Abstract
Interleukin-8 (IL-8) is a neutrophil chemokine that is encoded on the CXCL8 gene. Normally CXCL8 expression is repressed due to histone deacetylation, octamer-1 binding to the promoter and the inhibitory effect of nuclear factor-κB repressing factor (NRF). However, in response to a suitable stimulus, the human CXCL8 gene undergoes transcription due to its inducible promoter that is regulated by the transcription factors nuclear factor-κB (NF-κB), activating protein (AP-1), CAAT/enhancer-binding protein β (C/EBPβ, also known as NF-IL-6), C/EBP homologous protein (CHOP) and cAMP response element binding protein (CREB). CXCL8 mRNA is then stabilised by the activity of p38 mitogen-activated protein kinase (p38 MAPK). Cystic fibrosis (CF) lung disease is characterised by a neutrophil-dominated airway inflammatory response. A major factor contributing to the large number of neutrophils is the higher than normal levels of IL-8 that are present within the CF lung. Infection and inflammation, together with intrinsic alterations in CF airway cells are responsible for the abnormally high intrapulmonary levels of IL-8. Strategies to inhibit aberrantly high CXCL8 expression hold therapeutic potential for CF lung disease.
Keywords: interleukin-8, cystic fibrosis, lung
1. Introduction
Inflammation is an important consequence of tissue injury that can result from several causes such as infection, trauma, neoplasm, and autoimmune disease. This mechanism is highly regulated by small molecules called chemokines, which control trafficking of various types of leukocytes via interaction with transmembrane receptors. One important chemokine that plays a vital role in most inflammatory pathways is interleukin (IL)-8.
Based on its functions, in the past, IL-8 has been alternatively named as T-cell chemotactic factor, neutrophil-activity peptide 1, Beta-thromboglobulin-like protein, and tumour necrosis factor-induced gene, amongst others. However, the name and symbol chemokine (C-X-C motif) ligand 8 and CXCL8, respectively, were adopted and have been officially endorsed by the Chemokine Nomenclature Subcommittee of the International Union of Immunological Societies [1]; nonetheless the name interleukin-8 is still widely used when referring to the protein.
In humans, IL-8 is one of fifteen members of the CXC chemokine family that are mostly encoded on chromosome 4q, spanning a region of approximately 2.5 Mb [2]. The IL-8 gene (CXCL8) has been mapped to 4q12-q21 by using somatic cell hybridization and in situ hybridization. CXCL8 is 3211 bases in length and is encoded on 4 exons. Monocytes/macrophages, epithelial cells, smooth muscle cells and endothelial cells can all produce IL-8. It exists in two isoforms; a 72 amino acid form derived from endothelial cells and the more abundant 77 residue form secreted by monocytes and other cells [3,4]. The main receptors that interact with IL-8 are G-protein coupled seven transmembrane receptors CXCR1 and CXCR2, where the former has greater affinity and expression [5,6].
Due to the significant role IL-8 has in the inflammatory process in various diseases, importantly its expression can be regulated through a number of mechanisms. For example A/U rich elements and microRNA recognition elements in the 3' untranslated region of the CXCL8 mRNA have been reported to contribute to its post-transcriptional regulation [7,8]. However, of relevance here is the fact that the CXCL8 promoter is regulated by a selection of inducible transcription factors.
A major property of IL-8 during the inflammatory process is chemotaxis of target cells to the site of inflammation, in particular neutrophils. IL-8 also has chemotactic activity against T cells and basophils. Neutrophil adhesion to and transmigration across the endothelium are regulated by IL-8 and once neutrophils arrive to the site of inflammation, IL-8 further stimulates those cells to carry out phagocytosis, thus increasing the efficiency of tissue repair. Studies have also shown that IL-8 also has other immunomodulatory effects including the ability to induce matrix metalloproteinase-9 expression, release of TNF-related apoptosis-inducing ligand (TRAIL) and prime respiratory burst in neutrophils (reviewed in [9]).
2. Transcriptional Regulation of CXCL8
Inflammation recruits neutrophils to the site of injury, by a mechanism that is directed by chemokines. In the cystic fibrosis (CF) lung the most abundant chemokine is IL-8; it has variable characteristics, of which the most remarkable is its inducible expression which allows for variation of its expression levels. In non-pathological tissue, levels of IL-8 are not detectable, but are exponentially increased by 10- to 100-fold in response to pro-inflammatory cytokines such as tumour necrosis factor (TNF) and IL-1, bacterial or viral virulence factors, and oxidative stress. The expression of IL-8 is highly coordinated by many complex integrated signalling pathways [10] and is stimulus-dependent as shown, for example, by Kasahara et al. who demonstrated this phenomenon through nuclear run-on experiments in astrocytes [11].
The proximal region of the CXCL8 promoter spans approximately 200 nucleotides of the 5' flanking region of the CXCL8 gene, and is essential for transcriptional regulation of that gene [12]. Three of the major mechanisms responsible for CXCL8 regulation are: (i) repression of the CXCL8 promoter; (ii) transcriptional activation by inducible transcription factors; and (iii) mRNA stabilization. The following sections discuss each of these in more depth.
2.1. Repression of the CXCL8 Promoter
CXCL8 transcription is effectively repressed in unstimulated cells by a combination of three mechanisms: Deacetylation of histones, octamer-1 (Oct-1) binding, and active repression by NF-κB repressing factor (NRF). Gene transcription is normally enhanced by histone acetylation which improves the activity of transcriptional enhanceosomes (TE). TEs provide multi-protein surfaces that make optimal contact with the proteins of the basal transcriptional machinery and thus facilitate maximal gene transcription; deacetylation has the opposite effect. Histone deacetylase-1 (HDAC-1) inhibition derepresses expression of CXCL8, and following the recruitment of CREB binding protein (CBP)/p300 to the promoter results in hyperacetylation of histones and chromatin remodelling ultimately relieving the repression [13,14]. Regarding Oct-1, it has been shown that replacing the Oct-1 repressor with nuclear factor-κB (NF-κB) and CAAT/enhancer-binding protein (C/EBP) as a consequence of IL-1 stimulation induces transcription at the CXCL8 promoter [15]. Likewise binding of NRF to a negative regulatory element (NRE) in the CXCL8 promoter, which incompletely overlaps with the NF-κB response element, also represses CXCL8 expression [16]. Experiments have shown that reduced cellular NRF levels, achieved by expressing anti-sense RNA, causes spontaneous CXCL8 gene expression. Mutations in the NRE site result in loss of NRF binding and an increase in basal CXCL8 transcription. Unusually, NRF has a dual role in CXCL8 transcription: it acts as a repressor in the absence of a stimulus but actually functions as a co-activator to enhance IL-1-induced IL-8 protein production.
2.2. Transcriptional Activation of CXCL8 by Inducible Transcription Factors
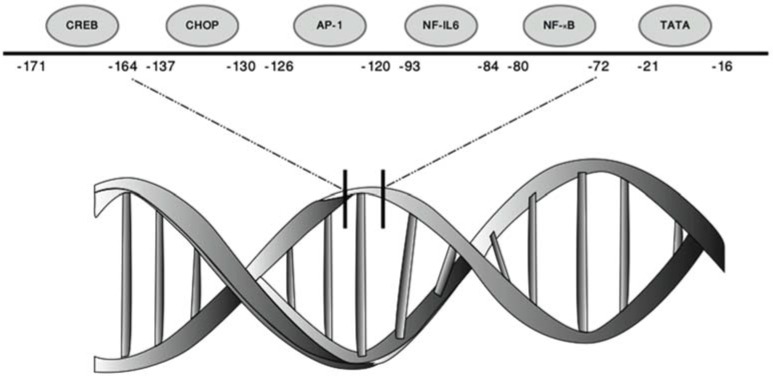
Through mutation and deletion analysis, it was discovered that the CXCL8 promoter element contains NF-κB, activating protein (AP-1), and C/EBPβ (also known as NF-IL-6) binding sites [10]. In addition the transcription factors C/EBP homologous protein (CHOP) [17] and cAMP response element binding protein (CREB) [18] can also bind the CXCL8 promoter (Figure 1 and Table 1).
Figure 1.
Architecture of the interleukin-8 promoter. Graphic representation of the CXCL8 promoter showing the locations of the binding sites for transcription factors that induce its expression (adapted from reference [12]).
Table 1.
Characteristics of transcription factors that regulate CXCL8 expression.
| Transcription Factors | Transcription Factor Consensus Sequence | Subunits/Isoforms | Structure | Position bp: Base Pair |
|---|---|---|---|---|
| NF-κB | GGAATTTCC | NF-κB1 (p50 & p105) | Dimeric | −80/−72 bp |
| NF-κB2 (p52 & p100) | ||||
| C-Rel | ||||
| RelA (p65) | ||||
| RelB | ||||
| NF-IL6/C/EBPβ | AGTTGCAAAT | 3 Isoforms: | Binds DNA as a dimer | −93/−84 bp |
| P17676-1 | Forms stable heterodimers with CEBPA, CEBPD and CEBPG | |||
| P17676-2 | ||||
| P17676-3 | ||||
| AP-1 | TGACTCA | c-Jun | Homodimer/heterodimer | −126/−120 bp |
| JunD | ||||
| JunB | ||||
| Atf-2 | ||||
| c-Fos | ||||
| Fra-1 | ||||
| Fra-2 | ||||
| CHOP | GTGTGATG | Isoforms: | Heterodimer | −137/−130 bp |
| P35638-1 | ||||
| P35638-2 | ||||
| CREB | TTTCGTCA | Isoforms: | Binds DNA as a dimer | −171/−164 bp |
| P16220-1 | ||||
| P16220-2 | ||||
| P16220-3 |
NF-κB is a dimeric transcription factor composed of various homo- or heterodimeric combinations of 5 subunits: RelA (also called p65), RelB, c-Rel, p50 (generated from the precursor p105/NF-κB1) and p52 (generated from p100/NF-κB2). It is stored in the cytoplasm in its inactivated form by binding to its inhibitory proteins, IκBα and β. During a stress stimulus, NF-κB is activated by the phosphorylation of IκB proteins by the IκB kinases, IKKα/β and IKKγ/Nemo. This results in the ubiquitination and rapid degradation of the inhibitory proteins by the proteosome, allowing NF-κB to translocate to the nucleus and bind to the endogenous CXCL8 promoter by its RelA subunit [19]. NF-κB is essential for activation of CXCL8 transcription, while AP-1 and C/EBP are required for maximal CXCL8 expression by synergising with NF-κB [10,20].
AP-1 is a homodimer or heterodimer composed of c-Jun, Jun-B, Jun-D, Atf-2, c-Fos, Fra-1, and Fra-2 [21]. In contrast to NF-κB which normally resides in the cytosol but translocates to the nucleus when IκB is released, AP-1 is directly bound to its target sequence on DNA and its trans-activating activity is regulated by its abundance, phosphorylation of transactivation domains, and the binding of protein kinases [22]. AP-1 is activated through MAPKs. Interestingly all three MAPK pathways have been shown to contribute to CXCL8 gene expression: the extracellular-regulated protein kinase (ERK 1 and ERK 2), JUN-N terminal protein kinase (JNK), and p38 MAPK pathways [10].
The ERK pathway on its own is not a very potent inducer of IL-8, but has the potential to enhance IL-8 induction stimulated by NF-κB [23]. Regarding JNK signalling, Krause et al. showed how JNK similar to NF-κB, is essential for CXCL8 expression [24], most likely via activation of c-Jun. A study by Holtmann et al. suggested that NF-κB and JNK work together for the induction of IL-8, as selectively blocking either pathway remarkably reduced IL-8 secretion [23]. p38 MAPK contributes to stabilisation of the CXCL8 transcript (see below).
C/EBPs and CHOP are basic leucine zipper transcription factors; C/EBP-β (also named NF-IL-6) not only regulates expression of interleukin-6 but also can bind to the CXCL8 promoter and co-operate with NF-κB for transcription of CXCL8 [25]. CHOP regulates CXCL8 expression independently of NF-κB [26]. Finally although CREB synergises with NF-κB to induce expression of interleukin-6, there is no synergy between these transcription factors in the process of CXCL8 expression induced by TNFα in astrocytes [27].
2.3. CXCL8 mRNA Stabilization
Stabilization of the CXCL8 mRNA is mediated by p38 MAPK. Both MKK6 and MK2 are also involved in this process. MKK6 is a dual specificity MAPK kinase that activates p38 MAPK, and MK2 (MAPK-activated protein kinase-2) is a downstream substrate of MKK6-p38 MAPK signalling [23,28,29]. Collectively this signalling pathway targets the A/U-rich 3' UTR of CXCL8 mRNA and stabilizes the transcript.
3. Interleukin-8 in the Cystic Fibrosis Lung
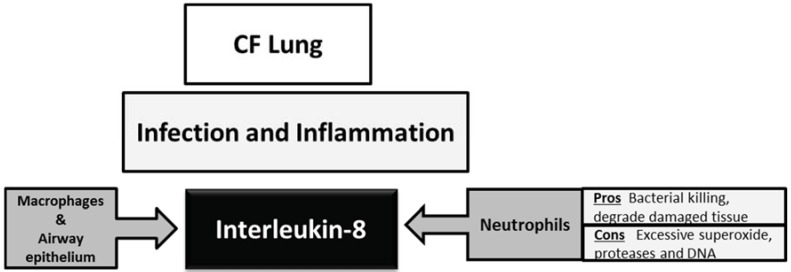
In cystic fibrosis defective or dysfunctional CFTR (cystic fibrosis transmembrane conductance regulator) impairs chloride secretion from airway epithelial cells, leading to excessive reabsorption of sodium and water, causing a reduction of airway surface liquid volume and an impairment of mucociliary clearance [30]. This predisposes to bacterial colonisation of the lung and is associated with neutrophil infiltration and inflammation. The purpose of neutrophil infiltration into the lung is to kill bacteria and degrade damaged tissues. However an over exaggerated influx of neutrophils can lead to excessive release of neutrophil granule contents including superoxide anions and enzymes such as neutrophil elastase (NE), causing damage to the epithelium and injuring the airway structure. Many studies have reported higher than normal levels of IL-8 in the CF lung and this is in large part responsible for the high neutrophil burden within the CF lung [31,32,33,34] (Figure 2).
Figure 2.
Effects of Interleukin-8 in the cystic fibrosis lung.
IL-8 is a neutrophil chemokine produced principally by macrophages and epithelial cells in the CF lung in response to infective and inflammatory stimuli. Ideally this leads to neutrophil-mediated bacterial killing via CXCR1 and removal and degradation of damaged tissue [35]. Due to exaggerated number of neutrophils in the CF lung, there are higher than normal levels of superoxide, neutrophil proteases and DNA [36,37,38,39].
Pseudomonas aeruginosa is the most common CF lung pathogen. However many other microbes can also colonise the CF airways including Staphylococcus aureus, Haemophilus influenzae, Burkholderia cepacia, Aspergillus fumigatus and other fungi, as well as various anaerobic bacterial species [36]. People with CF are also prone to viral lung infections. Activation of toll-like receptors (TLRs) on epithelial or innate immune cells in the lung by microbial- and host-derived factors, can induce proinflammatory gene expression and in particular CXCL8 expression. A number of studies have reported the expression and function of TLRs in the CF lung and in in vitro models of CF. mRNA for TLRs 1-10 is expressed by non-CF tracheal, bronchial and alveolar type II airway epithelial cells. Likewise all TLRs have been detected in primary and transformed CF airway epithelial cells [40,41,42]. TLR5, for example, is expressed by airway epithelial cells and can mediate inflammatory responses to flagellin-expressing Pseudomonas aeruginosa, Burkholderia cepacia and B. cenocepacia [43,44]. It has been shown that CF airway epithelial cells respond to P. aeruginosa, IL-1β or TNFα stimulation with an exaggerated NF-κB activation thereby strongly inducing CXCL8 transcription [45,46,47]. This may be related to NADPH oxidase which is necessary for IL-8 synthesis in response to TLR activation by P. aeruginosa [48], however the response does not involve increased C/EBP [49]. Taken together these studies underscore the importance of infection and inflammation in contributing to the exaggerated expression of CXCL8 in the CF lung.
A cycle of inflammation exists in the CF lung whereby neutrophil-derived products can drive CXCL8 expression and IL-8 protein production in airway epithelial cells leading to further neutrophil recruitment. A major factor responsible for this is NE, a serine protease released from activated neutrophils. Its normal function is to degrade elastin however it can also affect proinflammatory gene expression, including CXCL8, in airway epithelial cells. The McElvaney group have reported the mechanism by which NE can induce IL-8 protein production from bronchial epithelial cells [50,51,52]. They demonstrated that NE cleaves meprin-α on the epithelial cell surface leading to trans-activation of the epidermal growth factor receptor (EGFR) by release of a specific EGFR ligand, transforming growth factor-α. EGFR then co-localises with TLR4 and an intracellular signalling cascade leading to NF-κB activation and CXCL8 expression is implemented. This group also reported that NE can release heme from haemoglobin, possibly present in the CF lung due to micro bleeds, and that this in turn signals via a meprin/TLR/NF-κB pathway also leading to CXCL8 induction [53]. NE has other deleterious effects in the CF unrelated to IL-8, including up regulation of mucin expression and the ability to degrade immunoglobulins, antiproteases, chemokine receptors and innate immunity proteins [35,54,55,56].
Various intrinsic defects in CF cells have been reported that contribute to aberrant CXCL8 expression. For example, CF nasal airway epithelial cells have higher basal NF-κB activity and IL-8 protein expression compared to non-CF cells, however this effect is not evident in nasal epithelial cells stimulated with inflammatory or infective stimuli [57]. In CF airway epithelial cells Bartling and Drumm reported how defective CFTR contributes to oxidative stress resulting in intrinsic alterations in HDACs and increased acetylation of the CXCL8 promoter [58]. Finally it has been shown that stabilisation of CXCL8 mRNA in CF airway epithelial cells occurs due to constitutive p38 and ERK1/2 MAPK signalling [59].
4. Strategies Targeting CXCL8 in Cystic Fibrosis
Given the higher than normal levels of IL-8 in the CF lung and the deleterious consequences associated with this, the question arises as to whether IL-8 can be targeted therapeutically for CF? A number of groups have tested a variety of approaches including the use of anti-inflammatories, specific NF-κB inhibitors, transcription factor decoys and more recently microRNA-modulation approaches.
For example, in the CF lung CXCL8 is bound by gycosaminoglycans (GAGs); this does not interfere with its chemotactic activity and protects it from degradation by neutrophil proteases [60]. Treatment of people with CF with nebulized hypertonic saline can disrupt the interaction between IL-8 and GAGs rendering IL-8 susceptible to proteolytic degradation thereby decreasing neutrophil chemotaxis and facilitating resolution of inflammation [61]. More recent in vitro studies have shown that a CXCL8-based decoy, PA401, which displays no chemotactic activity but has GAG binding affinity can disrupt CXCL8:GAG complexes, rendering CXCL8 susceptible to proteolytic degradation [62]. CXCL8 normally promotes bacterial killing by neutrophils through CXCR1. However unopposed proteolytic activity in the CF airways cleaves CXCR1 on neutrophils and disables their bacterial-killing capacity. Hartl et al. demonstrated in vivo inhibition of proteases by inhalation of alpha1-antitrypsin leading to restored CXCR1 expression and improve bacterial killing in people with cystic fibrosis [35].
Regarding anti-inflammatory approaches, in vitro studies have tested the effects of genistein, fluvastatin and corilagin, amongst others [63,64,65]. Collectively these studies demonstrated that genistein reduces IL-8 production in cultured CF bronchial gland cells by increasing cytosolic IκBα protein levels, fluvastatin decreased IL-8 production in whole blood in response to Pseudomonas or Aspergillus antigens, and corilagin binds to NF-κB, thus inhibiting NF-κB/DNA interactions and can decrease CXCL8 gene expression in CF bronchial IB3-1 cells.
The transcription factor decoy approach is based on molecules mimicking the target sites of transcription factors and interfering with their activity when delivered into target cells. Intracellular delivery of double-stranded oligodeoxynucleotides (ODNs) designed to block NF-κB binding to the CXCL8 promoter in IB3-1 and CuFi-1 cells could partially inhibit the P. aeruginosa-dependent CXCL8 transcription [66]. Similar ODNs delivered in vitro to IB3-1 cells significantly inhibited P. aeruginosa LPS-induced CXCL8 mRNA expression [67]. Peptide nucleic acids (PNAs), in particular PNA-DNA-PNA (PDP) chimeras, are more stable than ODNs molecules. Finotti et al. have demonstrated that an NF-κB-targeting PDP chimera acts as a strong inhibitor of CXCL8 gene expression [68].
Regarding microRNA modulation approaches to decrease CXCL8 production in the CF lung, three recent studies have determined how miRNAs that are aberrantly expressed in the CF airways and that regulate expression of CXCL8, may be targeted for therapeutic benefit [69,70,71]. The first reported on miR-155, a miRNA that is highly expressed in CF lung, regulates SHIP1 and indirectly leads to increased IL-8 production. An inhibitor of miR-155 (antagomir-155) reversed this effect in vitro [69]. The other two studies on this topic have investigated miRNAs that are decreased in the CF lung that directly target CXCL8 mRNA. Fabbri et al. identified miR-93 as a miRNA that is decreased in IB3-1 and Cufi-1 cells infected with P. aeruginosa. Artificially increasing the levels of miR-93 (with pre-miR-93 transfection) could decrease IL-8 expression in these CF airway epithelial cells in vitro [70]. Lastly, Oglesby et al. [71] measured the expression and function of miRNAs decreased in the CF lung and in βENaC-transgenic mice that are predicted to target IL-8 mRNA. miR-17 was identified as a miRNA that regulates IL-8 and its expression was decreased in adult CF bronchial brushings, βENaC-transgenic mice and bronchial epithelial cells chronically stimulated with Pseudomonas-conditioned medium. Overexpression of miR-17 inhibited IL-8 protein production in F508del-CFTR homozygous bronchial epithelial cells. Thus modulating the expression of miRNAs that target CXCL8 mRNA in CF bronchial epithelial is likely to represent a new therapeutic strategy for CF [71].
5. Conclusions
Much is known about the transcriptional regulation of CXCL8 [12]. Detailed studies have identified the inducible regulatory factors involved and how these respond to different infective and pro-inflammatory stimuli. In the context of CF it is also known that intrinsic alterations due to defective CFTR can affect CXCL8 transcription and mRNA stabilization. Various studies have demonstrated the feasibility of inhibiting CXCL8 expression in CF airway epithelial cells and it is hoped that in the near future some of these strategies will be developed further and can be used for therapeutic benefit in individuals with CF.
Acknowledgments
Funding for research in Catherine M. Greene’s group is gratefully acknowledged from Science Foundation Ireland, the National Children’s Research Centre, the Royal College of Surgeons in Ireland and the European Respiratory Society.
Author Contributions
Karim Jundi and Catherine M. Greene drafted the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bacon K., Baggiolini M., Broxmeyer H., Horuk R., Lindley I., Mantovani A., Maysushima K., Murphy P., Nomiyama H., Oppenheim J., et al. Chemokine/chemokine receptor nomenclature. J. Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 2.Colobran R., Pujol-Borrell R., Armengol M.P., Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin. Exp. Immunol. 2007;148:208–217. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindley I., Aschauer H., Seifert J.M., Lam C., Brunowsky W., Kownatzki E., Thelen M., Peveri P., Dewald B., von Tscharner V., et al. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: Biological equivalence between natural and recombinant neutrophil-activating factor. Proc. Natl. Acad. Sci. USA. 1988;85:9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimbrone M.A., Jr., Obin M.S., Brock A.F., Luis E.A., Hass P.E., Hébert C.A., Yip Y.K., Leung D.W., Lowe D.G., Kohr W.J., et al. Endothelial interleukin-8: A novel inhibitor of leukocyte-endothelial interactions. Science. 1989;246:1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja S.K., Murphy P.M. The CXC chemokines growth-regulated oncogene (GRO) α, GROβ, GROγ, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 7.Winzen R., Thakur B.K., Dittrich-Breiholz O., Shah M., Redich N., Dhamija S., Kracht M., Holtmann H. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol. Cell. Biol. 2007;27:8388–8400. doi: 10.1128/MCB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu N., Zhang J., Cui W., Kong G., Zhang S., Yue L., Bai X., Zhang Z., Zhang W., Zhang X., et al. miR-520b Regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8. J. Biol. Chem. 2011;286:13714–13722. doi: 10.1074/jbc.M110.204131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman E., Dittrich-Brehiholz O., Holtmann H., Kracht M. Multiple Control of Interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 11.Kasahara T., Mukaida N., Yamashita K., Yagisawa H., Akahoshi T., Matsushima K.N. IL-1 and TNF-α induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology. 1991;74:60–67. [PMC free article] [PubMed] [Google Scholar]
- 12.Bezzerri V., Borgatti M., Finotti A., Tamanini A., Gambari R., Cabrini G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J. Immunol. 2011;187:6069–6081. doi: 10.4049/jimmunol.1100821. [DOI] [PubMed] [Google Scholar]
- 13.Ashburner B.P., Westerheide S.D., Baldwin A.S., Jr. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanden Berghe W., de Bosscher K., Boone E., Plaisance S., Haegeman G. The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J. Biol. Chem. 1999;274:32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 15.Wu G.D., Lai E.J., Huang N., Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 1997;272:2396–2403. [PubMed] [Google Scholar]
- 16.Nourbakhsh M., Kalble S., Dorrie A., Hauser H., Resch K., Kracht M. The NF-κB repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-κB-flanking sequence element. Biol. Chem. 2001;276:4501–4508. doi: 10.1074/jbc.M007532200. [DOI] [PubMed] [Google Scholar]
- 17.Vij N., Amoako M.O., Mazur S., Zeitlin P.L. CHOP transcription factor mediates IL-8 signaling in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2008;38:176–184. doi: 10.1165/rcmb.2007-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisatsune J., Nakayama M., Isomoto H., Kurazono H., Mukaida N., Mukhopadhyay A.K., Azuma T., Yamaoka Y., Sap J., Yamasaki E., et al. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-κB activation. J. Immunol. 2008;180:5017–5027. doi: 10.4049/jimmunol.180.7.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeuerle P.A., Baltimore D. NF-κB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 20.Mukaida N., Okamoto S., Ishikawa Y., Matsushima K.J. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 21.Karin M., Liu Z. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 22.Whitmarsh A.J., Davis R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 23.Holtmann H., Winzen R., Holland P., Eickemeier S., Hoffmann E., Wallach D., Malinin N.L., Cooper J.A., Resch K., Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause A., Holtmann H., Eickemeier S., Winzen R., Szamel M., Resch K., Saklatvala J., Kracht M. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 1998;273:23681–23689. doi: 10.1074/jbc.273.37.23681. [DOI] [PubMed] [Google Scholar]
- 25.Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caristi S., Piraino G., Cucinotta M., Valenti A., Loddo S., Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J. Biol. Chem. 2005;280:14433–14442. doi: 10.1074/jbc.M410725200. [DOI] [PubMed] [Google Scholar]
- 27.Spooren A., Kooijman R., Lintermans B., van Craenenbroeck K., Vermeulen L., Haegeman G., Gerlo S. Cooperation of NF-κB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal. 2010;22:871–881. doi: 10.1016/j.cellsig.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Winzen R., Kracht M., Ritter B., Wilhelm A., Chen C.Y., Shyu A.B., Müller M., Gaestel M., Resch K., Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jobin C., Holt L., Bradham C.A., Streetz K., Brenner D.A., Sartor R.B. TNF receptor-associated factor-2 is involved in both IL-1 beta and TNF-alpha signaling cascades leading to NF-κB activation and IL-8 expression in human intestinal epithelial cells. J. Immunol. 1999;162:4447–4454. [PubMed] [Google Scholar]
- 30.Mall M.A., Hartl D. CFTR: Cystic fibrosis and beyond. Eur. Respir. J. 2014;44:1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 31.Richman-Eisenstat J.B., Jorens P.G., Hébert C.A., Ueki I., Nadel J.A. Interleukin-8: An important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am. J. Physiol. 1993;264:L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- 32.Bonfield T.L., Panuska J.R., Konstan M.W., Hilliard K.A., Hilliard J.B., Ghnaim H., Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 33.Sagel S.D., Kapsner R., Osberg I., Sontag M.K., Accurso F.J. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am. J. Respir. Crit. Care Med. 2001;164:1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 34.Mayer-Hamblett N., Aitken M.L., Accurso F.J., Kronmal R.A., Konstan M.W., Burns J.L., Sagel S.D., Ramsey B.W. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartl D., Latzin P., Hordijk P., Marcos V., Rudolph C., Woischnik M., Krauss-Etschmann S., Koller B., Reinhardt D., Roscher A.A., et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 36.Hartl D., Gaggar A., Bruscia E., Hector A., Marcos V., Jung A., Greene C., McElvaney G., Mall M., Döring G. Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros. 2012;11:363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Roum J.H., Borok Z., McElvaney N.G., Grimes G.J., Bokser A.D., Buhl R., Crystal R.G. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J. Appl. Physiol. 1999;87:438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura H., Yoshimura K., McElvaney N.G., Crystal R.G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 1992;89:1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzenreiter R., Kienberger F., Marcos V., Schilcher K., Krautgartner W.D., Obermayer A., Huml M., Stoiber W., Hector A., Griese M., et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Greene C.M., Carroll T.P., Smith S.G., Taggart C.C., Devaney J., Griffin S., O’Neill S.J., McElvaney N.G. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 41.Muir A., Soong G., Sokol S., Reddy B., Gomez M.I., van Heeckeren A., Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 42.Hauber H.P., Tulic M.K., Tsicopoulos A., Wallaert B., Olivenstein R., Daigneault P., Hamid Q. Toll-like receptors 4 and 2 expression in the bronchial mucosa of patients with cystic fibrosis. Can. Respir. J. 2005;12:13–18. doi: 10.1155/2005/648984. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z., Louboutin J.P., Weiner D.J., Goldberg J.B., Wilson J.M. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 2005;73:7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De C. Ventura G.M., le Goffic R., Balloy V., Plotkowski M.C., Chignard M., Si-Tahar M. TLR 5, but neither TLR2 nor TLR4, is involved in lung epithelial cell response to Burkholderia cenocepacia. FEMS Immunol. Med. Microbiol. 2008;54:37–44. doi: 10.1111/j.1574-695X.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 45.Joseph T., Look D., Ferkol T. NF-κB activation and sustained IL-8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L471–L479. doi: 10.1152/ajplung.00066.2004. [DOI] [PubMed] [Google Scholar]
- 46.Muselet-Charlier C., Roque T., Boncoeur E., Chadelat K., Clement A., Jacquot J., Tabary O. Enhanced IL-1β-induced IL-8 production in cystic fibrosis lung epithelial cells is dependent of both mitogen-activated protein kinases and NF-κB signaling. Biochem. Biophys. Res. Commun. 2007;357:402–407. doi: 10.1016/j.bbrc.2007.03.141. [DOI] [PubMed] [Google Scholar]
- 47.Venkatakrishnan A., Stecenko A.A., King G., Blackwell T.R., Brigham K.L., Christman J.W., Blackwell T.S. Exaggerated activation of nuclear factor-κB and altered IκB-β processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 48.Roussel L., Martel G., Bérubé J., Rousseau S. P. aeruginosa drives CXCL8 synthesis via redundant toll-like receptors and NADPH oxidase in CFTR∆F508 airway epithelial cells. J. Cyst. Fibros. 2011;10:107–113. doi: 10.1016/j.jcf.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 49.DiMango E., Ratner A.J., Bryan R., Tabibi S., Prince A. Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Invest. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh D.E., Greene C.M., Carroll T.P., Taggart C.C., Gallagher P.M., O’Neill S.J., McElvaney N.G. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J. Biol. Chem. 2001;276:35494–35499. doi: 10.1074/jbc.M103543200. [DOI] [PubMed] [Google Scholar]
- 51.Devaney J.M., Greene C.M., Taggart C.C., Carroll T.P., O’Neill S.J., McElvaney N.G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003;544:129–132. doi: 10.1016/S0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 52.Bergin D.A., Greene C.M., Sterchi E.E., Kenna C., Geraghty P., Belaaouaj A., Taggart C.C., O’Neill S.J., McElvaney N.G. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 2008;283:31736–31744. doi: 10.1074/jbc.M803732200. [DOI] [PubMed] [Google Scholar]
- 53.Cosgrove S., Chotirmall S.H., Greene C.M., McElvaney N.G. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J. Biol. Chem. 2011;286:7692–7704. doi: 10.1074/jbc.M110.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao M.X., Nadel J.A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J. Immunol. 2005;175:4009–4016. doi: 10.4049/jimmunol.175.6.4009. [DOI] [PubMed] [Google Scholar]
- 55.Kelly E., Greene C.M., McElvaney N.G. Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets. 2008;12:145–157. doi: 10.1517/14728222.12.2.145. [DOI] [PubMed] [Google Scholar]
- 56.Greene C.M., McElvaney N.G. Proteases and antiproteases in chronic neutrophilic lung disease—Relevance to drug discovery. Br. J. Pharmacol. 2009;158:1048–1058. doi: 10.1111/j.1476-5381.2009.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrabino S., Carpani D., Livraghi A., di Cicco M., Costantini D., Copreni E., Colombo C., Conese M. Dysregulated interleukin-8 secretion and NF-κB activity in human cystic fibrosis nasal epithelial cells. J. Cyst. Fibros. 2006;5:113–119. doi: 10.1016/j.jcf.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Bartling T.R., Drumm M.L. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am. J. Respir. Cell Mol. Biol. 2009;40:58–65. doi: 10.1165/rcmb.2007-0464OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhattacharyya S., Gutti U., Mercado J., Moore C., Pollard H.B., Biswas R. MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;300:L81–L87. doi: 10.1152/ajplung.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McElvaney O.J., O’Reilly N., White M., Lacey N., Pohl K., Gerlza T., Bergin D.A., Kerr H., McCarthy C., O’Brien M.E., et al. The effect of the decoy molecule PA401 on CXCL8 levels in bronchoalveolar lavage fluid of patients with cystic fibrosis. Mol. Immunol. 2015;63:550–558. doi: 10.1016/j.molimm.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Reeves E.P., Williamson M., O’Neill S.J., Greally P., McElvaney N.G. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:1517–1523. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 62.Reeves E.P., Williamson M., Byrne B., Bergin D.A., Smith S.G., Greally P., O’Kennedy R., O’Neill S.J., McElvaney N.G. IL-8 dictates glycosaminoglycan binding and stability of IL-18 in cystic fibrosis. J. Immunol. 2010;184:1642–1652. doi: 10.4049/jimmunol.0902605. [DOI] [PubMed] [Google Scholar]
- 63.Tabary O., Escotte S., Couetil J.P., Hubert D., Dusser D., Puchelle E., Jacquot J. Genistein inhibits constitutive and inducible NF-κB activation and decreases IL-8 production by human cystic fibrosis bronchial gland cells. Am. J. Pathol. 1999;155:473–481. doi: 10.1016/S0002-9440(10)65143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jouneau S., Bonizec M., Belleguic C., Desrues B., Brinchault G., Galaine J., Gangneux J.P., Martin-Chouly C. Anti-inflammatory effect of fluvastatin on IL-8 production induced by Pseudomonas aeruginosa and Aspergillus fumigatus antigens in cystic fibrosis. PLoS ONE. 2011;6:e22655. doi: 10.1371/journal.pone.0022655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gambari R., Borgatti M., Lampronti I., Fabbri E., Brognara E., Bianchi N., Piccagli L., Yuen M.C., Kan C.W., Hau D.K., et al. Corilagin is a potent inhibitor of NF-κB activity and downregulates TNF-α induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012;13:308–315. doi: 10.1016/j.intimp.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Bezzerri V., Borgatti M., Nicolis E., Lampronti I., Dechecchi M.C., Mancini I., Rizzotti P., Gambari R., Cabrini G. Transcription factor oligodeoxynucleotides to NF-κB inhibit transcription of IL-8 in bronchial cells. Am. J. Respir. Cell Mol. Biol. 2008;39:86–96. doi: 10.1165/rcmb.2007-0176OC. [DOI] [PubMed] [Google Scholar]
- 67.De Stefano D., Ungaro F., Giovino C., Polimeno A., Quaglia F., Carnuccio R. Sustained inhibition of IL-6 and IL-8 expression by decoy ODN to NF-κB delivered through respirable large porous particles in LPS-stimulated cystic fibrosis bronchial cells. J. Gene Med. 2011;13:200–208. doi: 10.1002/jgm.1546. [DOI] [PubMed] [Google Scholar]
- 68.Finotti A., Borgatti M., Bezzerri V., Nicolis E., Lampronti I., Dechecchi M., Mancini I., Cabrini G., Saviano M., Avitabile C., et al. Effects of decoy molecules targeting NF-κB transcription factors in Cystic fibrosis IB3-1 cells: Recruitment of NF-κB to the IL-8 gene promoter and transcription of the IL-8 gene. Artif. DNA PNA XNA. 2012;3:97–296. doi: 10.4161/adna.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharyya S., Balakathiresan N.S., Dalgard C., Gutti U., Armistead D., Jozwik C., Srivastava M., Pollard H.B., Biswas R. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J. Biol. Chem. 2011;286:11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fabbri E., Borgatti M., Montagner G., Bianchi N., Finotti A., Lampronti I., Bezzerri V., Dechecchi M.C., Cabrini G., Gambari R. Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am. J. Respir. Cell Mol. Biol. 2014;50:1144–1155. doi: 10.1165/rcmb.2013-0160OC. [DOI] [PubMed] [Google Scholar]
- 71.Oglesby I.K., Vencken S., Agrawal R., Gaughan K., Molloy K., Higgins G., McNally P., McElvaney N.G., Mall M.A., Greene C.M. MiR-17 overexpression in cystic fibrosis airway epithelial cells decreases IL-8 production. Eur. Respir. J. 2015 doi: 10.1183/09031936.00163414. in press. [DOI] [PubMed] [Google Scholar]