Abstract
BACKGROUND AND PURPOSE:
Patients with SAH are at increased risk of delayed infarction. Early detection and treatment of delayed infarction remain challenging. We assessed blood-brain barrier permeability, measured as permeability surface area product, by using CTP in patients with SAH with delayed infarction.
MATERIALS AND METHODS:
We performed a retrospective study of patients with SAH with delayed infarction on follow-up NCCT. CTP was performed before the development of delayed infarction. CTP data were postprocessed into permeability surface area product, CBF, and MTT maps. Coregistration was performed to align the infarcted region on the follow-up NCCT with the corresponding location on the CTP maps obtained before infarction. Permeability surface area product, CBF, and MTT values were then obtained in the location of the subsequent infarction. The contralateral noninfarcted region was compared with the affected side in each patient. Wilcoxon signed rank tests were performed to determine statistical significance. Clinical data were collected at the time of CTP and at the time of follow-up NCCT.
RESULTS:
Twenty-one patients with SAH were included in the study. There was a statistically significant increase in permeability surface area product in the regions of subsequent infarction compared with the contralateral control regions (P < .0001). However, CBF and MTT values were not significantly different in these 2 regions. Subsequent follow-up NCCT demonstrated new delayed infarction in all 21 patients, at which time 38% of patients had new focal neurologic deficits.
CONCLUSIONS:
Our study reveals a statistically significant increase in permeability surface area product preceding delayed infarction in patients with SAH. Further investigation of early permeability changes in SAH may provide new insights into the prediction of delayed infarction.
Aneurysmal subarachnoid hemorrhage is a devastating illness with an average case fatality rate of 51% and approximately one-third of survivors needing life-long care.1 Patients who survive are at high risk for developing sequelae of cerebral vasospasm and delayed cerebral ischemia (DCI), leading to delayed infarction, which occurs in 40%–70% of patients with SAH, representing the leading cause of post-SAH morbidity and mortality.2 Recent studies reported a dissociation of angiographic vasospasm and poor neurologic outcome, supporting the assumption that infarction rather than vasospasm might reflect the ultimate end point of different proischemic pathomechanisms, including microvascular and neuronal dysfunction.3
Early and delayed microcirculatory dysfunction after SAH is increasingly thought to be associated with DCI resulting in delayed infarction, in the absence of proximal vasospasm.4 Microvascular dysfunction can be assessed by using CTP. CTP parameters currently used in clinical practice are MTT, CBF, and CBV,5 which provide useful findings regarding the presence and extent of DCI.5–10 However, screening tools are lacking for earlier detection of patients at particularly high risk of developing delayed infarction from DCI to prompt therapeutic pre-emptive measures to minimize the impending morbidity and mortality. Currently, the assessment of patients with SAH for DCI relies primarily on clinical examination, transcranial Doppler sonography, and NCCT. Clinical examination is limited because symptoms can be variable and difficult to detect,11 while transcranial Doppler sonography is limited by poor sensitivity and specificity.12–14 Although NCCT can detect delayed infarction, the management and treatment goals in patients with SAH are to prevent these sequelae of DCI.
Blood-brain barrier permeability (BBBP) is known to increase in certain conditions such as ischemia, malignancy, infection, and autoimmune disease.15 On the molecular level, the BBB is composed of the neurovascular unit, comprising tight junctions between astrocytes and vascular endothelial cells. Permeability surface-area product (PS), derived from CTP, measures the diffusion of contrast from the vascular into the interstitial space, thus providing an indirect measure of BBBP.16 Although several reports describe the high sensitivity and specificity of the CTP-derived CBF and/or MTT to detect perfusion abnormalities in DCI,5,7–10,17,18 alterations of the BBBP have not yet been studied in this context.
We hypothesized that BBBP increases before delayed infarction in patients with SAH. The purpose of this study was to assess whether alterations in BBBP, measured as PS by using CTP, precede development of infarction related to DCI after SAH.
Materials and Methods
Study Population
A retrospective analysis was performed on consecutive patients with SAH enrolled in a prospective diagnostic accuracy trial at our institution. SAH was determined by a combination of NCCT, CTA, DSA, and/or CSF analysis. In this prospective study design, CTP was performed during the baseline period on days 0–3 following aneurysmal rupture with day zero defined as the day of the initial hemorrhagic event, to assess baseline cerebral perfusion and to compare it with later perfusion examinations for interval change. A follow-up CTP was performed on the day of new focal neurologic deficits or on days 6–8 in asymptomatic patients. Institutional review board approval was obtained.
In our study, inclusion criteria were the following: 1) patients with delayed infarction related to DCI detected on NCCT, and 2) CTP, including assessment of PS performed before infarction. Follow-up NCCT was obtained as a standard of care at our institution to assess complications of SAH, including repeat hemorrhage, hydrocephalus, and new infarction.
Patients with SAH were classified as having DCI according to published criteria from recent expert consensus,19 consisting of a radiologic and clinical component. These criteria included either a new infarction on CT or MR imaging within 6 weeks after SAH or clinical manifestations of new focal neurologic impairment or a decrease of at least 2 points on the Glasgow Coma Scale. Regions of delayed infarction were defined as new areas of ischemia not present on imaging up to 48 hours after aneurysm occlusion and not attributable to other causes such as surgical clipping, endovascular treatment, ventricular catheter placement, and intraparenchymal hematoma.19 This definition has been used to effectively exclude primary brain damage from SAH and/or surgical interventions.19 Delayed infarction was dictated in the clinical reports of the NCCT by board-certified neuroradiologists on service at our institution. A second independent board-certified neuroradiologist blinded to all clinical and imaging data confirmed the presence and exact location of the infarcted region in each case for inclusion in this study.
Exclusion criteria were the following: 1) patients with an infratentorial infarction, given that the posterior fossa is not adequately imaged on CTP; and 2) severe motion degradation limiting CTP postprocessing.
Data collection included detailed review of each patient's clinical chart and recording of each patient's demographic characteristics, clinical symptoms at presentation, aneurysm location, clinical symptoms at the time of CTP and NCCT, infarction location, and modified Rankin Scale clinical outcome score. Subsequently, these clinical data were grouped and analyzed across all 21 patients.
CTP Image Acquisition, Postprocessing, and Analysis
Routine NCCT was performed from the foramen magnum to the vertex by using the following parameters: 120 kV(peak), 250 mAs, 1.0 rotation time, and 5.0-mm collimation. Extended CTP to measure PS was performed on a LightSpeed Discovery 750 64-section scanner (GE Healthcare, Milwaukee, Wisconsin) by using axial shuttle mode with the following parameters: 80 kVp, 400 mAs, 0.4 rotation time, 5.0-mm collimation with 17 cine cycles and 2.8-second interscan delay for the first pass. The second-pass technique included 10 cine cycles with a 10-second interscan delay. A total of 90 mL of nonionic contrast was intravenously administered at 4.0 mL/s followed by a 30-mL saline bolus.
All CTP data were postprocessed by using CT Perfusion 4D software (GE Healthcare) for generation of PS, CBF, and MTT maps. PS represents the flow across the blood vessel wall, from the intravascular space to the extravascular extracellular space, and thus constitutes a measure of BBBP. Its units are milliliter × milliliter−1 × minute−1 (volume of liquid per volume of tissue per minute). PS measurements are derived from the tail of the tissue attenuation curve obtained after the first pass of contrast. In normal cerebral vasculature, PS is negligible.20
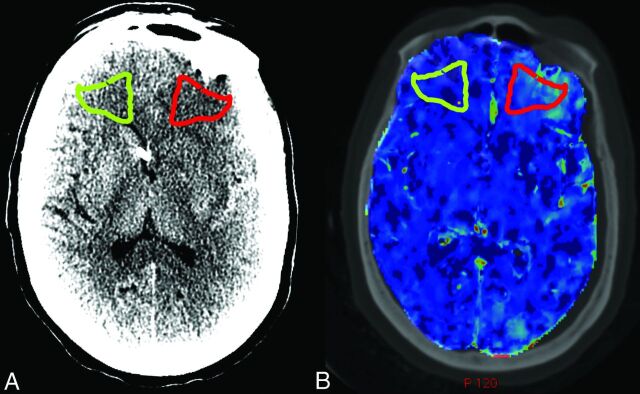
The follow-up NCCT showing the delayed infarction was used for ROI placement within the exact infarct location. Using the symmetry tool in AW2 software (GE Healthcare), we generated a “mirror ROI” in the corresponding location on the contralateral side of the brain to serve as the control for each patient. Figure 1A illustrates the ROI placement in a representative patient.
Fig 1.
ROI placement and coregistration in a representative patient. A, NCCT of a representative patient with SAH demonstrates ROI placement in the region of a new left frontal infarction, which was not present on admission (red) and the contralateral control ROI (green). B, Coregistration of the NCCT from A with the preinfarction CTP yields matched ROI placement on the PS map and the CBF and MTT maps (not shown).
Using the integrated registration tool in the AW2 software, we performed coregistration of the preinfarction CTP, and corresponding follow-up NCCT was performed in 3 planes for each patient. Integrated registration enabled propagation of the ROIs in the regions of subsequent infarction and the contralateral control from each follow-up NCCT onto the respective CTP maps obtained before infarction. Figure 1B illustrates coregistration of the NCCT from Fig 1A with the preinfarction CTP. CBF, MTT, and PS values were then measured within the ROI in the regions of subsequent infarction and the contralateral control. Statistical analysis included Wilcoxon signed rank tests for each CTP parameter to determine whether statistically significant differences in CTP parameter means (with 95% confidence intervals) were present between the regions of subsequent infarction and contralateral controls.
Results
Clinical Characteristics of the Study Population
Twenty-one patients with SAH with 23 delayed infarctions in the anterior circulation were included in the analysis. Patients were excluded if they had infratentorial infarctions (n = 1) or if severe motion artifacts impeded CTP postprocessing (n = 2). The mean age was 50 years (range, 35–88 years) with 76% (16/21) women and 76% (16/21) with anterior circulation aneurysms. The mean Hunt and Hess score at presentation was 3. NCCT performed at the time of the CTP examination did not demonstrate infarction in any of the patients included in this study. The median day for performing CTP after aneurysmal rupture was day zero (range, 0–3 days, with 71% [15/21] of patients undergoing their CTP on day 0 of admission). At the time of the preinfarction CTP, only 10% (2/21) of patients had developed new focal neurologic deficits not present on their admission clinical examination, while the remaining 90% (19/21) of patients had an unchanged clinical examination compared with their admission status. Follow-up NCCT demonstrated a new infarction at 4 days after CTP (range, 1–7 days) with 91% (21/23) MCA infarctions and 9% (2/23) anterior cerebral artery infarctions. By the time the NCCT showed the infarction, 38% (8/21) of patients exhibited new focal neurologic deficits. The median modified Rankin Scale score at the time of discharge was 3 (range, 0–6), indicating moderate disability.
CTP Analysis
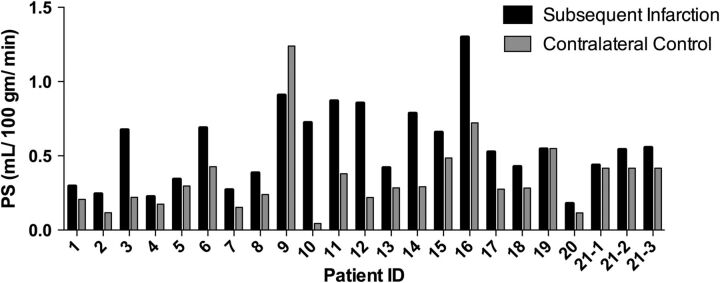
There was a statistically significant increase in PS in the region of subsequent infarction on the preinfarction CTP, compared with the contralateral control region (Table) in 96% of infarctions (22/23). Figure 2 demonstrates the consistency of elevated PS before infarction across the entire study population. However, no significant difference was seen for CBF and MTT between the regions of subsequent infarction and contralateral controls (Table). Figure 3 demonstrates PS elevation preceding the development of subsequent delayed infarction in a representative patient. In addition, the tissue attenuation curves representing the region of subsequent infarction and the contralateral control region demonstrate an elevation of the tail of the tissue attenuation curve (extended pass) in the region of subsequent infarction (Fig 3D).
Quantitative results of PS, CBF, and MTT in the region of subsequent infarction and contralateral control regiona
| Subsequent Infarction | Contralateral Control | P Value | |
|---|---|---|---|
| PS (mL × mL−1 × min−1) | 0.56 (0.45–0.68) | 0.35 (0.25–0.45) | <.0001 |
| CBF (mL × 100 g−1 × min−1) | 17.22 (14.00–20.43) | 17.39 (14.78–20.00) | .7048 |
| MTT (sec) | 10.85 (8.78–12.92) | 10.06 (7.42–12.70) | .128 |
Mean values are shown with 95% confidence intervals (in parentheses).
Fig 2.
PS values in the regions of subsequent infarction versus respective contralateral control regions for each individual patient.
Fig 3.
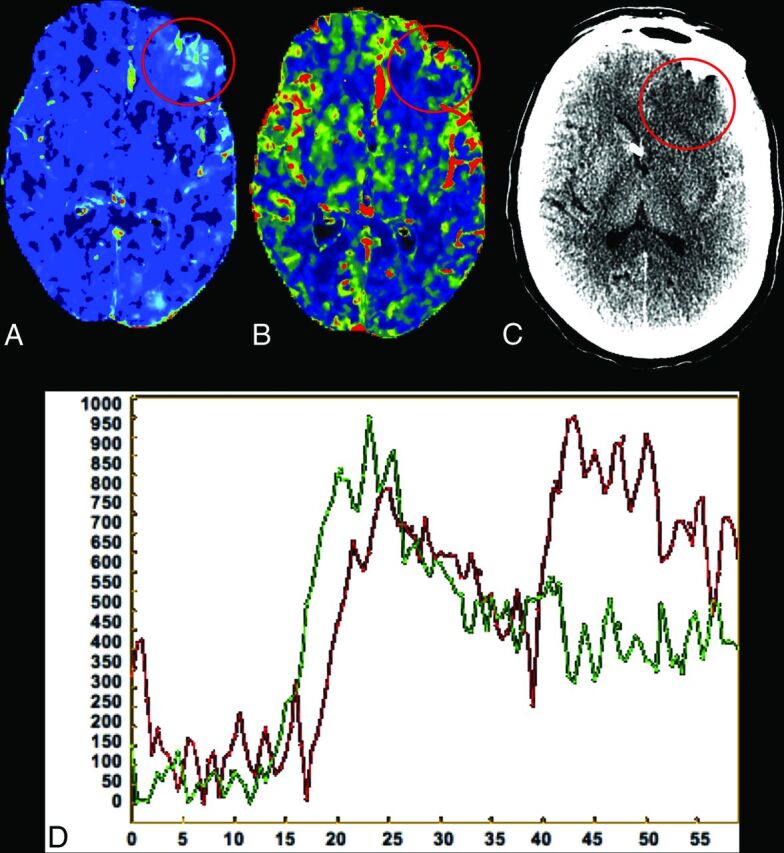
CTP performed on day zero of admission in a representative patient with SAH (same patient as shown in Fig 1). A, Elevated PS is demonstrated in the left frontal region (circled in red) compared with the contralateral side. B, CBF is not significantly different in the same region compared with the contralateral side. Follow-up NCCT (C) reveals an infarction in the exact location of the increased PS seen 2 days prior in this patient. D, Tissue attenuation curves representing the region of subsequent infarction (red) and the contralateral control region (green) obtained in this patient, demonstrating an elevation of the tail of the tissue attenuation curve (extended pass) in the region of subsequent infarction.
Discussion
Currently, the assessment of patients with SAH for DCI and delayed infarction is challenging, given the need to rely on clinical examination, transcranial Doppler sonography, and NCCT data to initiate early treatment and prognosticate patient outcomes. The value of clinical examination is limited due to the potential subjectivity of symptom assessment, low specificity of symptoms, and the frequent incidence of a depressed level of consciousness in patients in the intensive care unit, which can limit the performance of reliable neurologic examinations.11 Transcranial Doppler sonography is also limited by poor sensitivity and specificity.12–14 Thus, there is a strong need for the development and validation of novel imaging strategies allowing identification of patients with SAH at high risk for delayed infarction.
In this study, we have shown that increased PS assessed by CTP precedes the development of delayed infarction seen on NCCT in patients with SAH. These findings were present in 91% (21/23) of regions of subsequent infarction and were statistically significant across the entire study population. Most important, at the time of the preinfarction CTP, the perfusion parameters of CBF and MTT (the most sensitive parameters currently available to clinically assess perfusion deficits17) were not statistically different in the region of subsequent infarction compared with the contralateral control region. Furthermore, only 10% of patients manifested new focal neurologic deficits at the time of the preinfarction CTP, suggesting that an elevation in PS values could represent an early indicator of subsequent infarction. Thus, the detection of BBBP changes may prove a more sensitive method to detect delayed infarction related to DCI compared with conventional NCCT and CTP parameters of CBF and MTT, and before clinical symptoms. Furthermore, at the time of follow-up NCCT demonstrating the subsequent infarction, more patients (38%) had developed new focal neurologic deficits. Our findings are similar to those in prior studies reporting the development of neurologic deficits in up to 40% of patients with DCI.21,22 Moreover, patients with DCI have been shown to have a statistically increased rate of permanent deficits and worse clinical outcomes compared with patients without DCI.21,22
The BBB is composed of endothelial cells connected by tight junctions, providing an effective barrier against paracellular permeability. Transient or permanent cerebral injury can lead to alteration in molecular signaling pathways, such as the ubiquitin-proteasome pathway, leading to BBB disruption and degradation of the extracellular matrix, resulting in vasogenic edema.23 Experimental data have demonstrated increased BBBP and dysfunction of the endothelium and vascular components of the brain associated with the development of DCI and poor outcomes in animal models.15 Moreover, a decrease in BBB disruption and an associated decrease in the effects of cerebral ischemia were seen in animal models treated with novel pharmaceutical agents that stabilize the BBB.24,25 BBBP increase and cerebral ischemia thus appear to be closely intertwined; however, understanding the temporal resolution of these 2 pathophysiologic processes remains challenging. In human subjects, perfusion CT and MR imaging have been introduced as tools to measure BBBP.16,26
Although significant differences existed in PS values in the regions of subsequent infarction versus respective contralateral control regions, our study is limited by its small size and retrospective nature. Future studies are needed to establish the clinical value of monitoring PS as a predictor of delayed infarction related to DCI and poor outcomes and to compare PS values in patients with and without DCI, thus expanding our understanding of the utility of PS for prognostication in patients with SAH.
Conclusions
Our data indicate that the measurement of BBBP using CTP-derived PS has the potential to become a significant marker for the prediction of delayed infarction related to DCI before the onset of clinical symptoms and before alterations seen in MTT and CBF. Our results emphasize the need for continued prospective investigation of BBBP alteration in DCI to develop a clinical indicator for prediction and prognosis of SAH-related morbidity and mortality. Large prospective studies are needed to further validate these initial findings and to provide insight into the underlying pathomechanisms leading to delayed infarction related to DCI after SAH. More important, such future investigations could aid in the development of new strategies targeting the prevention of delayed infarction after SAH.
ABBREVIATIONS:
- BBBP
blood-brain barrier permeability
- DCI
delayed cerebral ischemia
- PS
permeability surface-area product
Footnotes
Disclosures: Jana Ivanidze—RELATED: Grant: National Institute of Neurological Disorders and Stroke (5K23NS058387)*; UNRELATED: Employment: New York-Presbyterian Hospital–Weill Cornell Medical College, Comments: Postgraduate Year-4 Radiology Resident; Grants/Grants Pending: Radiological Society of North America Resident Research Grant. Omar N. Kallas—RELATED: Grant: National Institute of Neurological Disorders and Stroke (5K23NS058387).* Ajay Gupta—UNRELATED: Grants/Grants Pending: I have active support provided for other neuroradiology-related grants from the Association of University Radiologists GE Radiology Research Academic Fellowship, the Foundation of the American Society of Neuroradiology Scholar Award, and CTSC National Institutes of Health grant UL1TR00457. Pina C. Sanelli—RELATED: Grant: National Institute of Neurologic Disorders and Stroke.* *Money paid to the institution.
This work was supported by grant number 5K23NS058387 from the National Institute of Neurological Disorders and Stroke, a component of the National Institutes of Health. The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Paper previously presented at: Annual Meeting of the Radiological Society of North America, December 1–6, 2013; Chicago, Illinois.
REFERENCES
- 1. Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660–64 [DOI] [PubMed] [Google Scholar]
- 2. Mocco J, Ransom ER, Komotar RJ, et al. Racial differences in cerebral vasospasm: a systematic review of the literature. Neurosurgery 2006;58:305–14 [DOI] [PubMed] [Google Scholar]
- 3. Petzold GC, Einhaupl KM, Dirnagl U, et al. Ischemia triggered by spreading neuronal activation is induced by endothelin-1 and hemoglobin in the subarachnoid space. Ann Neurol 2003;54:591–98 [DOI] [PubMed] [Google Scholar]
- 4. Dreier JP, Ebert N, Priller J, et al. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg 2000;93:658–66 [DOI] [PubMed] [Google Scholar]
- 5. Sanelli PC, Ugorec I, Johnson CE, et al. Using quantitative CT perfusion for evaluation of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2011;32:2047–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dankbaar JW, Rijsdijk M, van der Schaaf IC, et al. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 2009;51:813–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dankbaar JW, de Rooij NK, Velthuis BK, et al. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke 2009;40:3493–98 [DOI] [PubMed] [Google Scholar]
- 8. Dankbaar JW, de Rooij NK, Rijsdijk M, et al. Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2010;41:1927–32 [DOI] [PubMed] [Google Scholar]
- 9. Aralasmak A, Akyuz M, Ozkaynak C, et al. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 2009;51:85–93 [DOI] [PubMed] [Google Scholar]
- 10. Wintermark M, Ko NU, Smith WS, et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006;27:26–34 [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt JM, Wartenberg KE, Fernandez A, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg 2008;109:1052–59 [DOI] [PubMed] [Google Scholar]
- 12. Carrera E, Schmidt JM, Oddo M, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery 2009;65:316–23; discussion 323–24 [DOI] [PubMed] [Google Scholar]
- 13. Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke 2001;32:2292–98 [DOI] [PubMed] [Google Scholar]
- 14. Naval NS, Thomas CE, Urrutia VC. Relative changes in flow velocities in vasospasm after subarachnoid hemorrhage: a transcranial Doppler study. Neurocrit Care 2005;2:133–40 [DOI] [PubMed] [Google Scholar]
- 15. Yan J, Li L, Khatibi NH, et al. Blood-brain barrier disruption following subarchnoid hemorrhage may be facilitated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol 2011;230:240–47 [DOI] [PubMed] [Google Scholar]
- 16. Kishore S, Ko N, Soares BP, et al. Perfusion-CT assessment of blood-brain barrier permeability in patients with aneurysmal subarachnoid hemorrhage. J Neuroradiol 2012;39:317–25 [DOI] [PubMed] [Google Scholar]
- 17. Mir DI, Gupta A, Dunning A, et al. CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2014;35:866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Killeen RP, Mushlin AI, Johnson CE, et al. Comparison of CT perfusion and digital subtraction angiography in the evaluation of delayed cerebral ischemia. Acad Radiol 2011;18:1094–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–95 [DOI] [PubMed] [Google Scholar]
- 20. Jain R, Ellika SK, Scarpace L, et al. Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuroradiol 2008;29:694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanelli PC, Anumula N, Gold R, et al. Outcomes-based assessment of a new reference standard for delayed cerebral ischemia related to vasospasm in aneurysmal subarachnoid hemorrhage. Acad Radiol 2012;19:1066–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanelli PC, Anumula N, Johnson CE, et al. Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2013;34:292–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 2010;38:376–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Germanò A, d'Avella D, Imperatore C, et al. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir (Wien) 2000;142:575–80; discussion 580–81 [DOI] [PubMed] [Google Scholar]
- 25. Jin X, Liu J, Yang Y, et al. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol Dis 2012;48:309–16 [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann A, Bredno J, Wendland MF, et al. Validation of in vivo magnetic resonance imaging blood-brain barrier permeability measurements by comparison with gold standard histology. Stroke 2011;42:2054–60 [DOI] [PMC free article] [PubMed] [Google Scholar]