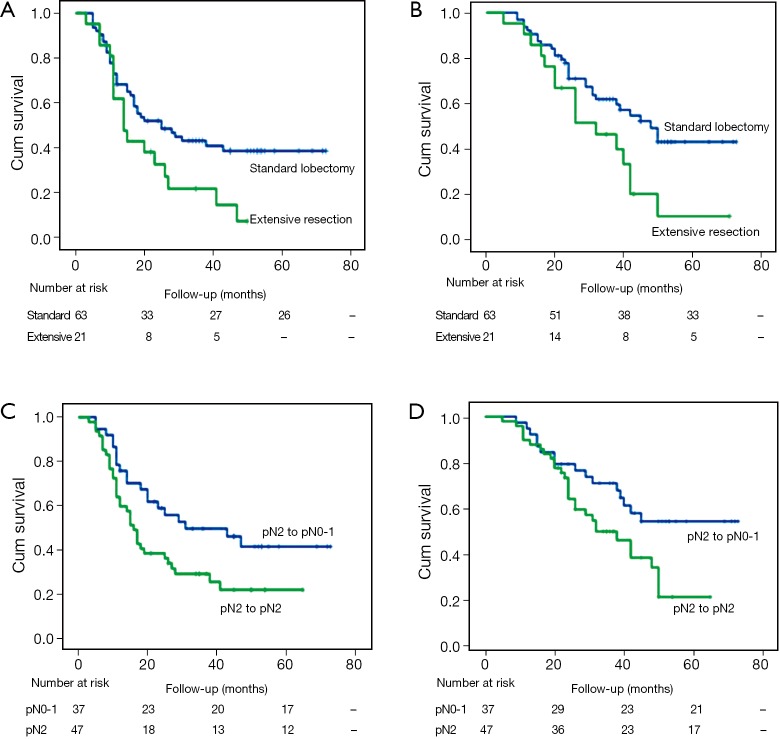
There was no significant difference in OS or PFS in cases with different margin status (R0 vs. R1) (P=0.118; P=0.369), clinical response (PR vs. SD/PD) (P=0.865; P=0.862), histologic subtype (P=0.685; P=0.208). There was a trend toward better OS in patients with single-station N2 involvement (n=14) compared those with multiple-station N2 involvement (n=33), but the difference was not significant (mean OS: 42.0 vs. 33.0 months, P=0.067; mean PFS: 28.7 vs. 24.1 months, P=0.245). There was a significant survival advantage for patients with lobectomy or pathologic nodal downstaging compared with a matched group with extensive resections or persistent N2 in PFS (mean 38.0 vs. 21.2 months, P=0.036; 41.8 vs. 26.4 months, P=0.025) and OS (mean 47.6 vs. 29.8 months, P=0.023; 51.0 vs. 36.2 months, P=0.024) analyses (Figure 1). Furthermore, in patients who underwent radical resection, the difference between standard lobectomy and extensive resection groups was more significant (P=0.016; P=0.022) (Figure 2).
Figure 1.
(A-D) Kaplan-Meier curves of progression-free survival (PFS) (A,C) and overall survival (OS) (B,D) in 84 patients, showing that there was a significant survival advantage for patients with lobectomy or pathologic nodal downstaging compared with a matched group with extensive resections or persistent N2 in PFS (P=0.036; P=0.025) and OS (P=0.023; P=0.024) analyses.
Figure 2.
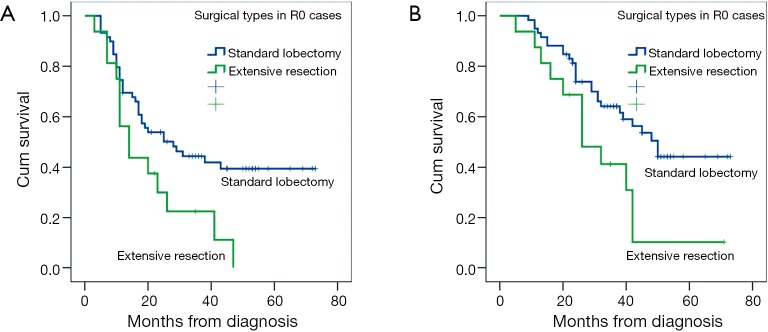
(A,B) Kaplan-Meier curves of progression-free survival (PFS) (A) and overall survival (OS) (B) in patients with radical resections (n=75). Patients performed with radical lobectomy had better PFS (P=0.036) and longer OS (P=0.023) than those with radical resection through an extensive way after neoadjuvant therapy.
On univariate Cox proportional hazards analysis (Table 3), lobectomy and pathologic nodal downstaging from pN2 to pN0-1 were favourably predictive factors both in PFS (P=0.043; P=0.030) and OS (P=0.028; P=0.029).
Table 3. Univariate analysis for association of baseline characteristics and treatment-related variables with PFS and OS (n=84).
| Variables | N (%) | PFS |
OS |
|||
|---|---|---|---|---|---|---|
| Mean (months) | P | Mean (months) | P | |||
| Age (years) | 0.460 | 0.876 | ||||
| ≤55 | 37 (44.0) | 31.1 | ||||
| >55 | 47 (56.0) | 36.5 | ||||
| Sex | 0.063 | 0.225 | ||||
| Male | 64 (76.2) | 37.5 | ||||
| Female | 20 (23.8) | 21.4 | ||||
| PS | 0.160 | 0.327 | ||||
| 0 | 31 (30.5) | 39.5 | ||||
| 1 | 53 (69.5) | 24.2 | ||||
| Smoking | 0.039 | 0.140 | ||||
| No | 31 (30.5) | 22.9 | ||||
| Yes | 53 (69.5) | 39.2 | ||||
| Histology | 0.807 | 0.489 | ||||
| Ad | 42 (50.0) | 30.8 | ||||
| SCC | 29 (34.5) | 41.6 | ||||
| Others | 12 (14.3) | 26.2 | ||||
| Surgical types | 0.028 | |||||
| Lobectomy | 63 (75.0) | 38 | 0.043 | 47.6 | ||
| Extensive resection | 21 (25.0) | 21.2 | 29.8 | |||
| Margin status | 0.379 | 0.129 | ||||
| R0 | 75 (89.3) | 35.1 | 45.3 | |||
| R1 | 9 (10.7) | 21.8 | 29.9 | |||
| Clinical response | 0.864 | 0.868 | ||||
| CR/PR | 39 (46.4) | 34.8 | 44.5 | |||
| SD/PD | 45 (53.6) | 33.5 | 43.3 | |||
| pN | 0.030 | 0.029 | ||||
| N2→N0-1 | 38 (45.2) | 41.8 | 51 | |||
| N2→N2 | 46 (54.8) | 26.4 | 36.2 | |||
R0, negative margin resection; R1, microscopically positive margin resection; PS, performance status; Ad, adenocarcinoma; SCC, squamous cell carcinoma; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; OS, overall survival.
Cox multivariate analysis identified only pathologic nodal downstaging to predict better PFS [hazard ratio (HR) 0.54, 95% confidence interval (CI): 0.31-0.94; P=0.030]; non-smoking (HR 1.89, 95% CI: 1.03-3.48, P=0.041) and lobectomy (HR 0.42, 95% CI: 0.22-0.80, P=0.008) were identified to be significantly prognostic for OS.
Discussion
Data from randomized controlled trials, have suggested that preoperative CTx improves survival. Neoadjuvant CTx is thought to convey several benefits, such as reduction of tumor size and increased chance of resectability, and eradication of micro-metastases. However, delays in undertaking surgery can render a tumor unresectable (10). Also the potential benefit of preoperative CTx must be balanced against possible toxicity. We could not assess toxicity in the current study, but clinical trials have described mild or acceptable toxicity and that CTx is, in general, well tolerated (12).
Radical resection is preferred while performing an oncological surgery. In this study, however, superiority of radical resection (R0, n=75) compared with incomplete resection (R1, n=9) couldn’t be confirmed, because the very small number of patients who had incomplete resection might have limited the analyses.
Clinical response (e.g., CR/PR and SD/PD) and pathological response (e.g., tumor regression, and downstaging of mediastinal nodes) are usually employed to evaluate the therapeutic effects of a neoadjuvant approach. Several studies have shown a positive association between nodal downstaging and improvement in OS in patients with pathologically confirmed stage IIIA N2 NSCLC after neoadjuvant CTx (13). In our study, there was no CR, but four cases had pT0 after neoadjuvant therapy. In addition, as shown above, in patients with initial N2 disease after induction therapy (n=84), those who had successful mediastinal downstaging (N2 to N0-1, n=37), compared with those with persistent mediastinal disease (N2 to N2, n=47), showed significantly better PFS and OS, whereas this phenomenon was not observed in corresponding patients with different levels of clinical response. Univariate analyses showed that favourable mediastinal downstaging (N2 to N0-1) was significantly predictive of better PFS and OS. However, in terms of PFS and OS, clinical response was not significantly predictive of better outcomes. Multivariate analyses showed mediastinal downstaging to be an independent favorable predictor of PFS. Our results suggested that clinical response (radiographic appearance) did not correlate with pathologic downstaging, and that pathologic (rather than clinical response) could be used to predict prognosis, a finding that is in accordance with several studies (14). Pathologic response represented not only local control but also eradication of mediastinal micrometastases sensitive to induction therapy.
The role of surgical intervention in stage IIIA N2 NSCLC remains uncertain (15-17). Reasons for the absence of benefit with surgery might be associated with the high prevalence of complications and late mortality after induction therapy, attributable mainly to the high prevalence of pneumonectomy and other causes. A recent study showed that neoadjuvant therapy doesn’t increase the risk of morbidity or, early/late mortality after resection (18). The present outcomes showed a higher prevalence of radical resection (75/84) with a low prevalence of pneumonectomy and no operative deaths compared with previous studies (4). In our series, survival outcomes of patients with stage IIIA N2 NSCLC treated with neoadjuvant therapy followed by surgery showed 3-year and 5-year OS of 57.7% and 34.2% respectively, which are comparable with those of some reports (7,19), but superior to those of several others authors’ (4,16). The outcomes were more promising and better in terms of patients with R0: OS at 3-year and 5-year was 59.2% and 37.1% respectively; 3-year and 5-year PFS was 53.1% and 33.9%, respectively. Patients who had radical lobectomy after induction therapy had significantly better survival outcomes than those with extensive resection in terms of PFS and OS. Similar results have been reported (16). Univariate analyses identified lobectomy, rather than its extensive counterpart, as a significantly favorable predictor for better PFS and OS. Lobectomy was a significantly favorable prognostic factor for OS according to Cox multivariate analysis. Patients who potentially required radical lobectomy on CT after preoperative neoadjuvant therapy seemed to be the best candidates for surgery. Comparison between surgery after induction therapy and definitive CTx would be required to verify this finding. Further studies will allow clearer definition of patients most likely to derive benefit from neoadjuvant therapy plus appropriate management.
Limitations of our study were its retrospective design, single-institution experience, and lack of comparison with definitive CTx alone in cases with stage III N2 NSCLC.
Conclusions
Our results, in combination with those of several other studies, showed that neoadjuvant therapy followed by surgery can be considered in patients with stage IIIA N2 NSCLC who have technically resectable disease. Carefully selection of patients needing radical resection, especially those may require radical lobectomy could result in longer survival after induction therapy. Given that radical resection and nodal downstaging could be confirmed only postoperatively in the present study, further studies are required to accurately of diagnose mediastinal downstaging by non-invasive and non-surgical approaches such as PET (20).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1-6. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw 2014;12:1738-61. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Ann Oncol 2004;15:1645-53. [DOI] [PubMed] [Google Scholar]
- 6.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S. [DOI] [PubMed] [Google Scholar]
- 7.Kocher F, Pircher A, Mohn-Staudner A, et al. Multicenter phase II study evaluating docetaxel and cisplatin as neoadjuvant induction regimen prior to surgery or radiochemotherapy with docetaxel, followed by adjuvant docetaxel therapy in chemonaive patients with NSCLC stage II, IIIA and IIIB (TAX-AT 1.203 Trial). Lung Cancer 2014;85:395-400. [DOI] [PubMed] [Google Scholar]
- 8.Darling GE, Li F, Patsios D, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer†. Eur J Cardiothorac Surg 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Donington JS, Pass HI. Surgical resection of non-small cell lung cancer with N2 disease. Thorac Surg Clin 2014;24:449-56. [DOI] [PubMed] [Google Scholar]
- 10.NSCLC Meta-analysis Collaborative Group . Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol 2013;8:915-22. [DOI] [PubMed] [Google Scholar]
- 12.Askoxylakis V, Tanner J, Kappes J, et al. Trimodal therapy for stage III-N2 non-small-cell lung carcinoma: a single center retrospective analysis. BMC Cancer 2014;14:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [DOI] [PubMed] [Google Scholar]
- 14.Margaritora S, Cesario A, Galetta D, et al. Ten year experience with induction therapy in locally advanced non-small cell lung cancer (NSCLC): is clinical re-staging predictive of pathological staging? Eur J Cardiothorac Surg 2001;19:894-8. [DOI] [PubMed] [Google Scholar]
- 15.Toyokawa G, Takenoyama M, Ichinose Y. Multimodality treatment with surgery for locally advanced non-small-cell lung cancer with n2 disease: a review article. Clin Lung Cancer 2015;16:6-14. [DOI] [PubMed] [Google Scholar]
- 16.Vansteenkiste J, Betticher D, Eberhardt W, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Thorac Oncol 2007;2:684-5. [DOI] [PubMed] [Google Scholar]
- 17.Van Meerbeeck JP, Van Schil PE, Senan S, et al. Reply: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Thorac Oncol 2007;2:1138-9. [DOI] [PubMed] [Google Scholar]
- 18.Peer M, Stav D, Cyjon A, et al. Morbidity and mortality after major pulmonary resections in patients with locally advanced stage IIIA non-small cell lung carcinoma who underwent induction therapy. Heart Lung Circ 2015;24:69-76. [DOI] [PubMed] [Google Scholar]
- 19.Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim HJ, Lee HY, Lee KS, et al. Predictive factors for survival in stage IIIA N2 NSCLC patients treated with neoadjuvant CCRT followed by surgery. Cancer Chemother Pharmacol 2015;75:77-85. [DOI] [PubMed] [Google Scholar]