Abstract
There have been many factors that have allowed for progressive improvement in outcomes and lower complication rates. These include the improvement in left ventricular assist device (LVAD) technologies, combined with better understanding of patient management, all these. Nowadays the numbers of LVAD implantations exceed the number of annual heart transplants worldwide. Minimally invasive procedures are shown to improve the surgical outcome in both LVAD insertion and replacement. These minimally invasive techniques can be grouped grossly into shifting from on-pump to off-pump implantation, alternative access for implantation other than sternotomy, and a combination of both, which should be the ultimate aim of minimally invasive LVAD implantation. Here we describe the alternative techniques and configurations of minimally invasive and sites of implantation.
Keywords: Left ventricular assist device (LVAD), minimally invasive, off-pump, ventricular assist device (VAD)
Introduction
The use of left ventricular assist devices (LVADs) in patients with congestive heart failure (CHF) has excellent outcomes when compared to medical therapy (1-3). LVADs have become an important option for the treatment of patients with advanced heart failure (4-7). Nowadays the number of LVAD implantations exceeds the number of annual heart transplants worldwide. The improved pump technology, enhanced performance of the pumps, increased device durability, reduced size of the new LVAD devices, combined with a better understanding of patient management, all these have allowed for progressive improvement in long-term outcomes and pump durability after implantation (3,4,8). These advances led to new implantation strategies which considered the novel art in LVAD surgery (9-16).
The concept of minimally invasive implantation was introduced by Hill et al. (17) by using a combination of a right minithoracotomy and small left subcostal incision to implant the Thoratec paracorporeal LVAD. Gregoric et al. (12) subsequently described a less invasive approach for implanting axial flow LVADs without median sternotomy utilizing a subcostal incision and a right minithoracotomy when they implanted six patients with HeartMate II. Nowadays that minimally invasive surgery is possible for the implantation, explanation, and exchange of LVADs. The minimally invasive techniques can be grouped grossly into shifting from on pump to off-pump implantation, and alternative access for implantation other than sternotomy. Here we discuss all these alternative techniques and configurations of minimally invasive and sites of implantation as well as their advantages and disadvantages.
Minimally invasive off-pump strategy
Implantation of ventricular assist devices (VADs) is typically performed with the patient on cardiopulmonary bypass (CPB). However, CPB is associated with harmful effects well documented in the literature, including activation of inflammatory mediators, increased pulmonary vascular resistance, platelet activation, coagulopathy and impaired renal function (18-21). The postoperative course of these ill patients usually accounts for many complications because of their preoperative comorbidities, or for surgical complications (18,22). The patient population that requires VAD implantation often has evidence of end-organ dysfunction, including liver congestion, renal insufficiency, and pulmonary edema. VAD placement under CPB often exacerbates these pre-existing conditions, resulting in post-operative coagulopathy, bleeding and worsening right heart failure. VAD implantation without the use of CPB could help to minimize these post-operative complications without hemodynamic compromise or excessive bleeding during implantation (15,20,23). The short term benefits of an off-pump strategy to reduce blood-product transfusion, reoperation for perioperative bleeding, acute kidney injury and respiratory complications have been demonstrated in a large randomized study (24). Minimizing blood product transfusions and reducing exposure to blood antigens decreases the risk of recipient sensitization, thus preserving donor pool availability for bridge-to-transplantation candidates undergoing LVAD implantation (8).
Sun et al. (25) in their series of 25 patients off-pump implanted LVAD, concluded that placement of long-term LVADs can be performed without the use of CPB, and concluded that these techniques can be implemented with acceptable outcomes and minimal blood utilization in selected patients who do not require correction of additional cardiac pathology, there are multiple ways used for off-pump LVAD implantation, we discuss here the most commonly used as well some promising techniques. Reliant on the experience in transcatheter valve implantation by the use of rapid pacing, Centofanti et al. (26) reported inserting off-pump Jarvik LVAD with rapid pacing for the insertion; this was also reported multiple authors (15,27,28).
Implantation of LVAD with non-fibrillatory technique by administrating of an intravenous bolus of adenosine to induce a short bradycardic arrest during off-pump LVAD placement was also described (8,29). In spite of Adenosine-induced asystole it further reduces blood loss by reducing both the volume of blood ejected from the heart during LVAD implant (reduction in blood pressure) and decreasing the heartbeats. In this method Adenosine mediates pulmonary vasodilatation, which may reduce pulmonary pressures and protect the RV function (30,31). Anastasiadis et al. (32) reported successful insertion of Jarvik 2000 device by using ECMO in spite of CPB for cardiac support during the procedure. This technique was associated with decreased duration of operation, reduction in blood transfusion, need for inotropic support, duration of mechanical ventilation, and length of hospital stay.
Other promising techniques (in animal lab) also reported by Cohn (33) who reported a new modified technique of off-pump LVAD insertion by using vacuum-assisted coring tool with occlusion endovascular balloon, inserted via femoral artery to minimize the bleeding and to maintain steady hemodynamics during the insertion on the inflow cannula.
There are circumstances where an off-pump LVAD placement is not appropriate. These include patients who require concomitant surgery for left ventricular or atrial appendage clot and patients who have significant AI, mitral stenosis, or severe tricuspid regurgitation, right-to-left shunts with worsening hypoxemia, and thrombus formation (8,29).
Minimally invasive LVAD insertion technique
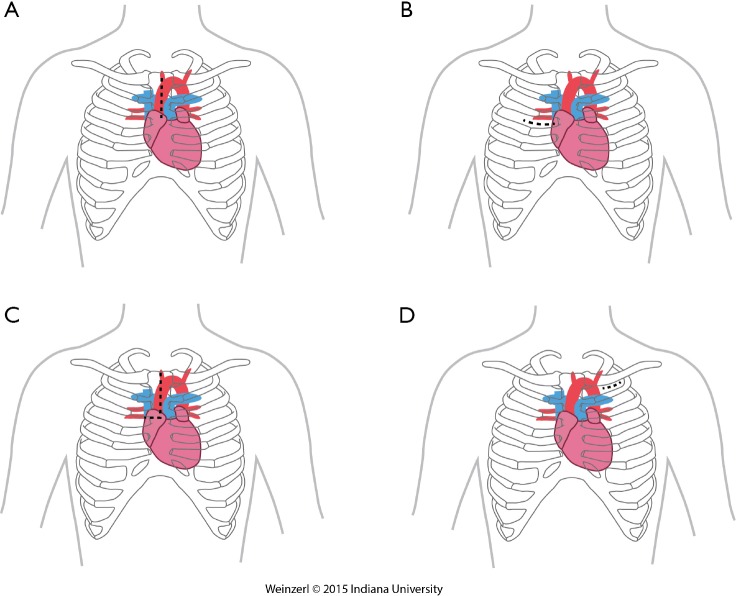
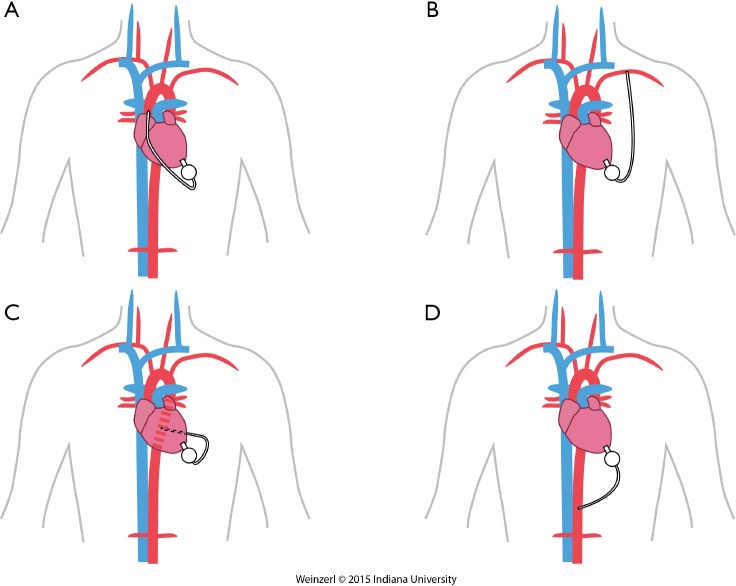
The LVAD insertion is divided into three steps: (I) inflow cannula and pump insertion; (II) outflow graft (OG) insertion; (III) drive line insertion. The minimally invasive procedure could be done for the first two steps. Inflow cannula sites approaches are shown in (Figure 1), OG site insertion locations are shown in (Figure 2), and all minimally invasive LVAD insertion configurations are shown in (Figure 3).
Figure 1.
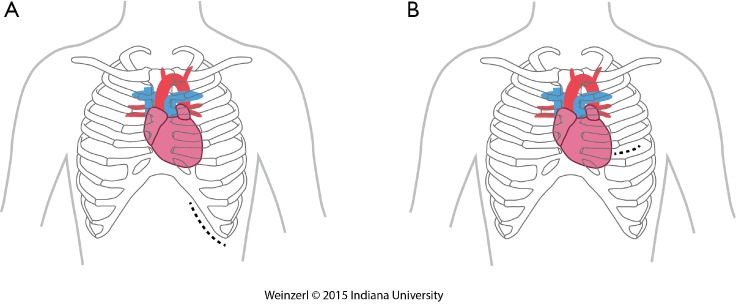
Inflow cannula sites. (A) Sub-diaphragmatic; (B) left thoracotomy.
Figure 2.
OG site insertion. (A) Upper hemisternotomy; (B) right minithoracotomy; (C) upper hemisternotomy & right hemithoracotomy J shape incision; (D) axillary. OG, outflow graft.
Figure 3.
Minimally invasive LVAD insertion configurations. (A) OG to ascending aorta; (B) OG to axillary artery; (C) OG to descending aorta; (D) OG to supra-celiac aorta. LVAD, Left ventricular assist device; OG, outflow graft.
Inflow and pump insertion
There are two insertion approaches for the minimally invasive techniques, a left subcostal incision mainly used now for HeartMate II (14,17,34) this incision will help in preparing the pocket for HMII (Figure 1A). The other approach is via left anterior thoracotomy at 5th or 6th intercostal space (Figure 1B).
The advantages of this access are to avoid redo-sternotomy in a difficult sternal entry, to avoid opening the pericardium, it can be used for a porcelain aorta with OG in the axillary artery or the descending aorta, this approach, if done without sternotomy is particularly appealing in patients with a history of coronary artery bypass surgery, and is preferentially utilized in bridged patients to spare the sternal incision for heart transplantation.
OG insertion
This could be done by multiple techniques and in different locations, which include right minithoracotomy, split upper hemisternotomy, axillary and subclavian arteries, Supra-celiac abdominal aorta, innominate artery, and descending aorta (35).
Upper hemisternotomy for OG (Figure 2A)
This is the most commonly used for on-pump minimally invasive strategy and was already described by Schmitto (9,16,36). It can be associated with either inflow pump incisions, this technique permit to initiate CPB off-pump placement of the LV apex sewing ring, and tunneling of the driveline to the right or left upper quadrant. For the majority of patients, the OG is tunneled and passed within the pericardium and anastomosed to the proximal ascending aorta. The OG could be passed in the anterior mediastinum behind the sternum in case of previous sternotomy (8).
Right minithoracotomy OG implants (Figure 2B)
The OG could be also inserted through a 4-6 cm right anterior minithoracotomy. The incision is made at 2nd-3rd intercostal space which allows access to the ascending aorta to initiate the CPB and/or to perform the outflow anastomosis (Figure 3A) (16,36,37). This technique is preferentially utilized in bridged patients to spare the sternal incision for heart transplantation. This approach is particularly appealing in patients with a history of coronary artery bypass surgery (8).
Upper hemisternotomy with right hemithoracotomy J shape incision (Figure 2C)
A combination of the previous two incisions (38).
Left axillary artery (Figure 2D)
Left subclavian OG anastomosis has been increasingly utilized for specific patient situations when OG anastomosis to the ascending or the descending aorta is prohibited.
The OG is tunneled through the right or the left pleural cavity, passed through the 2nd intercostal space to be anastomosed to the subclavian artery (Figure 3B). Major concerns about this technique include, the compression of the OG by the surrounding structures, excessive blood flow to the arm, and flow disturbances with extensive elevation of the arm. Banding of the subclavian artery is mandatory if there is a mean pressure difference of more than 20 mmHg to avoid excessive blood flow to the arm (28). Some authors described progressive increase of the pump speed over a few days to allow gradual distal adaptation to the increased of pump flow and decrease the risks of arm hypoperfusion.
In this case if CBP is required it is initiated by cannulating both the common femoral artery and vein.
Single left thoracotomy incision with descending aorta anastomosis for high-risk patients (Figure 3C)
For this approach, the femoral vessels are exposed for CPB if needed. Surface echocardiography is used to enter the left pleural space and expose the LV apex. The inferior pulmonary ligament is freed to optimize exposure. The LVAD positioning is mainly done by using TEE and guide wire if needed. The OG is placed in the left pulmonary fissure, measured and anastomosed end-to-side to the descending aorta or the aortic arch using a partial cross clamp (8,39).
Supra-celiac abdominal aorta (Figure 3D)
This is used by making left subcostal incision and dividing the diaphragm to make the pocket. This allows the LVAD to be implanted on the diaphragmatic surface of the left ventricle and the supra-celiac aorta is then exposed by extra peritoneal exposure. The outflow is trimmed and anastomosed end to side anastomosis using side biting clamp on the supra-celiac aorta (14).
Conclusions
LVAD insertion by minimally invasive procedures is feasible and safe, minimally invasive techniques improve the survival and reduced mortality in LVAD insertion when compared to reoperative sternotomy, this was also proven for LVAD exchange (40,41). LVAD implantation without the use of CPB has the potential to minimize post-operative complications such as excessive bleeding during implantation without compromising hemodynamics. By minimizing the needs for blood transfusions, patients have decreased exposure to blood antigens which ultimately reduces the risk of sensitization in transplant candidates. We believe that a combination of minimally invasive LVAD insertion with off-pump should be the ultimate goal of minimally invasive LVAD implantation.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [DOI] [PubMed] [Google Scholar]
- 2.Birks EJ, George RS, Hedger M, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation 2011;123:381-90. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141-56. [DOI] [PubMed] [Google Scholar]
- 4.Wever-Pinzon O, Drakos SG, Kfoury AG, et al. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation 2013;127:452-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 2010;29:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant 2002;21:516-21. [DOI] [PubMed] [Google Scholar]
- 7.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg 1999;68:734-41. [DOI] [PubMed] [Google Scholar]
- 8.Maltais S, Davis ME, Haglund N. Minimally invasive and alternative approaches for long-term LVAD placement: the Vanderbilt strategy. Ann Cardiothorac Surg 2014;3:563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [DOI] [PubMed] [Google Scholar]
- 10.Umakanthan R, Haglund NA, Stulak JM, et al. Left thoracotomy HeartWare implantation with outflow graft anastomosis to the descending aorta: a simplified bridge for patients with multiple previous sternotomies. ASAIO J 2013;59:664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabashnikov A, Mohite PN, Simon AR, et al. HeartWare miniaturized intrapericardial ventricular assist device: advantages and adverse events in comparison to contemporary devices. Expert Rev Med Devices 2013;10:441-52. [DOI] [PubMed] [Google Scholar]
- 12.Gregoric ID, La Francesca S, Myers T, et al. A less invasive approach to axial flow pump insertion. J Heart Lung Transplant 2008;27:423-6. [DOI] [PubMed] [Google Scholar]
- 13.Gregoric ID, Cohn WE, Frazier OH. Diaphragmatic implantation of the HeartWare ventricular assist device. J Heart Lung Transplant 2011;30:467-70. [DOI] [PubMed] [Google Scholar]
- 14.Anyanwu AC. Technique for less invasive implantation of Heartmate II left ventricular assist device without median sternotomy. Semin Thorac Cardiovasc Surg 2011;23:241-4. [DOI] [PubMed] [Google Scholar]
- 15.Cheung A, Lamarche Y, Kaan A, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg 2011;91:1294-6. [DOI] [PubMed] [Google Scholar]
- 16.Krabatsch T, Drews T, Potapov E, et al. Different surgical strategies for implantation of continuous-flow VADs-Experience from Deutsches Herzzentrum Berlin. Ann Cardiothorac Surg 2014;3:472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JD, Avery GJ, Egrie G, et al. Less invasive Thoratec LVAD insertion: a surgical technique. Heart Surg Forum 2000;3:218-23. [PubMed] [Google Scholar]
- 18.Pintar T, Collard CD. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin North America 2003;21:453-64. [DOI] [PubMed] [Google Scholar]
- 19.Clive Landis R, Murkin JM, Stump DA, et al. Consensus statement: minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum 2010;13:E116-23. [DOI] [PubMed] [Google Scholar]
- 20.Momeni M, Carlier C, Baele P, et al. Fibrinogen concentration significantly decreases after on-pump versus off-pump coronary artery bypass surgery: a systematic point-of-care ROTEM analysis. J Cardiothorac Vasc Anesth 2013;27:5-11. [DOI] [PubMed] [Google Scholar]
- 21.Belhaj A. Actual knowledge of systemic inflammation reaction during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov 2012;7:165-9. [DOI] [PubMed] [Google Scholar]
- 22.Selzman CH, Sheridan BC. Off-pump insertion of continuous flow left ventricular assist devices. J Card Surg 2007;22:320-2. [DOI] [PubMed] [Google Scholar]
- 23.Van Meter CH, Jr, Robbins RJ, Ochsner JL. Technique of right heart protection and deairing during heartmate vented electric LVAD implantation. Ann Thorac Surg 1997;63:1191-2. [DOI] [PubMed] [Google Scholar]
- 24.Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-Pump or On-Pump Coronary-Artery Bypass Grafting at 30 Days. N Engl J Med 2012;366;1489-97. [DOI] [PubMed] [Google Scholar]
- 25.Sun BC, Firstenberg MS, Louis LB, et al. Placement of long-term implantable ventricular assist devices without the use of cardiopulmonary bypass. J Heart Lung Transplant 2008;27:718-21. [DOI] [PubMed] [Google Scholar]
- 26.Centofanti P, La Torre M, Attisani M, et al. Rapid pacing for the off-pump insertion of the Jarvik left ventricular assist device. Ann Thorac Surg 2011;92:1536-8. [DOI] [PubMed] [Google Scholar]
- 27.Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without the use of cardiopulmonary bypass. Ann Thorac Surg 2003;75:1028-30. [DOI] [PubMed] [Google Scholar]
- 28.Riebandt J, Sandner S, Mahr S, et al. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann Thorac Surg 2013;96:1094-6. [DOI] [PubMed] [Google Scholar]
- 29.Awad H, Abd El Dayem M, Heard J, et al. Initial experience with off-pump left ventricular assist device implantation in single center: retrospective analysis. J Cardiothorac Surg 2010;5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galiè N, Ussia G, Passarelli P, et al. Role of pharmacologic tests in the treatment of primary pulmonary hypertension. Am J Cardiol 1995;75:55A-62A. [DOI] [PubMed] [Google Scholar]
- 31.Reeves JT, Groves BM, Weir EK. Adenosine and selective reduction of pulmonary vascular resistance in primary pulmonary hypertension. Circulation 1991;84:1437-9. [DOI] [PubMed] [Google Scholar]
- 32.Anastasiadis K, Antonitsis P, Argiriadou H, et al. Use of minimal extracorporeal circulation circuit for left ventricular assist device implantation. ASAIO J 2011;57:547-9. [DOI] [PubMed] [Google Scholar]
- 33.Cohn WE. New tools and techniques to facilitate off-pump left ventricular assist device implantation. Tex Heart Inst J 2010;37:559-61. [PMC free article] [PubMed] [Google Scholar]
- 34.Anyanwu AC, Itagaki S, Pinney S, et al. Initial experience with routine less invasive implantation of HeartMate II left ventricular assist device without median sternotomy. Eur J Cardiothorac Surg 2014;46:985-90. [DOI] [PubMed] [Google Scholar]
- 35.El-Sayed Ahmed MM, Aftab M, Singh SK, et al. Left ventricular assist device outflow graft: alternative sites. Ann Cardiothorac Surg 2014;3:541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas SV, Avsar M, Hanke JS, et al. Minimally-invasive approach for left ventricular assist device implantation: Lower mortality and improved early outcome in adult patients with severe heart failure. Thorac cardiovasc Surg 2013;61-OP52. [Google Scholar]
- 37.Popov AF, Hosseini MT, Zych B, et al. HeartWare left ventricular assist device implantation through bilateral anterior thoracotomy. Ann Thorac Surg 2012;93:674-6. [DOI] [PubMed] [Google Scholar]
- 38.Wagner CE, Bick JS, Kennedy J, et al. Minimally invasive thoracic left ventricular assist device implantation; case series demonstrating an integrated multidisciplinary strategy. J Cardiothorac Vasc Anesth 2015;29:271-4. [DOI] [PubMed] [Google Scholar]
- 39.Popov AF, Mohite PN, Sabashnikov A, et al. Minimally invasive HeartWare LVAD implantation through single left thoracotomy. J Artif Organs 2015;18:170-2. [DOI] [PubMed] [Google Scholar]
- 40.Schechter MA, Patel CB, Blue LJ, et al. Improved early survival with a nonsternotomy approach for continuous-flow left ventricular assist device replacement. Ann Thorac Surg 2015;99:561-6. [DOI] [PubMed] [Google Scholar]
- 41.Ota T, Yerebakan H, Akashi H, et al. Continuous-flow left ventricular assist device exchange: clinical outcomes. J Heart Lung Transplant 2014;33:65-70. [DOI] [PubMed] [Google Scholar]