Summary
Domestication is an excellent model for studies of adaptation because it involves recent and strong selection on a few, identified traits [1–5]. Few studies have focused on the domestication of fungi, with notable exceptions [6–11], despite their importance to bioindustry [12] and to a general understanding of adaptation in eukaryotes [5]. Penicillium fungi are ubiquitous molds among which two distantly related species have been independently selected for cheese making—P. roqueforti for blue cheeses like Roquefort and P. camemberti for soft cheeses like Camembert. The selected traits include morphology, aromatic profile, lipolytic and proteolytic activities, and ability to grow at low temperatures, in a matrix containing bacterial and fungal competitors [13–15]. By comparing the genomes of ten Penicillium species, we show that adaptation to cheese was associated with multiple recent horizontal transfers of large genomic regions carrying crucial metabolic genes. We identified seven horizontally transferred regions (HTRs) spanning more than 10 kb each, flanked by specific transposable elements, and displaying nearly 100% identity between distant Penicillium species. Two HTRs carried genes with functions involved in the utilization of cheese nutrients or competition and were found nearly identical in multiple strains and species of cheese-associated Penicillium fungi, indicating recent selective sweeps; they were experimentally associated with faster growth and greater competitiveness on cheese and contained genes highly expressed in the early stage of cheese maturation. These findings have industrial and food safety implications and improve our understanding of the processes of adaptation to rapid environmental changes.
Keywords: convergence, parallel adaptation, Wallaby, CheesyTer, Penicillium, HGT, food spoiler, gene expression
Highlights
-
•
New HTRs are found in cheese fungi
-
•
HTRs are flanked by specific transposable elements
-
•
HTRs have spread in cheese-associated fungi through recent selective sweeps
-
•
Experiments link two HTRs to growth and competitive advantages on cheese
Ropars et al. report newly discovered horizontally transferred regions, flanked by specific transposable elements that allow cheese-making fungi and food spoilers to grow faster and be better competitors on cheese. These findings have industrial and food safety implications and also improve our understanding of adaptation processes.
Results and Discussion
Multiple Recent Horizontal Gene Transfers between Distant Penicillium Species, Flanked by Specific Retrotransposons
We report here five newly sequenced and assembled Penicillium genomes, which we compared with previously available data [16–19]. The full dataset included the genome sequences of ten Penicillium species, six of which are either used as industrial inocula for cheese making (Penicillium roqueforti and Penicillium camemberti) or occur as contaminants in cheeses (Figure 1; Table S1). P. camemberti is only found in cheese and is thought to include a single clonal lineage originating from selection programs at the end of the 19th century from the blue-gray cheese molds used at that time, i.e., Penicillium biforme and Penicillium fuscoglaucum [20, 21]. By contrast, P. roqueforti also occurs in habitats other than cheese, such as silage or wood, and displays substantial genetic diversity [22, 23]. For reconstructing a rooted phylogeny of these ten Penicillium species, we used four Aspergillus species as an outgroup. We concatenated alignments of 3,089 single-copy genes shared by at least ten species and reconstructed a fully resolved and well-supported maximum-likelihood phylogeny (Figures 1A and 1B).
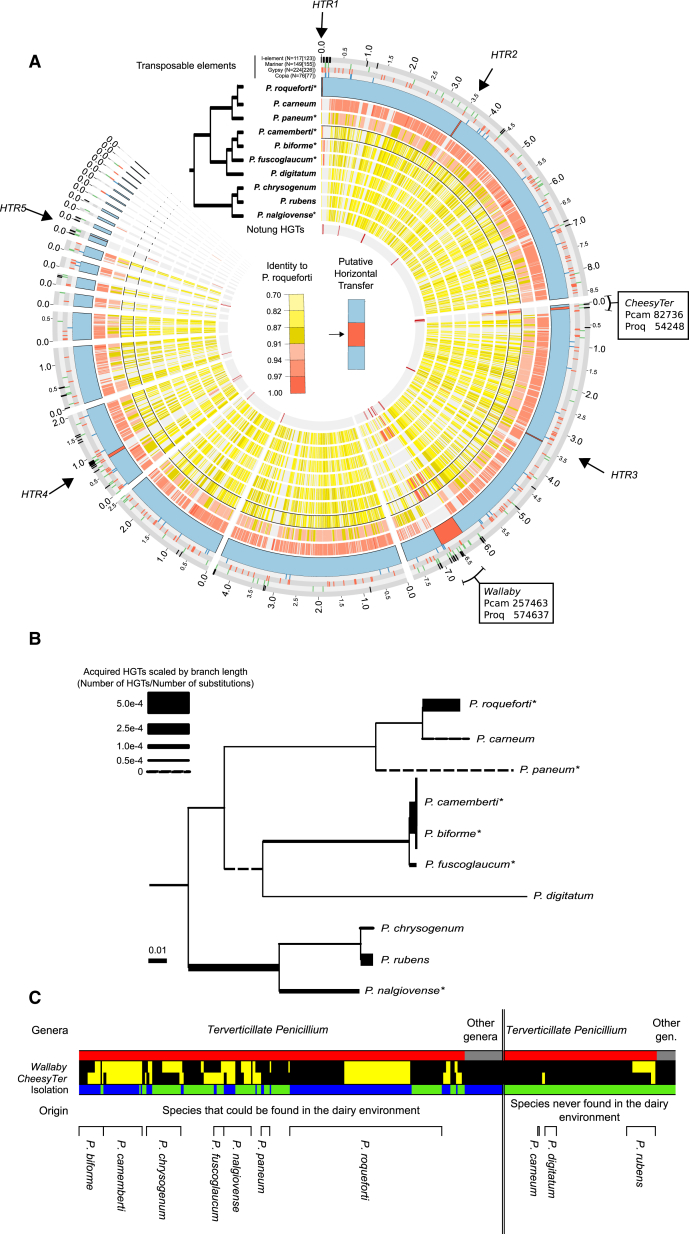
Figure 1.
Horizontal Gene Transfers between Penicillium Fungi
(A) The syntenic blocks in Penicillium genomes larger than 10,000 bp are shown as heat-colored circles, aligned against the 23 P. roqueforti scaffolds that are larger than 10,000 bp (outer blue circle). The percentage of identity to the P. roqueforti genome is indicated by heat colors, from yellow for low identity level to red for high identity level. The seven large regions represented in red on the P. roqueforti outer circle (blue otherwise) display levels of identity above 97% between several distantly related species, while being absent from others, and are indicated as horizontally transferred regions (HTRs) numbers 1 to 5, Wallaby and CheesyTer, respectively. These regions are also characterized by the clustering of i LINE retrotransposons at their edges; the four most abundant transposable element families are shown on the four outermost gray circles. The red bars in the inner circle indicate the location of the horizontal gene transfer (HGT) events inferred by Notung. The topology of the species phylogeny obtained with 100% bootstrap support based on 3,089 single-copy genes is represented. Asterisks indicate the strains collected from cheese.
(B) Phylogenetic tree of Penicillium fungi based on the 3,089 single-copy genes, with branch lengths as estimated by RAxML. The asterisks are as in (A). Branch widths in the tree are proportional to HGT acquisition rate, i.e., number of genes acquired by HGT as inferred by Notung divided by the number of substitutions along the branch.
(C) Presence of Wallaby and CheesyTer in 416 strains from 65 fungal species. Each column represents a strain; positive (for at least one primer pair) and negative (for all three primer pairs) PCR amplifications are indicated in yellow and black, respectively. Species are ordered by origin (i.e., dairy environment above the blue line versus other environments above the green line) and by taxon (i.e., terverticillate Penicillium below the red lines versus other genera below gray lines).
We used this rooted phylogeny to investigate the occurrence of horizontal gene transfers (HGTs) between Penicillium species. As HGTs (also known as xenology [24]) result in incongruences between gene genealogies and species trees, we compared all individual gene genealogies with the species tree. For this goal, we used the Notung software [25–27] to infer the number of duplication, loss, and HGT events that reconciled the gene genealogies with the species tree. Notung is conservative regarding the inference of HGTs because it tests their temporal feasibility, assumes that HGTs occur with a low probability, and forces the poorly supported nodes to follow the species tree. Only orthologous groups with at least one homolog in at least eight genomes were analyzed, further rendering our estimates of HGTs a lower bound. We found overall 104 HGTs between Penicillium species, distributed among 77 orthologous groups. Notung inferred the highest number of HGTs relative to branch length in the clade encompassing P. camemberti, P. biforme, and their common ancestor (Figure 1B). P. roqueforti also acquired many xenologs relative to its branch length. 8 of the 21 horizontally acquired genes detected in P. roqueforti were inferred to come from P. camemberti, P. biforme, or their common ancestor, indicating recent transfers from species sharing the same ecological niche (Figure S1). Only five of these eight genes could be assigned putative functions, i.e., a protein kinase, two transcription factors, a cation transporter, and an integrase-like protein. Cation transport seems particularly relevant for growth in cheeses as several ions are limiting in this medium (e.g., iron ions), and pH drastically drops during cheese maturation [28].
Another line of evidence for the abundance of HGTs in Penicillium fungi came from the finding of multiple large genomic islands that were almost 100% identical at the nucleotidic level between distant species, while being absent from closely related species (Figure 1A). The only substitutions detected in these genomic islands corresponded to repeat-induced point mutations, i.e., C-to-T transitions induced by a specific fungal defense mechanism against transposable elements (TEs) that can substitute multiple base pairs in a single meiosis [29]. In P. roqueforti, for example, seven genomic islands larger than 10 kb and displaying above 97% nucleotide identity with multiple other species were found. Only the largest region had previously been identified and was called Wallaby [16]. Such a high level of identity suggests that these genomic islands correspond to recent horizontally transferred regions (HTRs), although they could alternatively be recent introgressions. Two lines of evidence, however, support the HTR hypothesis rather than introgression: (1) the presence of several of these regions at non-homologous locations in the different Penicillium genomes (Figure S2; [16]) and (2) the low mean genome sequence identity between the Penicillium species sharing these regions, being less than 90%, an identity level at which no successful interspecific crosses have ever been reported in fungi [30]. Interestingly, these HTRs were flanked in P. roqueforti by copies of TEs from a particular family that were rare elsewhere in the genomes (Figures 1A and S3), the i non-LTR retrotransposons [6]. The other abundant TEs (e.g., mariner DNA transposons and copia retrotransposons) were in contrast scattered in the genomes (Figure 1A). The genes present in these genomic islands of high sequence similarity partially overlapped with the HGTs detected by Notung. In P. roqueforti for example, 17% of the HGTs inferred by Notung were located in the seven large HTRs (Figure 1A). The fact that not all genes in HTRs were detected by Notung mainly results from the filter of this analysis where we used only orthologous groups with homologs in at least eight species. Further, 11% of the inferred HGTs in P. roqueforti clustered within 50 kb of the HTRs (Figure 1A), suggesting that these genomic regions may be prone to integrate foreign DNA. This is consistent with the previous finding that the genomic region where Wallaby is inserted in P. roqueforti carries other species-specific genes in each of P. camemberti, P. rubens, and P. roqueforti [16].
The identification of multiple very recent HTRs, with almost 100% identity in multiple species (Figure 1A), together with Notung inferences of horizontally transferred genes occurring also elsewhere in the genomes (at least 104 in total among Penicillium fungi), indicates that HGTs occur frequently among Penicillium. The clustering of i elements in the flanking regions of HTRs suggests that they may be involved in the mechanism of horizontal transfers. It has been shown that TEs can pass across species boundaries [31] and that they promote genomic rearrangements and recombination [32–34]. The capacity for mycelia fusions may also facilitate the exchange of genetic material in fungi [35].
Two Horizontally Transferred Genomic Regions Are Likely Involved in Cheese Adaptation and Have Spread in Cheese-Associated Penicillium through Recent Selective Sweeps
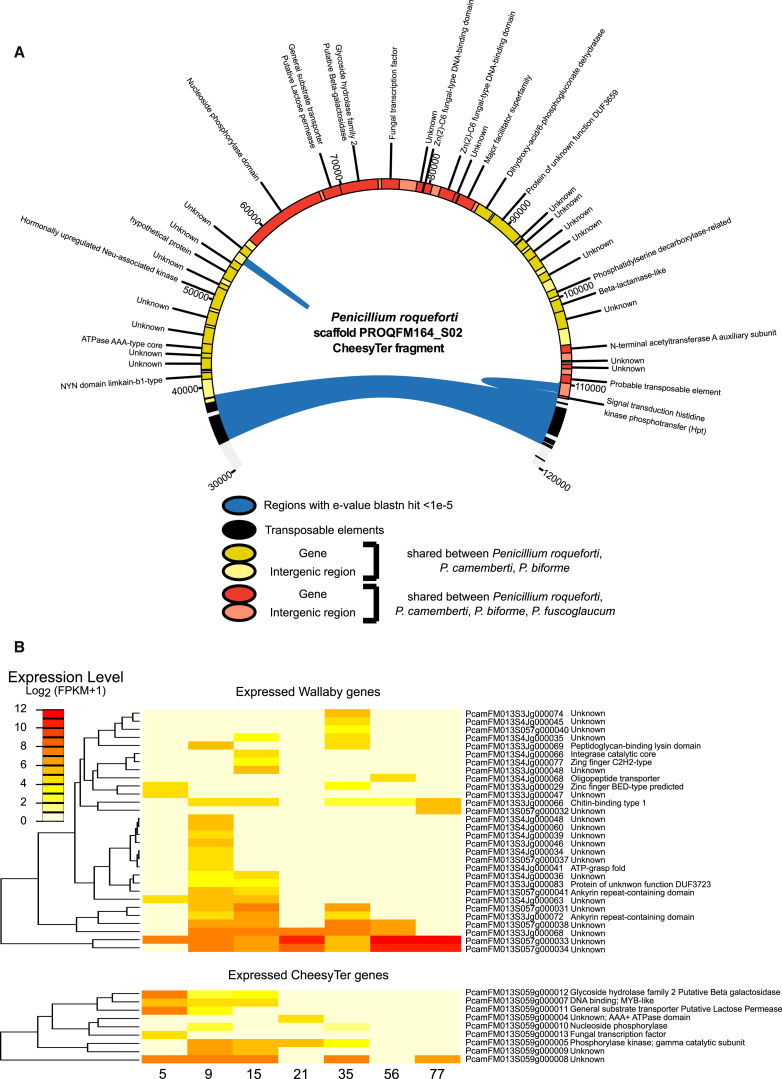
Five of the seven large HTRs detected in P. roqueforti were shared between Penicillium strains isolated from cheese, P. camemberti carrying four of them (Figure 1A). Two HTRs appeared of special relevance for cheese adaptation. Wallaby [16] carries a gene encoding an antifungal protein, known for inhibiting the growth of competitors. The second largest HTR was found at terminal edges of scaffolds and was therefore named CheesyTer. This 80-kb region, found as a single block in all the genomes studied, carried 37 putative genes, among which two had relevant putative functions for adaptation to cheese, i.e., lactose permease and beta-galactosidase (Figure 2A). Lactose is present during the first few days of cheese maturation, and it acts as a primary carbon source, being rapidly consumed by lactic acid bacteria [37]. The two lactose metabolism genes present in CheesyTer were among the most strongly expressed in P. camemberti during the first step of cheese rind maturation, during which lactose is available (Figure 2B; Table S2; Supplemental Experimental Procedures), indicating a role in the use of the cheese substrate.
Figure 2.
Structure of CheesyTer and Gene Expression of Wallaby and CheesyTer in P. camemberti
(A) Structure of the CheesyTer island in P. roqueforti (scaffold PROQFM164_S02 from 30,000 bp to 120,000 bp). CheesyTer is entirely shared and syntenic between P. roqueforti, P. biforme, and P. camemberti. The region in P. fuscoglaucum lacks some fragments (shown in yellow) but is syntenic otherwise (fragments shown in red). The putative functions of the genes are shown. CheesyTer is flanked by i transposable elements, represented in black, showing a high level of identity (e value < 1e−5). This suggests that they are recently duplicated copies.
(B) Expression of Wallaby and CheesyTer genes in P. camemberti during the first 77 days of cheese rind maturation in industrial Camembert, represented as a heatmap of log2(FPKM+1), a measure of transcript abundance (fragments per kilobase of exon per million fragments mapped). These data were generated in a previous study [36]. The putative functions of the genes are shown; the two genes of CheesyTer whose functions are likely involved in lactose metabolism, i.e., the putative lactose permease and beta-galactosidase, are highlighted in gray.
We investigated the presence of CheesyTer and Wallaby by PCR in 416 strains from 65 fungal species from various environments (Figure 1C; Table S3; Supplemental Experimental Procedures). The presence of the transfers was found highly significantly associated with dairy environment both among species (χ2 = 55.7; degrees of freedom [df] = 1; p value = 8.571e−14) and among strains within species (χ2 = 45.2; df = 1, p value = 1.774e−11). Amplicons were actually obtained only for Penicillium species that are frequently isolated in the dairy environment, with the only exception of P. rubens, the penicillin-producer fungus, in which 16 strains out of 20 carried either one of the two HTRs. The CheesyTer and Wallaby fragments obtained by PCR showed zero substitution among all strains from all species, including synonymous sites and non-coding regions, as previously found for Wallaby [16]. This result confirms that the presence of CheesyTer and Wallaby is not ancestral in Penicillium species and that these genomic islands have instead been acquired very recently. This also indicates that the two HTRs have spread in several species through recent selective sweeps. P. roqueforti was found polymorphic for the presence of Wallaby and CheesyTer, with all tested strains carrying either both of these regions or neither of them (Table S3). Within P. roqueforti, these regions were present only in strains isolated from the cheese environment, suggesting a role in adaptation to the cheese environment.
The Wallaby and CheesyTer Horizontally Transferred Regions Are Experimentally Associated with Faster Growth and Greater Competitiveness on Cheese
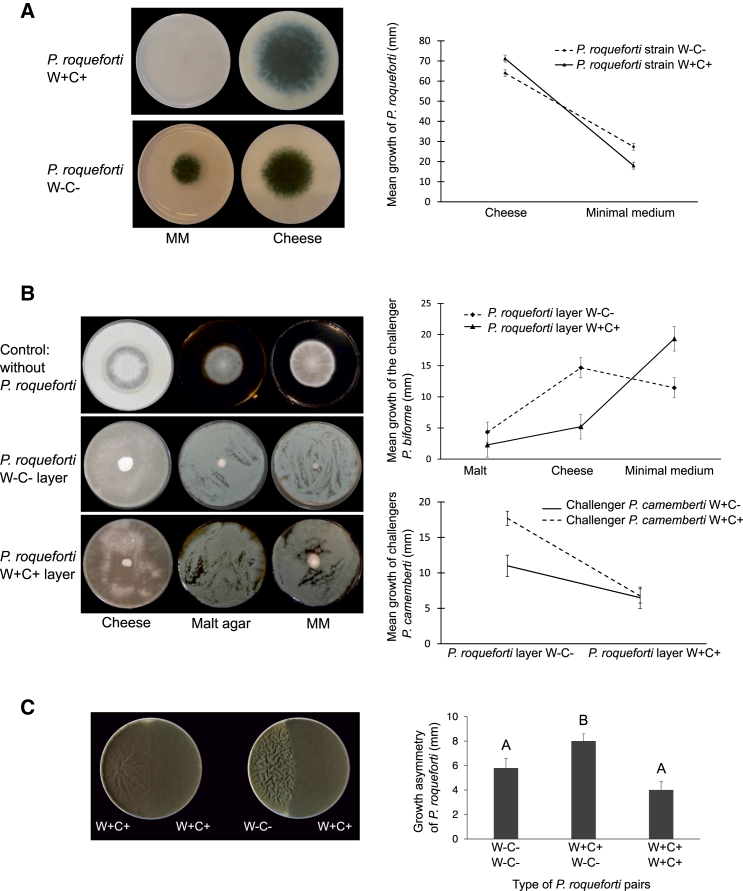
We therefore investigated whether strains carrying Wallaby and CheesyTer showed higher fitness in terms of growth on cheese substrate or for competitiveness. We set up three experiments, focusing on P. roqueforti, because a large collection of strains was available, isolated from various environments, and including strains carrying both Wallaby and CheesyTer (hereafter named W+C+) and strains lacking them (hereafter named W−C−).
We first compared the growth of 50 P. roqueforti strains on a cheese medium and on a minimal medium (26 W+C+ and 24 W−C−; Table S4, tab a). Neither the presence of Wallaby and CheesyTer, as a main effect independent of the medium, nor the origin of the strain (i.e., cheese versus other environments) significantly influenced the growth of P. roqueforti (Table S1). By contrast, the effect on growth of the medium and its interaction with the presence of the two genomic islands were significant (Figure 3; Table S1): W+C+ strains had a growth advantage on cheese medium but a slower growth on minimal medium.
Figure 3.
Fitness Advantages of P. roqueforti Carrying Wallaby and CheesyTer, for the Use of the Cheese Substrate and for Outgrowing Competitors
(A) Left: pictures of two P. roqueforti strains, with (LCP06166, top) and without (LCP06040, bottom) Wallaby and CheesyTer on minimal medium and cheese medium. Right: mean growth ± SE (in mm) of P. roqueforti strains with and without Wallaby and CheesyTer on the two media.
(B) Left: pictures of a P. biforme challenger (LCP05529, without Wallaby and CheesyTer) on two different P. roqueforti lawns, on cheese, malt agar, and minimal media (bottom: strain LCP06149 with Wallaby and CheesyTer; top: strain LCP05885 without the genomic islands; the first line is a control, i.e., with no P. roqueforti lawn). Right: mean growth ± SE (in mm) of a P. biforme (top) or a P. camemberti (bottom) challenger on P. roqueforti strain lawns with or without Wallaby and CheesyTer.
(C) Growth asymmetry (mean ± SE in mm of deviations from the middle of the Petri dish) in pairwise confrontations of P. roqueforti strains with (W+C+) or without (W−C−) Wallaby and CheesyTer, on cheese medium, for the three types of possible pairs. The A and B letters correspond to significantly different means according to a Tukey-Kramer test. The picture shows examples of confrontations, at left LCP06271 (W+C+) against LCP06157 (W+C+) and at right LCP00148 (W+C+) against LCP06157 (W−C−).
Second, we investigated whether P. roqueforti strains carrying Wallaby and CheesyTer had a higher ability to exclude competitors. We measured the growth of three fungal strains belonging to species commonly found in cheese but lacking Wallaby and CheesyTer (P. nalgiovense FM193, P. biforme LCP05529, and Geotrichum candidum FM074) on plates covered with lawns of P. roqueforti either W+C+ (n = 11) or W−C− (n = 12) strains (Table S4, tab b). These experiments were carried out on minimal, cheese, and malt agar media. No difference in growth was detected for the yeast G. candidum between lawns of W+C+ or W−C− P. roqueforti strains (Table S1). By contrast, W+C+ P. roqueforti strains significantly impaired the growth of the two Penicillium challengers on the cheese and malt media (Figure 3B). This was not the case on the minimal medium: the interaction between the presence of the transfers and the medium was significant (Table S1). Using the same experimental design, we then investigated the effect of the two genomic islands when present in the challengers. For this goal, we inoculated on P. roqueforti lawns (W+C+, n = 2, or W−C−, n = 2), on cheese medium, different strains of species displaying a polymorphism in the Wallaby and/or CheesyTer presence (Table S4, tab c). We used as challengers different strains of P. camemberti (W+C−, n = 1, or W+C+, n = 3), P. biforme (W−C−, n = 2, or W+C+, n = 2), and P. rubens (W−C−, n = 1, or W+C−, n = 3). For all three species, we found that the P. roqueforti lawns significantly inhibited the growth of challengers and significantly more so when the P. roqueforti lawn carried Wallaby and CheesyTer. Interestingly, the presence of either CheesyTer or Wallaby in the challengers allowed better growth on W−C− P. roqueforti lawns while neither had significant effect on the growth on W+C+ P. roqueforti lawns (Table S1).
Third, we investigated competition among P. roqueforti strains carrying (W+C+, n = 8) or lacking (W−C−, n = 11) Wallaby and CheesyTer. We grew P. roqueforti strains on cheese medium as pairwise face-to-face confrontations, and we measured the deviations from symmetrical growth (Table S4, tab d; Figure 3C; Supplemental Experimental Procedures). For the W+C+ versus W+C+ confrontations, the mean growth deviation from a boundary in the exact middle of the Petri dish was not significantly different from zero (t test, t = 1.5; df = 36; p value = 0.14). Similar results were obtained for the W−C− versus W−C− confrontations (t test, t = 0.25; df = 70; p value = 0.80). For the W+C+ versus W−C− confrontations, deviations were measured by taking the W+C+ strain as the focal strain; the mean growth deviation was significantly different from zero and positive, the W+C+ strains thus growing farther than the W−C− strain (t test, t = 12.32; df = 90; p value < 0.0001). The mean deviations were significantly higher in the W−C− versus W+C+ confrontations than in the W+C+ versus W+C+ or W−C− versus W−C− confrontations (Tukey-Kramer test, p value < 0.0001), while the means between these two latter were not significantly different. This experiment shows that the competitive advantage of W+C+ strains against W−C− strains also holds within the species P. roqueforti. Altogether, these experimental results strongly support the existence of fitness advantages for the Penicillium strains carrying the horizontally transferred genomic islands, both in the use of cheese substrate and in competition with fungal competitors.
Conclusions
Our present study on domesticated fungi shows how adaptation can occur rapidly in eukaryotes. The two cheese species studied here underwent parallel adaptation to the cheese medium, and this involved the transfers of identical regions across species boundaries. HGT events have been reported in fungi [35, 38, 39], particularly in environments created by humans, in domesticated yeasts and fungal pathogens of crops [10, 40, 41]. The extent and timing of gene transfers and the number of species having received the same HTRs are here particularly striking. Furthermore, we provide experimental evidence of fitness advantages for strains carrying these HTRs on a human-made medium. These findings altogether are potentially useful for guiding modern strain improvement programs. Indeed, together with the protocol for inducing sex in P. roqueforti [22, 42], the identification here of several key candidate genes important for cheese metabolism and competition may allow further selecting interesting traits for cheese industry using the great genetic variability present in P. roqueforti strains without Wallaby or CheesyTer [22]. In addition, our results suggest that caution is required concerning the introduction of genes into microorganisms, as these genes could readily be transferred to other species in the food environment. Indeed, the rapid spread of Wallaby and CheesyTer into many species of the dairy environment, even when occurring only as contaminants, indicates that transgenes may readily cross species boundaries in the food chain. Finally, the findings here of rapid adaptation through frequent horizontal gene transfers among distant species under selection in novel, human-made media contribute to our understanding of the evolutionary genomic mechanisms allowing rapid adaptation to environmental changes in eukaryotes.
Author Contributions
Conceptualization, T.G., J.D., and J.R.; Methodology, A.B., T.G., M.L.-V., R.C.R.d.l.V., and J.R.; Software, A.B. and R.C.R.d.l.V.; Validation, É.D. and S.L.; Formal Analysis, A.B., T.G., M.L.-V., R.C.R.d.l.V., and J.R.; Investigation, A.B., M.L.-V., R.C.R.d.l.V., and J.R.; Resources, R.D., J.D., and J.G.; Data Curation, A.B., J.G., R.C.R.d.l.V., and E.S.; Writing – Original Draft, A.B., T.G., R.C.R.d.l.V., and J.R.; Writing – Review & Editing, A.B., T.G., M.L.-V., R.C.R.d.l.V., and J.R.; Visualization, A.B., M.L.-V., R.C.R.d.l.V., and J.R.; Supervision, A.B. and T.G.; Project Administration, A.B. and T.G.; Funding Acquisition, A.B., J.D., T.G., J.G., and R.C.R.d.l.V.
Acknowledgments
This work was supported by the ANR FROMA-GEN grant (ANR-12-PDOC-0030) awarded to A.B., an “attractivité” grant from Paris-Sud University to A.B., the ERC starting grant GenomeFun 309403 awarded to T.G., a Marie Curie postdoctoral fellowship to R.C.R.d.l.V. (FP7 COFUND PRES-SUD No. 246556), and the ANR grant “Food Microbiomes” (ANR-08-ALIA-007-02) awarded to J.D. We thank Marco van den Berg for sharing raw data for the Penicillium chrysogenum Wisconsin 54-1255 strain (recently renamed P. rubens).
Published: September 24, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, three figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.08.025.
Contributor Information
Antoine Branca, Email: antoine.branca@u-psud.fr.
Tatiana Giraud, Email: tatiana.giraud@u-psud.fr.
Accession Numbers
The accession numbers for the Penicillium genome sequences reported in this paper are GenBank: HG813601–HG814182 for P. biforme FM169; HG816029–HG818118 for P. carneum LCP05634; HG814183–HG815135 for P. fuscoglaucum FM041; HG815136–HG815288 and HG815290–HG816004 for P. nalgiovense FM193; and HG813308–HG813531 for P. paneum FM227.
Supplemental Information
Time-wise expression of Penicillium camemberti genes during cheese rind maturation; these data were generated in a previous study [36].
Presence/absence of the horizontally transferred regions Wallaby and CheesyTer in 416 different isolates from diverse origins belonging to 65 fungal species. Strains isolated from the cheese environment are shown in purple, and positive PCR amplifications are represented in yellow. Type, neotype, and epitype strains are indicated, respectively, with a “T,” a “NeoT,” and a “EpiT” after the strain number. All strains included in this study are available in the public fungal strain collection of the National Museum of Natural History in Paris, except the strains labeled “FM,” corresponding to a private collection provided by French anonymous stakeholders. Only the six FM strains whose genomes are available (e.g., FM013, FM041, FM164, FM169, FM193, and FM227, indicated with an asterisk) have LCP numbers and are publicly available.
Growth measures (in mm) obtained in the fitness experiments. (a) Experiments assessing the ability of using the cheese substrate: growth measures of 50 Penicillium roqueforti strains on minimal medium (MM) and cheese medium. (b) Experiments assessing competitive ability: growth measures of three challengers, i.e., P. biforme, P. nalgiovense, and Geotrichum candidum, inoculated in the middle of Petri dishes on P. roqueforti lawns (n = 23). (c) Experiments assessing competitive ability: growth measures of 12 challengers belonging to P. biforme, P. camemberti, or P. rubens, inoculated on P. roqueforti lawns (2 W+C+ and 2 W−C−). (d) Experiments assessing competitive ability: growth measures of 19 P. roqueforti strains on cheese medium, inoculated pairwise in two opposite points of Petri dishes.
References
- 1.Lin T., Zhu G., Zhang J., Xu X., Yu Q., Zheng Z., Zhang Z., Lun Y., Li S., Wang X. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014;46:1220–1226. doi: 10.1038/ng.3117. [DOI] [PubMed] [Google Scholar]
- 2.Bosse M., Megens H.J., Frantz L.A.F., Madsen O., Larson G., Paudel Y., Duijvesteijn N., Harlizius B., Hagemeijer Y., Crooijmans R.P., Groenen M.A. Genomic analysis reveals selection for Asian genes in European pigs following human-mediated introgression. Nat. Commun. 2014;5:4392. doi: 10.1038/ncomms5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu G.A., Prochnik S., Jenkins J., Salse J., Hellsten U., Murat F., Perrier X., Ruiz M., Scalabrin S., Terol J. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014;32:656–662. doi: 10.1038/nbt.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian F., Stevens N.M., Buckler E.S., 4th Tracking footprints of maize domestication and evidence for a massive selective sweep on chromosome 10. Proc. Natl. Acad. Sci. USA. 2009;106(Suppl 1):9979–9986. doi: 10.1073/pnas.0901122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladieux P., Ropars J., Badouin H., Branca A., Aguileta G., de Vienne D.M., Rodríguez de la Vega R.C., Branco S., Giraud T. Fungal evolutionary genomics provides insight into the mechanisms of adaptive divergence in eukaryotes. Mol. Ecol. 2014;23:753–773. doi: 10.1111/mec.12631. [DOI] [PubMed] [Google Scholar]
- 6.Galagan J.E., Calvo S.E., Cuomo C., Ma L.J., Wortman J.R., Batzoglou S., Lee S.I., Baştürkmen M., Spevak C.C., Clutterbuck J. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 7.Borneman A.R., Desany B.A., Riches D., Affourtit J.P., Forgan A.H., Pretorius I.S., Egholm M., Chambers P.J. Whole-genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1001287. doi: 10.1371/journal.pgen.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons J.G., Salichos L., Slot J.C., Rinker D.C., McGary K.L., King J.G., Klich M.A., Tabb D.L., McDonald W.H., Rokas A. The evolutionary imprint of domestication on genome variation and function of the filamentous fungus Aspergillus oryzae. Curr. Biol. 2012;22:1403–1409. doi: 10.1016/j.cub.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fay J.C., Benavides J.A. Evidence for domesticated and wild populations of Saccharomyces cerevisiae. PLoS Genet. 2005;1:66–71. doi: 10.1371/journal.pgen.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novo M., Bigey F., Beyne E., Galeote V., Gavory F., Mallet S., Cambon B., Legras J.L., Wincker P., Casaregola S., Dequin S. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc. Natl. Acad. Sci. USA. 2009;106:16333–16338. doi: 10.1073/pnas.0904673106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsit S., Mena A., Bigey F., Sauvage F.X., Couloux A., Guy J., Legras J.L., Barrio E., Dequin S., Galeote V. Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Mol. Biol. Evol. 2015;32:1695–1707. doi: 10.1093/molbev/msv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stajich J.E., Berbee M.L., Blackwell M., Hibbett D.S., James T.Y., Spatafora J.W., Taylor J.W. The fungi. Curr. Biol. 2009;19:R840–R845. doi: 10.1016/j.cub.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McSweeney P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004;57:127–144. [Google Scholar]
- 14.Wolfe B.E., Button J.E., Santarelli M., Dutton R.J. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell. 2014;158:422–433. doi: 10.1016/j.cell.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irlinger F., Mounier J. Microbial interactions in cheese: implications for cheese quality and safety. Curr. Opin. Biotechnol. 2009;20:142–148. doi: 10.1016/j.copbio.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Cheeseman K., Ropars J., Renault P., Dupont J., Gouzy J., Branca A., Abraham A.L., Ceppi M., Conseiller E., Debuchy R. Multiple recent horizontal transfers of a large genomic region in cheese making fungi. Nat. Commun. 2014;5:2876. doi: 10.1038/ncomms3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcet-Houben M., Ballester A.R., de la Fuente B., Harries E., Marcos J.F., González-Candelas L., Gabaldón T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genomics. 2012;13:646. doi: 10.1186/1471-2164-13-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerqueira G.C., Arnaud M.B., Inglis D.O., Skrzypek M.S., Binkley G., Simison M., Miyasato S.R., Binkley J., Orvis J., Shah P. The Aspergillus Genome Database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res. 2014;42:D705–D710. doi: 10.1093/nar/gkt1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg M.A., Albang R., Albermann K., Badger J.H., Daran J.M., Driessen A.J.M., Garcia-Estrada C., Fedorova N.D., Harris D.M., Heijne W.H.M. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008;26:1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- 20.Giraud F., Giraud T., Aguileta G., Fournier E., Samson R., Cruaud C., Lacoste S., Ropars J., Tellier A., Dupont J. Microsatellite loci to recognize species for the cheese starter and contaminating strains associated with cheese manufacturing. Int. J. Food Microbiol. 2010;137:204–213. doi: 10.1016/j.ijfoodmicro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Pitt J.I., Cruickshank R.H., Leistner L. Penicillium commune, P. camemberti, the origin of white cheese moulds, and the production of cyclopiazonic acid. Food Microbiol. 1986;3:363–371. [Google Scholar]
- 22.Ropars J., López-Villavicencio M., Dupont J., Snirc A., Gillot G., Coton M., Jany J.L., Coton E., Giraud T. Induction of sexual reproduction and genetic diversity in the cheese fungus Penicillium roqueforti. Evol. Appl. 2014;7:433–441. doi: 10.1111/eva.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillot G., Jany J.-L., Coton M., Le Floch G., Debaets S., Ropars J., López-Villavicencio M., Dupont J., Branca A., Giraud T., Coton E. Insights into Penicillium roqueforti morphological and genetic diversity. PLoS ONE. 2015;10:e0129849. doi: 10.1371/journal.pone.0129849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koonin E.V. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 2005;39:309–338. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- 25.Durand D., Halldórsson B.V., Vernot B. A hybrid micro-macroevolutionary approach to gene tree reconstruction. J. Comput. Biol. 2006;13:320–335. doi: 10.1089/cmb.2006.13.320. [DOI] [PubMed] [Google Scholar]
- 26.Vernot B., Stolzer M., Goldman A., Durand D. Reconciliation with non-binary species trees. J. Comput. Biol. 2008;15:981–1006. doi: 10.1089/cmb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolzer M., Lai H., Xu M., Sathaye D., Vernot B., Durand D. Inferring duplications, losses, transfers and incomplete lineage sorting with nonbinary species trees. Bioinformatics. 2012;28:i409–i415. doi: 10.1093/bioinformatics/bts386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnet C., Landaud S., Bonnarme P., Swennen D. Growth and adaptation of microorganisms on the cheese surface. FEMS Microbiol. Lett. 2015;362:1–9. doi: 10.1093/femsle/fnu025. [DOI] [PubMed] [Google Scholar]
- 29.Galagan J.E., Selker E.U. RIP: the evolutionary cost of genome defense. Trends Genet. 2004;20:417–423. doi: 10.1016/j.tig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Le Gac M., Giraud T. Existence of a pattern of reproductive character displacement in Homobasidiomycota but not in Ascomycota. J. Evol. Biol. 2008;21:761–772. doi: 10.1111/j.1420-9101.2008.01511.x. [DOI] [PubMed] [Google Scholar]
- 31.Schaack S., Gilbert C., Feschotte C. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol. Evol. 2010;25:537–546. doi: 10.1016/j.tree.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baudry C., Malinsky S., Restituito M., Kapusta A., Rosa S., Meyer E., Bétermier M. PiggyMac, a domesticated piggyBac transposase involved in programmed genome rearrangements in the ciliate Paramecium tetraurelia. Genes Dev. 2009;23:2478–2483. doi: 10.1101/gad.547309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L., Mitra R., Atkinson P.W., Hickman A.B., Dyda F., Craig N.L. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature. 2004;432:995–1001. doi: 10.1038/nature03157. [DOI] [PubMed] [Google Scholar]
- 34.Rajaei N., Chiruvella K.K., Lin F., Aström S.U. Domesticated transposase Kat1 and its fossil imprints induce sexual differentiation in yeast. Proc. Natl. Acad. Sci. USA. 2014;111:15491–15496. doi: 10.1073/pnas.1406027111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisecaver J.H., Rokas A. Fungal metabolic gene clusters-caravans traveling across genomes and environments. Front Microbiol. 2015;6:161. doi: 10.3389/fmicb.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lessard M.H., Viel C., Boyle B., St-Gelais D., Labrie S. Metatranscriptome analysis of fungal strains Penicillium camemberti and Geotrichum candidum reveal cheese matrix breakdown and potential development of sensory properties of ripened Camembert-type cheese. BMC Genomics. 2014;15:235. doi: 10.1186/1471-2164-15-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leclercq-Perlat M.N., Buono F., Lambert D., Latrille E., Spinnler H.E., Corrieu G. Controlled production of Camembert-type cheeses. Part I: Microbiological and physicochemical evolutions. J. Dairy Res. 2004;71:346–354. doi: 10.1017/s0022029904000196. [DOI] [PubMed] [Google Scholar]
- 38.Marcet-Houben M., Gabaldón T. Acquisition of prokaryotic genes by fungal genomes. Trends Genet. 2010;26:5–8. doi: 10.1016/j.tig.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Wisecaver J.H., Slot J.C., Rokas A. The evolution of fungal metabolic pathways. PLoS Genet. 2014;10:e1004816. doi: 10.1371/journal.pgen.1004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friesen T.L., Stukenbrock E.H., Liu Z., Meinhardt S., Ling H., Faris J.D., Rasmussen J.B., Solomon P.S., McDonald B.A., Oliver R.P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 2006;38:953–956. doi: 10.1038/ng1839. [DOI] [PubMed] [Google Scholar]
- 41.Coelho M.A., Gonçalves C., Sampaio J.P., Gonçalves P. Extensive intra-kingdom horizontal gene transfer converging on a fungal fructose transporter gene. PLoS Genet. 2013;9:e1003587. doi: 10.1371/journal.pgen.1003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goarin A., Silar P., Malagnac F. Gene replacement in Penicillium roqueforti. Curr. Genet. 2015;61:203–210. doi: 10.1007/s00294-014-0456-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-wise expression of Penicillium camemberti genes during cheese rind maturation; these data were generated in a previous study [36].
Presence/absence of the horizontally transferred regions Wallaby and CheesyTer in 416 different isolates from diverse origins belonging to 65 fungal species. Strains isolated from the cheese environment are shown in purple, and positive PCR amplifications are represented in yellow. Type, neotype, and epitype strains are indicated, respectively, with a “T,” a “NeoT,” and a “EpiT” after the strain number. All strains included in this study are available in the public fungal strain collection of the National Museum of Natural History in Paris, except the strains labeled “FM,” corresponding to a private collection provided by French anonymous stakeholders. Only the six FM strains whose genomes are available (e.g., FM013, FM041, FM164, FM169, FM193, and FM227, indicated with an asterisk) have LCP numbers and are publicly available.
Growth measures (in mm) obtained in the fitness experiments. (a) Experiments assessing the ability of using the cheese substrate: growth measures of 50 Penicillium roqueforti strains on minimal medium (MM) and cheese medium. (b) Experiments assessing competitive ability: growth measures of three challengers, i.e., P. biforme, P. nalgiovense, and Geotrichum candidum, inoculated in the middle of Petri dishes on P. roqueforti lawns (n = 23). (c) Experiments assessing competitive ability: growth measures of 12 challengers belonging to P. biforme, P. camemberti, or P. rubens, inoculated on P. roqueforti lawns (2 W+C+ and 2 W−C−). (d) Experiments assessing competitive ability: growth measures of 19 P. roqueforti strains on cheese medium, inoculated pairwise in two opposite points of Petri dishes.