Abstract
Background
A 2006 trial in healthy medical students found that anodal slow oscillating tDCS delivered bi-frontally during slow wave sleep had an enhancing effect in declarative, but not procedural memory. Although there have been supporting animal studies, and similar findings in pathological groups, this study has not been replicated, or refuted, in the intervening years. We therefore tested these earlier results for replication using similar methods with the exception of current wave form (square in our study, nearly sinusoidal in the original).
Objective/Hypothesis
Our objective was to test the findings of a 2006 trial suggesting bi-frontal anodal tDCS during slow wave sleep enhances declarative memory.
Methods
Twelve students (mean age 25, 9 women) free of medical problems underwent two testing conditions (active, sham) in a randomized counterbalanced fashion. Active stimulation consisted of oscillating square wave tDCS delivered during early Non-Rapid Eye Movement (NREM) sleep. The sham condition consisted of setting-up the tDCS device and electrodes, but not turning it on during sleep. tDCS was delivered bi-frontally with anodes placed at F3/F4, and cathodes placed at mastoids. Current density was 0.517mA/CM2, and oscillated between zero and maximal current at a frequency of 0.75Hz. Stimulation occurred during five-five minute blocks with one-minute inter-block intervals (25 minutes total stimulation). The primary outcomes were both declarative memory consolidation measured by a paired word association test (PWA), and non-declarative memory, measured by a non-dominant finger-tapping test (FTT). We also recorded and analyzed sleep EEG.
Results
There was no difference in the number of paired word associations remembered before compared to after sleep [(active = 3.1±3.0SD more associations) (sham = 3.8±3.1S.D more associations)]. Finger tapping improved, (non-significantly) following active stimulation [(3.6±2.7 S.D. correctly typed sequences) compared to sham stimulation (2.3± 2.2 S.D. correctly typed sequences)].
Conclusion
In this study, we failed to find improvements in declarative or performance memory and could not replicate an earlier study using nearly identical settings. Specifically we failed to find a beneficial effect on either overnight declarative or non-declarative memory consolidation via square-wave oscillating tDCS intervention applied bi-frontally during early NREM sleep. It is unclear if the morphology of the tDCS pulse is critical in any memory related improvements.
Keywords: transcranial direct current stimulation, sleep, slow wave sleep, memory consolidation, cognitive enhancement
Introduction
Previous trials have found that the application of both transcranial direct current stimulation (tDCS) and slow oscillating transcranial direct current stimulation (SOtDCS) during early Non-Rapid Eye Movement (NREM) sleep selectively increases the consolidation of declarative memory. This technique has been applied successfully in healthy adult students during a night of sleep [1,2], healthy adult students during a nap opportunity [3], schizophrenic patients during a night of sleep [4], and in children with Attention Deficit Hyperactive Disorder (ADHD) [5]. However, one other trial utilizing the technique in healthy older adults did not find an improvement in declarative memory using the same overnight stimulation protocol [6]. In addition to human studies, there have also been several rodent studies with a demonstrated improvement in a surrogate for declarative memory [7,8].
This area of research has been limited by a number of factors including, limited study outside of the original stimulation paradigm (sine wave, or sine wave like wave forms), studies using small sample sizes, and one published negative study. Additionally there has yet to be a published successful replication of the first declarative memory enhancement paper (Marshall et al., 2006) which reported a large effect. We therefore tested whether we could replicate the 2006 manuscript findings using nearly identical methods, with only a few differences. Specific differences included a different stimulator, delivering a divergent current wave-form, and a slightly dissimilar participant population.
Materials and Methods
Overall Study Design and Procedure
This was a randomized, single-blind, counterbalanced, crossover study that was closely adapted to the protocol described by [1]. This protocol was approved by the institutional review board of the Medical University of South Carolina (MUSC), and was conducted under the principles of the declaration of Helsinki.
Participants
We recruited healthy adult students over the age of 18 utilizing email advertisements from the MUSC community. We excluded anyone who was pregnant, had active neurologic, psychiatric, medical, or sleep disorders, or anyone taking any medication. We additionally excluded anyone with a history of seizures, closed head injuries with loss of consciousness, any known brain tumors/lesions, any metal implants/implanted devices above the neck, a history of eczema, or other sensitive skin conditions, an allergy to latex, those who used tobacco, those who used illicit drugs, those who consumed greater than 500mg of caffeine daily, or those who met criteria for alcohol abuse or dependence in their lifetime.
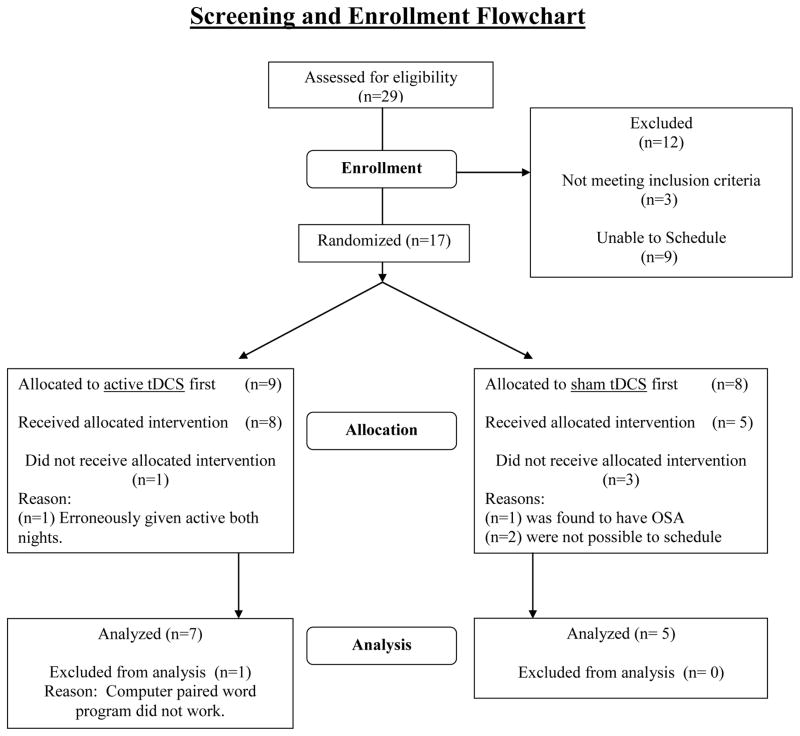
We screened a total of 29 interested individuals, 18 of whom passed the initial phone screen. Of those meeting all inclusion criteria, and failing to meet any exclusion criteria, 17 signed written informed consent, three of which were lost to follow-up, or were unable to find dates that worked with their schedule, and one participant was found to have obstructive sleep apnea on the screening polysomnogram. The remaining sample of 14 enrolled (Figure 1). Two of those participants were excluded from data analysis prior to any data analysis due to technical difficulties. One received active stimulation during both nights, and the other had data that was not usable (There was a computer failure during the morning declarative memory testing session so the participant had already seen the word list, but was unable to have memory testing). The mean age of the 12 remaining participants was 25±2.3, three of whom were men (Table 1). After signing written informed consent approved by the MUSC IRB, subjects received a general medical history and physical exam, as well as a Structured Clinical Interview of the Diagnostic and Statistical Manual (SCID I) for Axis one conditions.
Figure 1.
Consort Flow Chart
Table 1.
Behavioral Task Measures (Mean Value ± Standard Deviation)
| Measure | Active Pre | Active Post | Active Change | Sham Pre | Sham Post | Sham Change | p-Value |
|---|---|---|---|---|---|---|---|
| Paired Word Association Test | 32.8±3.3 | 36.0±4.7 | 3.3±3.0 | 31.2±3.7 | 34.9±4.7 | 3.8±3.1 | p= 0.37 |
| Finger Tap Test (Correctly typed) | 21.7±3.7 | 25.1±3.1 | 3.4±2.7 | 22.1±4.5 | 23.9±4.1 | 2.0±2.2 | p= 0.14 |
| Finger Tap Test (Number of Errors) | 1.9±2.3 | 1.1±1.0 | −0.8±1.7 | 0.9±0.8 | 0.8±0.6 | −0.1±0.9 | p= 0.41 |
| PANAS Positive |
27.5±6.1 | 26.3±6.7 | −1.3±3.6 | 25.8±6.8 | 24.2±6.9 | −2.5±4.3 | p= 0.39 |
| PANAS Negative |
11.3±1.2 | 11.1±2.5 | −0.2±2.3 | 11.6±2.8 | 10.7±1.2 | −0.4±2.4 | p= 0.45 |
| Word Fluency | 6.3±3.0 | 6.8±2.9 | 0.6±2.5 | 7.9±4.4 | 7.9±4.9 | 1.3±3.7 | p= 0.59 |
Following their screening visit, participants underwent an adaptation night in the sleep lab where they were connected to a standard polysomnogram (PSG), but no other intervention was performed. After their adaptation night, participants underwent two additional nights in the sleep lab. One night they received active tDCS stimulation as described below, and the other night they received sham tDCS stimulation. The order of the nights was determined by randomization. There were at least 7 nights in between experimental visits.
Each night in the sleep lab consisted of the following: Participants arrived in the lab between 7 and 8pm. We then applied standard PSG leads including electroencephalogram (EEG) leads at F3′, F4′ (1cm anterior to standard F3 and F4), C3, C4, O1, O2, A1, A2, right and left outer canthi, and sub-mental EMG leads. Following the application of PSG leads, we administered the learning phase of both a paired word association test and a non-dominant hand finger tap test. They were then allowed to go to sleep, and either underwent active or sham tDCS stimulation during early non-REM sleep. After being awakened between 6:30am and 7:00am by the overnight technologist, and given approximately 30 minutes of wake time, we administered the retrieval portion of both the paired word association test and the non-dominant finger tap test. Memory testing was performed as described by Marshal et al. [1].
Stimulation
tDCS was delivered via a battery driven Chattanooga Ionto™ Iontophoresis System – Phoresor. The output of the Iontophoresis device was then modulated via a logic gate controlled by a Windows operating system custom program that alternately oscillated the current between 0 and 0.6 milliamps, at a frequency of 0.75Hz (0.66 s-on/0.66 s-off). The resulting waveform was by definition a squarewave that oscillated between a current density of 0, and 0.517mA/cm2 of maximum current density. Current was delivered through 8mm diameter Ag/AgCl ring electrodes in 1.2cm diameter of 10–20 paste (Weaver). The current was split and delivered bilaterally with anodes at F3, and F4, and cathodes at mastoids (A1, A2), using the international 10–20 system of electrode placement. Current output was verified using both a multimeter, as well as an oscilloscope. When receiving active stimulation, current was delivered in five, five-minute blocks with one-minute inter-stimulation intervals, occurring after four consecutive minutes (8 standard PSG epochs) of unequivocal stage N2, or N3 sleep. Stage of sleep was scored online by GLS who has extensive experience with polysomnography and is a registered polysomnographic technologist (RPSGT). Stimulation delivery and frequency was confirmed by a strong stimulation artifact observed in the sleep EEG. When receiving sham stimulation, electrodes were fastened to the subject but were not connected to a device. Participants were blinded to condition, however neither the technical staff, nor the administrators of the memory testing were blinded to condition. Participants were asked to guess their condition following each night of the procedure.
EEG data analysis
EEG was recorded using a Compumedics Profusion PSG3 system. We recorded eight channels with a sampling rate of 256 Hz. EEG analysis was performed in two different fashions and is similar to the analysis performed in the original 2006 manuscript. First we removed epochs of sleep obtained during stimulation, and the corresponding epochs during the sham night (due to artifact during stimulation). Next we visually scored both active and sham night polysomnograms using standard scoring criteria [9]. Following visual scoring Fast Fourier Transformation (FFT) of the one minute prior to stimulation as well as the 5 interstimulation intervals (50 seconds each) of EEG were performed for the signal obtained from the EEG lead F3′ (1cm anterior to the EEG coordinate F3). For analysis we included the longest block of artifact free EEG (Ranging from 35–60 seconds, average 55.7 seconds each). The FFT window used for analysis was 1000 data points (approximately 4 seconds). Power spectra were separated into bands consistent with the 2006 manuscript (0.5–1Hz, 1–4Hz, 4–8Hz, 8–12Hz, 12–15Hz, 15–25Hz). For statistical reasons mean power for the 1 min prior to stimulation was subtracted from each 1-minute interstimulation interval.
Statistical analysis
We report means and standard deviations to describe the sample. Due to the small sample size, we used a non-parametric Wilcoxon signed rank test to examine behavioral outcomes as well as visually scored sleep variables. For EEG spectral analysis data a within subject repeated measures ANOVA was applied.
Results
Memory testing
Please see table 1 for memory testing data.
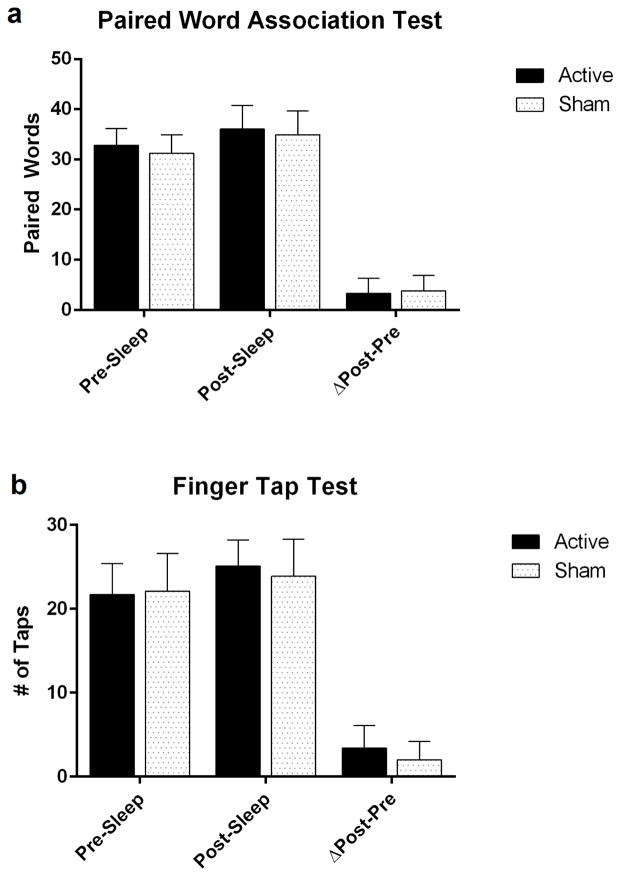
Paired word association test: In the active stimulation condition, the number of paired words recalled in the morning increased 3.1±3.0 words from the baseline-learning phase of the experiment, whereas the sham condition increased 3.8±3.1 words (Figure 2a). Non-dominant finger tapping test: The number of correctly typed sequences increased an average of 3.4±2.7 in the active stimulation condition, and 2.0±2.2 in the sham condition (Figure 2b).
Figure 2.
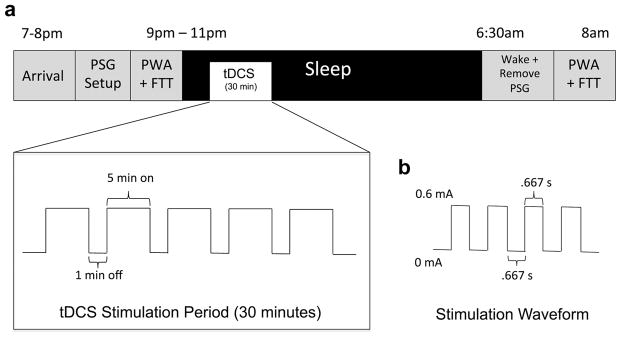
Study Night Time Line
Description:
a. This timeline represents the nightly order of events.
b. A square tDCS waveform oscillating between 0mA and 0.6mA was generated by the constant current stimulator and delivered via 1.2cm diameter electrodes resulting in a maximum current density of 0.517mA/cm2. The frequency of stimulation was .75Hz (.667s on, .667 off).
EEG data
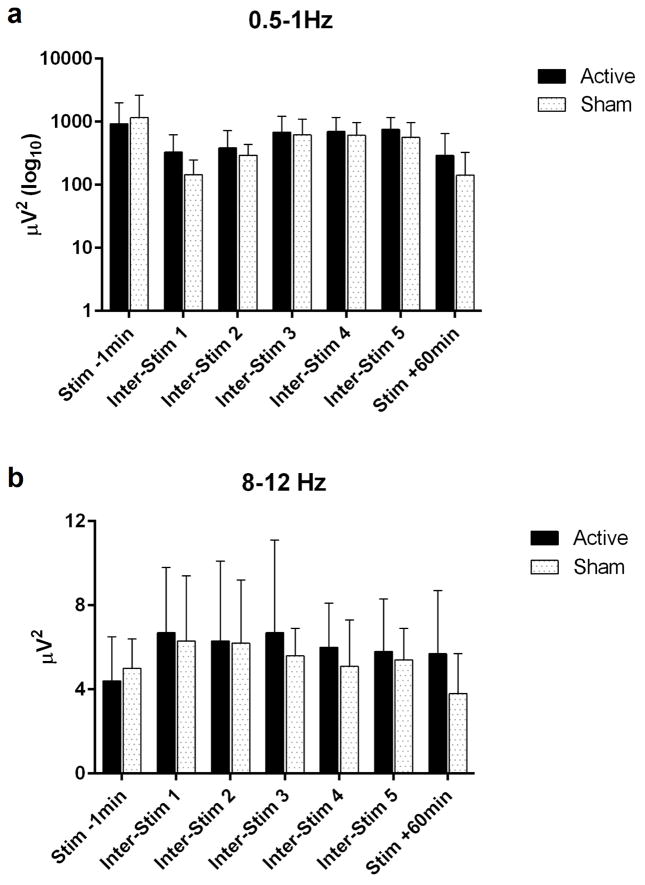
Please see table 2 for sleep EEG data. There was a small significant difference (p=.04) in stage 3 NREM in the sleep EEG 1 hour following stimulation as compared to sham stimulation (active=6.1±4.3 minutes, 10.1±7.1% vs sham=3.8±2.8 minutes, 6.3±4.7%). Mathematical but not statistically significant differences were seen in the power spectra bins of 0.5–1Hz (slow delta), as well as in 8–12Hz (slow frontal spindle range) in the F3′ recording electrode (see figures 3a,3b), with an apparent increase in each of these bins in the inter-stimulation intervals of the active stimulation night compared to the sham night (See table 3).
Table 2.
Sleep Efficiency and Stages
| Active (Entire night*) | Sham (Entire Night*) | p---Value | Active (Hour following Stimulation) | Sham (Hour Following Stimulation) | p---Value | |
|---|---|---|---|---|---|---|
| Sleep Efficiency | 87.3±4.2SD | 85.5±6.4SD | Ns | 94.3±8.7SD | 95.1±8.1SD | Ns |
| Total Sleep Time (Mins) | 402±35SD | 379.3±37.2 | Ns | 56.6±5.2SD | 57.0±4.8SD | Ns |
| NREM Stage 1 (%) | 2.5±1.0SD | 2.7±1.7 | Ns | 1.9±3.8SD | 1.9±2.4SD | Ns |
| NREM Stage 2 (%) | 50.2±7.3SD | 54.6±8.8 | Ns | 35.3±13.4SD | 39.0±19.0SD | Ns |
| NREM Stage 3 (%) | 7.0±2.6SD | 6.6±2.6 | Ns | 10.1±7.1SD | 6.3±4.7SD | P=.04 |
| NREM Stage 4 (%) | 17.4±5.8SD | 15.4±5.5 | Ns | 39.3±22.0SD | 35.9±20.7SD | Ns |
| NREM SWS (%) | 24.4±6.7SD | 21.9±6.9 | Ns | 49.4±16.9SD | 42.2±19.2SD | Ns |
| REM Sleep (%) | 23.9±5.0SD | 20.7±5.2 | Ns | 7.7±10.3SD | 11.9±13.7SD | Ns |
Figure 3.
Paired Word Association Test (PWAT) graph & Finger Tap Test (FTT) graph
Table 3.
QEEG Power Data (Mean Value ± Standard Deviation) μV2
| Frequency Range |
Condition | 1 min Prior to Stimulation |
Inter- stimulation interval 1 |
Inter- stimulation interval 2 |
Inter- stimulation interval 3 |
Inter- stimulation interval 4 |
Inter- stimulation interval 5 |
60 min post- Stimulation |
p-Value |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 – 1.0 Hz | Active | 932.5±10614.5 | 331.1±291.5 | 383.2±342.1 | 686.3±534.5 | 701.5±464.1 | 756.2±405.3 | 292.6±359.9 | ns |
| Sham | 1159.3±1475.3 | 144.8±101.1 | 294.5±143.1 | 620.1±468.6 | 611.1±357.8 | 562.6±412.6 | 141.8±185.6 | ns | |
| 1.0 – 1.5 Hz | Active | 601.0±599.0 | 251.5±123.7 | 323.6±191.6 | 571.2±271.5 | 661.6±507.3 | 958.8±665.8 | 253.2±279.7 | ns |
| Sham | 481.2±296.0 | 184.2±137.9 | 368.4±189.3 | 663.0±410.3 | 621.1±399.1 | 781.0±953.2 | 191.7±298.4 | ns | |
| 1.0 – 4.0 Hz | Active | 186.3±170.1 | 103.7±47.4 | 128.0±71.3 | 202.0±100.1 | 214.1±138.6 | 288.9±154.0 | 94.6±73.2 | ns |
| Sham | 153.7±90.3 | 79.1±51.5 | 141.3±63.9 | 226.6±123.6 | 222.2±134.7 | 260.1±283.0 | 78.7±102.0 | ns | |
| 4 – 8 Hz | Active | 12.74±5.8 | 18.3±9.2 | 16.4±6.5 | 18.2±8.0 | 18.3±6.2 | 19.3±4.7 | 13.8±3.8 | ns |
| Sham | 13.1±3.1 | 14.3±4.0 | 15.4±5.4 | 18.3±6.2 | 17.5±6.1 | 15.9±6.7 | 11.6±4.7 | ns | |
| 8 – 12 Hz | Active | 4.4±2.1 | 6.7±3.1 | 6.3±3.8 | 6.7±4.4 | 6.0±2.1 | 5.8±2.5 | 5.7±3.0 | ns |
| Sham | 5.0±1.4 | 6.3±3.1 | 6.2±3.0 | 5.6±1.3 | 5.1±2.2 | 5.4±1.5 | 3.8±1.9 | ns | |
| 12 – 15 Hz | Active | 3.0±1.9 | 4.8±2.0 | 4.0±2.6 | 4.5±4.2 | 4.0±2.5 | 3.7±2.1 | 5.3±3.7 | ns |
| Sham | 4.2±3.7 | 6.0±3.4 | 4.5±2.8 | 3.1±1.4 | 3.4±2.3 | 3.6±2.0 | 3.4±2.4 | ns | |
| 15 – 25 Hz | Active | 0.7±0.6 | 0.8±0.5 | 0.4±0.2 | 1.1±2.5 | 0.5±0.2 | 0.3±0.1 | 0.7±0.4 | ns |
| Sham | 0.7±0.2 | 0.6±0.4 | 0.4±0.1 | 0.3±0.1 | 0.4±0.2 | 0.4±0.2 | 0.7±0.4 | ns |
All conditions failed to achieve statistical significance
Blinding
5 out of 12 (With a confidence of 0.8/4 ± 1.0SD on a Likert scale) participants were able to correctly guess both conditions on the morning following stimulation.
Discussion
It is a provocative notion to externally synchronize direct current stimulation with ongoing brain activity in order to boost brain activity. There have been several examples of this in the human and animal literature. An initial study in this field was a 2006 manuscript which found that healthy medical students who received active synchronized slow oscillating tDCS during slow wave sleep (when declarative memory consolidation occurs) were able to retrieve more word pairs the following morning compared to when they received sham stimulation. We sought to test whether these results were able to replicate given the lack of follow-up studies in the literature. With similar but not identical methods we failed to find a beneficial effect of active greater than sham stimulation, and in fact found a small negative effect of stimulation despite finding similar EEG differences. Although our sample was small, it was nearly as large as the original study. Even with this small of a sample, we can reasonably conclude that a large effect does not exist with these methods and in healthy students. Given the consistently found improvements in pathological groups as well as two trials in animal models [4,5,7,8] it is becoming increasingly more likely that an effect of stimulation exists on declarative memory consolidation. It is however particularly interesting that now two trials experimenting with healthy participants failed to find en effect [6]. In our current trial we were able to demonstrate that externally delivered oscillating current is able to reach the brain and induce similar EEG changes (mathematical but not statistically significant increases in frontal slow delta activity, and slow frontal spindle frequencies in active but not sham stimulation). Despite these changes in brain activity thought to correlate with improved declarative memory consolidation, we did not observe the expected behavioral effect. We discuss briefly the few ways in which this trial differs from the 2006 manuscript, and how the differences might affect the outcome (See table 4). It is not uncommon for brain stimulation methods to be able to reverse or treat a pathological state, while failing to improve peak performance in health. Perhaps that is the case with slow wave tDCS during sleep and memory consolidation.
Table 4.
Experimental Procedure Differences
| Experimental Difference | Marshal et al 2006 | Current Experiment |
|---|---|---|
| Device | neuroConn DC Stimulator (neuroConn GmbH, Germany) | Chattanooga Ionto™ device (DJO Global, USA) with custom built logic gate driven by a Windows laptop |
| Number of Drivers | Two synchronized drivers | One driver split into two parallel circuits |
| Stimulation waveform | Sine wave | Square wave |
| Conduction paste | Not reported | Ten20 Paste (Weaver, USA) |
| Student Population | Medical students | Medical and other graduate students |
| Language | German | English |
| Nationality | German | American |
| Behavioral Tests | Paired word, Finger Tap Test, PANAS, EWL, Digit Span, Word Fluency Task. | Paired word, Finger Tap Test, PANAS, Word Fluency Task. |
| Blinding | Double blind | Single blind |
| QEEG data recording site | Fz | One cm anterior to F3 |
In our experiment we stimulated using a direct current device that was controlled by a logic gate resulting in a square wave, rather than the near-sine wave used in the original 2006 manuscript. At this time it is unclear whether there is a difference in the physiologic effect of square wave stimulation as opposed to near-sine wave stimulation. Even if there is a physiological difference due to wave form, it is unlikely that square wave stimulation would result in a true square wave reaching neural tissue, as any direct electrical current is likely smoothed out while passing through the scalp, skull, and cerebral spinal fluid. Unless square wave stimulation is in some way disruptive to memory consolidation we would have still expected to find a positive effect on memory consolidation (though less) as even in earlier work performed by the same group using continuous direct current stimulation cycled on and off at 30 second intervals resulted in a positive effect in declarative memory consolidation [2]. This notion is supported by basic science work that implies that continuous direct stimulation results in increased power spectra of neuronal networks, and alternating current stimulation at or near the endogenous frequency results in a larger increase in power spectra [10,11]. Other recent work demonstrated that total charge delivered may be more important than wave form when comparing the effect of tDCS to slow oscillating tDCS on motor evoked potentials[12]. We delivered nearly the same total charge with our square wave stimulation as compared to near-sine wave stimulation, subsequently if total charge is the critical component to effect, we would have expected the same result. If wave form is a critical component and there is a medium effect with intermittent direct stimulation, and a large effect of slow oscillating direct stimulation, we would certainly not expect a small negative effect of square wave stimulation. This is particularly likely in light of our findings that stimulation resulted in a mathematical difference in frontal EEG activity as described above. It still remains unclear however whether this altered the results of our experiment.
In addition to wave-form there are also additional differences in our stimulation methods compared to theirs. We used a different device (Chattanooga Ionto™ Iontophoresis System – Phoresor device with a logic gate as a current regulator) as opposed to the device used in the original 2006 study, and the neuroConn device used in the other cited studies. Furthermore the other studies discussed used two drivers that were externally synched whereas we used a single driver that delivered the current in parallel. We believe neither of these differences were critical differences in the stimulation paradigm. First, we were able to verify current output and waveform using an oscilloscope, which demonstrated we were able to deliver the correct current output bilaterally with the same waveform. Additionally we are confident that the device was able to consistently produce constant current during sleep. This was determined by the observable artifact on the sleep EEG that was of the expected frequency. Finally as described above we were able to produce mathematical increases in EEG power spectra during inter stimulation intervals in the previously described frequency bins of 0.5–1.0Hz (Slow oscillations) and 8–12Hz (Slow Frontal Spindles) during active but not sham stimulation. We subsequently believe this to be sufficient evidence to conclude that the current penetrated the skull and stimulated the cortex, resulting in a similar, although smaller, effect on cortical activity than the findings of the earlier manuscript. One possible explanation for the observed EEG effects without corresponding behavioral effects would be that there is a non-linear response to stimulation where there requires a certain threshold of stimulation to exert an effect (rather than a dose response effect). Subsequently if our stimulation parameters produced a less robust effect on cortical networks we may not have produced enough of a change to result in the expected behavioral effect.
Another difference in our protocol compared to the original manuscript is the subject population. In their study they recruited exclusively German medical students, whereas we recruited American medical students as well as American students from other graduate programs including doctoral and masters level students. Our patient population had slightly more women than theirs (They had 7 women out of 13, and we had 9 women out of 12). We would not expect either of these variables to alter the effects of stimulation, as it is unlikely that there would be significant differences in baseline declarative memory, or socioeconomic status. As the population is much the same in other respects, we generally would not expect this to explain the differential response we found. It is however possible that either the students tested in the initial experiment were more sleep deprived, or they had a higher baseline level of declarative based learning throughout the day (medical students are known to sleep less than the normal population, and medical school is particularly rigorous and includes a great deal of declarative learning).
The main other variable that differed between our experiment and the original experiment in 2006 was blinding. In their experiment they were able to have a true double blind, whereas in our experiment the participant was blinded, but the memory-testing administrator was not. An unblinded investigator could certainly have an influence on the performance of a participant, however the testing was strictly objective. Subsequently we would not expect our unblinded administrator to negatively influence the results of this experiment.
Although far from definitive, our trial demonstrates the need for further study in this area. Optimally a future trial would include a group that was randomized to receive either square wave oscillating tDCS, or near-sine wave tDCS delivered according to this protocol, and sham. Such a trial would have the dual advantage of both testing the initial study for replication with an identical device, and to determine if the waveform is a critical component. Additionally an experiment with a homogenous group of medical students, as well as healthy young adults, would tease out the impact of healthy participant population.
Conclusions
In our small trial we failed to find an effect of 0.75Hz square wave oscillating transcranial Direct Stimulation (tDCS) delivered during early Non Rapid Eye Movement Sleep (NREM) on declarative memory consolidation. It is unclear if the waveform is critical for an effect, or if the effect observed in a previous trial is smaller than initially thought in a young healthy population.
Figure 4.
EEG Data
Highlights.
Previous published trials have found a beneficial effect on declarative memory consolidation when Slow Oscillating transcranial Direct Current Stimulation (SOtDCS) is applied during Slow Wave Sleep (SWS). Follow-up studies have demonstrated an effect in pathologic groups, to date, however there have been no published papers replicating the original findings in healthy participants.
Using similar methods we sought to test the initial findings that SOtDCS delivered during SWS would improve declarative memory consolidation in healthy participants for replication.
In our cohort of healthy students we failed to find an effect.
Acknowledgments
Support: NIDA R25 DA020537-06 (PI’s Back and Brady), Medical University of South Carolina, Department of Psychiatry Sleep and Anxiety Treatment, and Research program.
We would like to thank the MUSC Sleep and Anxiety Treatment and Research Program for all of their help in the development, and implementation of this project. We would also like to thank the MUSC Drug and Alcohol Research Training Program with special thanks to Nicky Thornley and Drs. Sudie Back, Sarah Book, and Kathleen Brady along with the grant that supports it (NIDA R25 DA020537-06 (PI’s Back and Brady)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 2.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonenko D, Diekelmann S, Olsen C, Born J, Molle M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37:1142–1151. doi: 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- 4.Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, et al. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144:153–154. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, et al. Transcranial Oscillatory Direct Current Stimulation During Sleep Improves Declarative Memory Consolidation in Children With Attention-deficit/hyperactivity Disorder to a Level Comparable to Healthy Controls. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, et al. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimul. 2013;6:938–945. doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Binder S, Berg K, Gasca F, Lafon B, Parra LC, et al. Transcranial Slow Oscillation Stimulation During Sleep Enhances Memory Consolidation in Rats. Brain Stimul. 2014;7:508–515. doi: 10.1016/j.brs.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Binder S, Rawohl J, Born J, Marshall L. Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats. Front Behav Neurosci. 2014;7:220. doi: 10.3389/fnbeh.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechtschaffen AKA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NIH Publ US Government Printing Office; Washington: 1968. p. 204. [Google Scholar]
- 10.Schmidt SF, Fröhlich F. Brain Stimulation by Network Resonance with Weak Electric Fields Probed by Optogenetics in Vitro. IEEE; [Google Scholar]
- 11.Ali MM, Sellers KK, Frohlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33:11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groppa S, Bergmann TO, Siems C, Molle M, Marshall L, et al. Slow-oscillatory transcranial direct current stimulation can induce bidirectional shifts in motor cortical excitability in awake humans. Neuroscience. 2010;166:1219–1225. doi: 10.1016/j.neuroscience.2010.01.019. [DOI] [PubMed] [Google Scholar]