Abstract
A simple, noninvasive imaging technique was used to obtain in situ measurements of organic-liquid saturation in a two-phase system under dynamic conditions. Efficacy of the light reflection visualization (LRV) imaging method was tested through comparison of measured and known volumes of organic liquid for experiments conducted with a two-dimensional flow cell. Two sets of experiments were conducted, with source-zone configurations representing two archetypical residual-and-pool architectures. LRV measurements were collected during the injection of organic liquid and during a dissolution phase induced by water flushing. There was a strong correlation between measured and known organic-liquid volumes, with the LRV-measured values generally somewhat lower than the known volumes. Errors were greater for the system wherein organic liquid was present in multiple zones comprised of porous media of different permeabilities, and for conditions of multiphase flow. This method proved effective at determining organic-liquid distribution in a two-phase system using minimal specialized equipment.
Key words: : NAPL, imaging, saturation
Introduction
The contamination of groundwater by hazardous organic chemicals and the associated risks to human health and the environment are issues of great importance. One of the most critical issues associated with hazardous waste sites is the potential presence of organic-liquid contamination in the subsurface. Organic liquids, such as chlorinated solvents, creosote, coal tars, and fuels, once introduced into the subsurface become entrapped, and serve as long-term sources of contamination. The distribution of organic liquid in the source zone is a key factor controlling the generation of contaminant plumes and the efficacy of source-zone remediation.
Intermediate-scale flow cells are often used in studies of the behavior of organic liquid in porous media. For example, flow cells have been used to examine migration of organic liquid in saturated and variably saturated systems, organic-liquid dissolution in heterogeneous systems, and the impact of remediation technologies on mass removal. Several noninvasive techniques such as dual-energy γ-radiation, magnetic resonance imaging, and X-ray tomography have been developed to measure fluid saturations in flow-cell systems, as recently reviewed by Werth et al. (2010). These methods have been used successfully to measure both water and organic-liquid saturations in one-dimensional (1D) and two-dimensional systems. However, these methods have some disadvantages, such as relatively long data acquisition times and the need for specialized instrumentation, that limit their use for some applications (e.g., Werth et al., 2010).
Noninvasive imaging techniques based on light reflection visualization (LRV) and light transmission visualization (LTV) theory are also available for measuring fluid saturation (e.g., Glass et al., 2000; Kechavarzi et al., 2000, 2005; Darnault et al., 2001; Niemet and Selker, 2001; Conrad et al., 2002; Bob et al., 2008). An advantage of these methods is short data acquisition times, which allows for characterization of fluid saturations under dynamic conditions. Another advantage is limited need for specialized instrumentation. However, there are also associated disadvantages.
Many of the prior tests of LRV/LTV methods have been applied only to systems wherein organic liquid is present in a single, homogeneous porous medium. Thus, only a relatively few studies have examined systems with organic liquid present in multiple porous media types (e.g., Glass et al., 2000; Darnault et al., 2001; Conrad et al., 2002). The purpose of this research was to evaluate a noninvasive, light-reflection-based imaging technique for determining organic-liquid saturation during flow-cell experiments conducted for two-fluid-phase systems. The method was tested specifically for systems with heterogeneous permeability fields wherein the organic liquid was nonuniformly distributed.
Materials and Methods
Flow cell materials
Three natural sand media with different median particle diameters were used in these experiments, 713 μm (20/30 mesh), 359 μm (40/50 mesh), and 172 μm (70/100 mesh) (Unimin Corp.). Trichloroethene (TCE), American Chemical Society (ACS) grade (Aldrich), was used as the organic liquid. The organic liquid was dyed with Sudan IV (Aldrich) at a concentration of 100 mg/L, which has been shown to have minimal impact on fluid properties and behavior (e.g., Schwille, 1988; Kennedy and Lennox, 1997).
The flow cell was constructed of stainless steel, with dimensions of 40 cm long by 20 cm high by 2.6 cm wide. A sheet of tempered glass was attached to the front of the flow cell, and another sheet was attached to the back. The flow cell was equipped with multiple, evenly spaced injection/extraction ports on each end. In addition, three ports were evenly spaced at the top of the flow cell to allow injection of organic liquid. Water-tight seals were made with Teflon tape and silicon sealant. The calibration vials and flow cells were lit from the front using 100 W incandescent bulb clamp lamps. The experiments were conducted in a darkroom to minimize variations in lighting due to sunlight.
Organic-liquid injection and dissolution
The performance of the LRV method was tested by comparing measured and known volumes of organic liquid. Two experiments with different porous-media and organic-liquid configurations, representing two archetypical “residual and pool” distributions, were conducted for this study. The flow cell for the Mixed Source experiment comprised a homogeneous pack of 40/50 sand with a 1-cm-thick capillary barrier composed of 70/100 sand placed at the bottom of the flow cell. The flow cell for the Heterogeneous experiment comprised a matrix of 40/50 sand with lenticular zones of 20/30 and 70/100 sand.
The organic-liquid distributions for both experiments were developed by injecting TCE through the top injection ports using a gas-tight syringe and needle. The injection needle was driven to a depth of ∼7 cm from the top of the flow cell and secured to the injection port. Organic liquid was injected in 1-mL increments at a rate of 1 mL/min and allowed to naturally distribute within the porous media. For the Mixed Source experiment, all 12 mL of the organic liquid was injected through the central injection port. Injection of the organic liquid (∼15 mL) in the Heterogeneous experiment was partitioned between two ports: 67% of the total organic liquid volume was injected in the far left port and 33% was injected through the center port. After completion of TCE injection, the flow cell remained undisturbed for 48 h to attain a stable fluid distribution. Deionized, de-aired water was then injected at a constant rate equivalent to an average pore-water velocity of ∼9 cm/h, representative of induced-gradient conditions typical for field sites, to initiate the dissolution phase of the experiment. Integration of the effluent concentration data provided cumulative mass removed as a function of time.
For all experiments, effluent samples were collected with a glass syringe and injected into glass autosampler vials. The glass autosampler vials were stored at 4°C until analyzed using a gas chromatograph (Shimadzu 14A) equipped with an autosampler and a flame ionization detector. Analytical methods for TCE were similar to previous studies (e.g., Brusseau et al., 2008). The quantifiable detection limit was ∼1.7 mg/L.
Image analysis method: image processing
Light transmission and reflection theory has been described in detail by other researchers (e.g., Niemet and Selker, 2001; Bob et al., 2008). Therefore, only a description of the imaging method is provided herein. A high-resolution digital camera (Nikon D70 with an AF-S Nikkor 18–70 mm lens) was used for raw data collection. All images included an optical density photographic card (Kodak) to correct for lighting differences and to convert pixel intensity to optical density. Images were imported into MATLAB as three-dimensional matrices, with the dimensions representing the red (R), green (G), and blue (B) color values, respectively. Next, the images were converted into a 1D matrix of intensity (I) values:
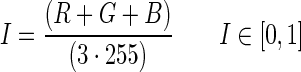 |
(1) |
The optical density of each pixel was calculated using the average intensity of the white step of the optical density card:
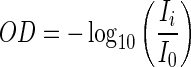 |
(2) |
where OD is the pixel optical density, Ii is the intensity of the pixel, and I0 is the average intensity of the white step of the optical density card.
Image analysis method: calibration
An optical density and organic-liquid saturation calibration curve was obtained for the two sands that contained TCE liquid in the flow cell experiments (Fig. 1). A series of 40-mL glass vials were prepared by mixing known volumes of deionized water, organic liquid, and sand in a beaker. Then, a quarter of the sand mixture at a time was transferred into the vials and compacted using a glass bar. When the vial was filled, the cap was placed onto the vial and sealed using parafilm. Vials were prepared to represent ∼50%, 30%, 20%, and 10% organic liquid saturation. A vial representing 100% organic liquid saturation was prepared by filling the vial with a known volume of organic liquid. Next, sand was added to the vial and compacted using a glass bar. A 0% organic liquid saturation vial was prepared in a similar fashion, using deionized water instead of organic liquid. Once prepared, the vials were photographed next to an optical density photographic card. The actual organic-liquid saturation for each vial was then determined by solvent extraction using methanol. The extraction efficiency for these relatively ideal media was >95%.
FIG. 1.
Relationship between organic-liquid saturation and pixel optical density.
Image analysis method: flow cell
For the flow cell experiments, a series of three high-resolution (pixel size of ∼175 μm) photographs were taken of the front face of the flow cell at each time of interest. Image sets were collected at several times throughout organic-liquid injection and dissolution. As noted above, each photograph contained the optical density photographic card to convert pixel intensity to optical density. The images were imported into MATLAB and the OD value of each pixel was determined, as described above. An average pixel-by-pixel optical density value for a given time was determined by averaging the optical density of the three images of a given image-time set. The impact of slight variations in lighting across the front of the flow cell was accounted for by normalizing the OD values to a background set of OD values collected before TCE injection. High OD values registered near the edge of the image where no organic liquid existed due to reflection of the flow cell frame. These anomalous values were removed from the image.
Variations in the light source among image sets (i.e., from one collection time to another) were corrected by selecting an observation window within the flow cell that was organic-liquid free throughout the course of the experiment. The average OD within this window was monitored over time. To correct the image data, OD values were multiplied by the ratio of the average OD of the calibration standard with no organic liquid (the reference standard) and the average OD of the observation window for the specific collection time.
The entire image was smoothed by averaging OD over a 5×5 pixel area. A threshold value was selected to remove noise due to changes in the lighting or the camera. Organic-liquid saturation was calculated based on the aforementioned organic-liquid saturation versus optical density calibration curve. The volume of organic liquid was calculated by multiplying the organic-liquid saturation by the pixel volume (∼7.2×10−4 cm3).
Results
Error analysis
The impact of system noise on the measured organic-liquid saturation was determined by evaluating the results obtained for the observation window, which was organic-liquid free throughout the entirety of the experiment. Since this region was unaffected by organic liquid, any deviation in the optical density of this region was the result of camera noise and/or temporal variations in the light source. Variations in lighting due to sunlight were minimized by performing experiments in a dark room, as noted above.
The calibration curve was used to calculate average organic-liquid saturation and the average standard deviation of saturation for the observation window for each image. The mean organic-liquid saturation calculated for this region before image smoothing was normally distributed with a mean value of ∼0 (Fig. 2). Obtaining a mean value of 0 for this organic-liquid-free region supports the robustness of the OD-organic liquid calibration curve. Image smoothing decreased the observed noise (Fig. 3). The average standard deviation after image smoothing was 0.093% for the observation window. This value was taken as the standard deviation associated with each saturation measurement. Therefore, a threshold saturation value of 0.2% was used in this analysis to remove background noise from the images. This value is assumed to be the lower limit of detection for this method.
FIG. 2.
Histogram of organic-liquid saturation (%) for the observation window before image smoothing.
FIG. 3.
Histogram of organic-liquid saturation (%) for the observation window after image smoothing.
Comparison of calculated and known volumes
For the Mixed Source experiment, a total of ∼12 mL of TCE was injected in 1-mL increments, and photographs were taken after each addition. During injection, organic liquid migrated downward through the matrix and spread laterally along the top of the capillary barrier, forming a pool (Fig. 4). For the Heterogeneous experiment, a total of ∼15 mL of TCE was injected in 1-mL increments, and photographs were taken after each addition. During injection, organic liquid migrated downward on the left side and pooled along the top boundary of the 70/100 zone. In the center of the flow cell, organic liquid migrated downward to the 70/100 zone. The organic liquid moved laterally along this boundary, spilled over the edge, and then wicked into the adjacent 20/30 zone and pooled (Fig. 5).
FIG. 4.
Organic-liquid distribution during injection for the Mixed Source experiment determined using the light reflection visualization (LRV) method. The matrix was composed of 40/50 sand, and the gray zone was composed of 70/100 sand. The organic-liquid saturation is defined using the color scale, where warm colors indicate high organic-liquid saturation and cool colors represent low saturation. Color images available online at www.liebertonline.com/ees
FIG. 5.
Organic-liquid distribution during injection for the Heterogeneous experiment determined using the LRV method. The matrix was composed of 40/50 sand. The black outlined zones were composed of 20/30 and gray zones were composed of 70/100 sand. The organic-liquid saturation is defined using the color scale, where warm colors indicate high organic-liquid saturation and cool colors represent low saturation. Color images available online at www.liebertonline.com/ees
Once the organic-liquid distribution stabilized, deionized, de-aired water was injected at a constant rate, equivalent to an average pore-water velocity of ∼9 cm/h, to initiate dissolution of the organic liquid. Integration of the effluent concentration data provided cumulative mass removed as a function of time. Images were taken throughout the course of flushing, and image-calculated organic-liquid volumes were compared to the volume remaining calculated from moment analysis of the elution data (Figs. 6 and 7).
FIG. 6.
Organic-liquid distribution during mass removal for the Mixed Source experiment determined using the LRV method. The matrix was composed of 40/50 sand and the gray zone was composed of 70/100 sand. The organic-liquid saturation is defined using the color scale, where warm colors indicate high organic-liquid saturation and cool colors represent low saturation. Color images available online at www.liebertonline.com/ees
FIG. 7.
Organic-liquid distribution during mass removal for the Heterogeneous experiment determined using the LRV method. The matrix was composed of 40/50 sand. The black outlined zones were composed of 20/30 and gray zones were composed of 70/100 sand. The organic-liquid saturation is defined using the color scale, where warm colors indicate high organic-liquid saturation and cool colors represent low saturation. Color images available online at www.liebertonline.com/ees
The measured volumes of organic liquid, determined from image analysis, at any given time during both the injection and dissolution phases were plotted against the known volumes, determined from the volumes associated with the controlled release and elution curves (Fig. 8). There was a strong correlation between the calculated and known volumes for both experiments (R2>0.91). The values measured with LRV are generally somewhat smaller than the known volumes, with deviations from one-to-one correspondence of ∼94% and 76% for the Mixed Source and Heterogeneous experiments, respectively. This is consistent with other optical-based imaging methods (e.g., Bob et al., 2008). Some of the error is associated with the calibration step, wherein the efficiency of the solvent-extraction method was <100%, such that the measured TCE saturations used for the calibration curves were slightly smaller than the actual values.
FIG. 8.
Comparison of calculated and known organic-liquid volumes. The thick gray line represents the one-to-one between known and calculated volume. The solid black line represents the best fit line for the Mixed Source experimental data. The dashed black line represents the best fit line for the Heterogeneous experimental data.
The percent error between measured and known volumes was generally greater for the smallest volumes of organic liquid. In addition, the error was generally greater for the measurements collected during the injection phase for both experiments. This is likely a result of the multiphase flow conditions present during organic-liquid injection, and the possibility that organic liquid had not yet distributed completely from the injection point to the flow-cell walls. Conversely, the dissolution phases of the experiments were conducted after 48 h to allow the organic liquid distributions to stabilize. As noted above, the error was greater for the Heterogeneous experiment, for which the organic liquid was located in different permeability zones. It is possible that the distribution of the organic liquid across the flow-cell width may have been less uniform for this system because of the greater complexity of multiphase flow associated with the heterogeneous permeability field.
Conclusions
A simple, noninvasive imaging technique was used to measure in situ organic-liquid distribution in a two-fluid-phase system. This method required minimal use of special equipment and allowed for quantification of source-zone architecture in heterogeneous flow-cell systems under dynamic conditions. The efficacy of the LRV imaging method was tested based on known volumes of organic liquid, and there was a strong correlation between measured and known organic-liquid volumes, with the LRV-measured values generally somewhat lower than the known volumes. Errors were greater for the system wherein organic liquid was present in multiple zones comprised of porous media of different permeabilities, and for conditions of multiphase flow.
A significant difference between the LRV and LTV methods is that the former measures saturation only at the interface between the flow-cell wall and the porous medium. Thus, nonuniform distribution of fluid within the porous media in the z dimension (normal to the flow-cell/porous-medium interface) will lead to greater error in calculated saturations. Images taken of the front and back of the flow cells (data not shown) displayed comparable organic-liquid distributions, indicating relatively uniform fluid distribution in the z dimension for these experiments. For cases where there is deviation between the front and back sides, image analysis can be conducted for both sides to reduce uncertainty.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences Superfund Basic Research Program (ES04940), with additional funding provided by the U.S. Department of Defense Strategic Environmental Research and Development Program. We wish to thank Larry Acedo and Aldo Perazzone from the University Research Instrumentation Center at the University of Arizona.
Author Disclosure Statement
No competing financial interests exist.
References
- Bob M.M. Brooks M.C. Mravik S.C. Wood A.L. A modified light transmission visualization method for DNAPL saturation measurements in 2-D models. Adv. Water Resour. 2008;31:727. [Google Scholar]
- Brusseau M.L. DiFilippo E.L. Marble J.C. Oostrom M. Mass-removal and mass-flux-reduction behavior for idealized source zones with hydraulically-poorly-accessible immiscible liquid. Chemosphere. 2008;71:1511. doi: 10.1016/j.chemosphere.2007.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S.H. Glass R.J. Peplinski W.J. Bench-scale visualization of DNAPL remediation processes in analog heterogeneous aquifers: surfactant floods and in situ oxidation using permanganate. J. Contam. Hydrol. 2002;58:13. doi: 10.1016/s0169-7722(02)00024-4. [DOI] [PubMed] [Google Scholar]
- Darnault C.J.G. DiCarlo D.A. Bauters T.W.J. Jacobson A.R. Throop J.A. Montemagno C.D. Parlange J.Y. Steenhuis T.S. Measurement of fluid contents by light transmission in transient three-phase oil-water-air systems in sand. Water Resour. Res. 2001;37:1859. [Google Scholar]
- Glass R.J. Conrad S.H. Peplinski W. Gravity-destabilized nonwetting phase invasion in macroheterogeneous porous media: experimental observations of invasion dynamics and scale analysis. Water Resour. Res. 2000;36:3121. [Google Scholar]
- Kechavarzi C. Soga K. Illangasekare T.H. Two-dimensional laboratory simulation of LNAPL infiltration and redistribution in the vadose zone. J. Contam. Hydrol. 2005;76:211. doi: 10.1016/j.jconhyd.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kechavarzi C. Soga K. Wiart P. Multispectral image analysis method to determine dynamic fluid saturation distribution in two-dimensional three-fluid phase flow laboratory experiments. J. Contam. Hydrol. 2000;4:265. [Google Scholar]
- Kennedy C.A. Lennox W.C. A pore-scale investigation of mass transport from dissolving DNAPL droplets. J. Contam. Hydrol. 1997;24:221. [Google Scholar]
- Niemet M.R. Selker J.S. A new method for quantification of liquid saturation in 2D translucent porous media systems using light transmission, Adv. Water Resour. 2001;24:651. [Google Scholar]
- Schwille F. In: Dense Chlorinated Solvents in Porous and Fractured Media. Pankow J.F., translator. Chelsea, MI: Lewis Publications; 1988. p. 144. [Google Scholar]
- Werth C.J. Zhang C. Brusseau M.L. Oostrom M. Baumann T.A. Review of non-invasive imaging methods and applications in contaminant hydrogeology research. J. Contam. Hydrol. 2010;113:1. doi: 10.1016/j.jconhyd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]










