Abstract
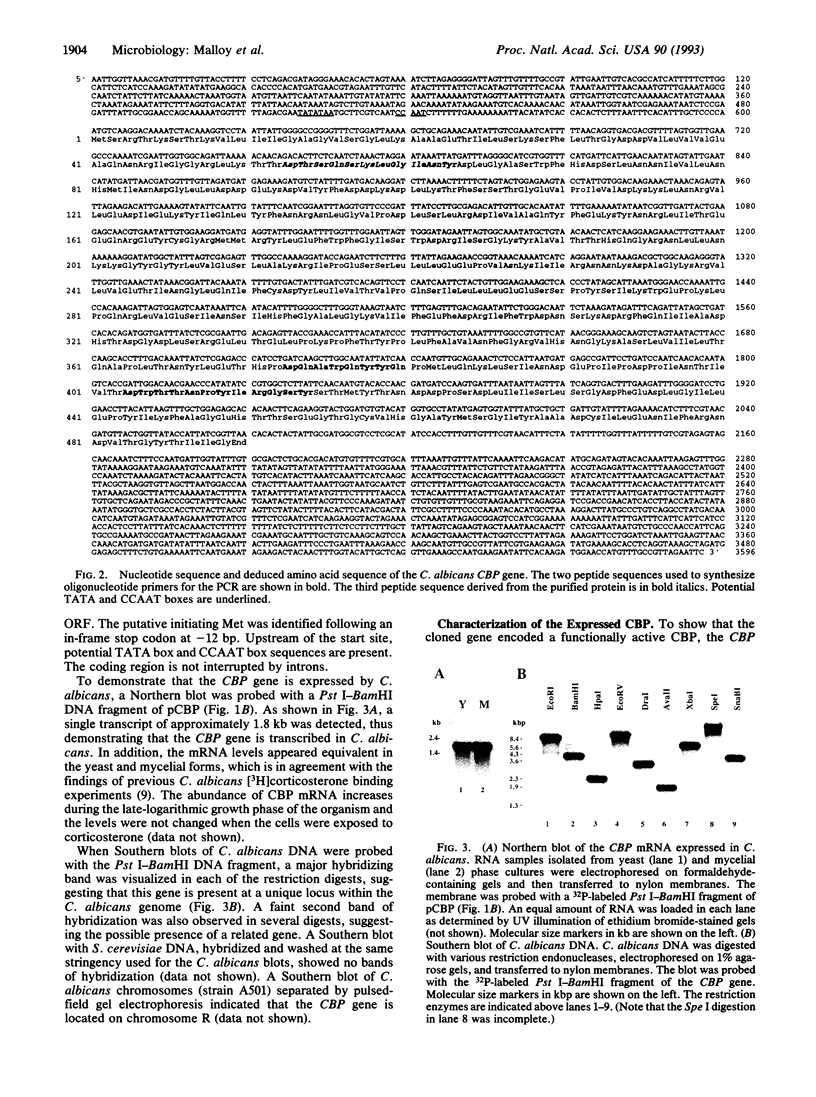
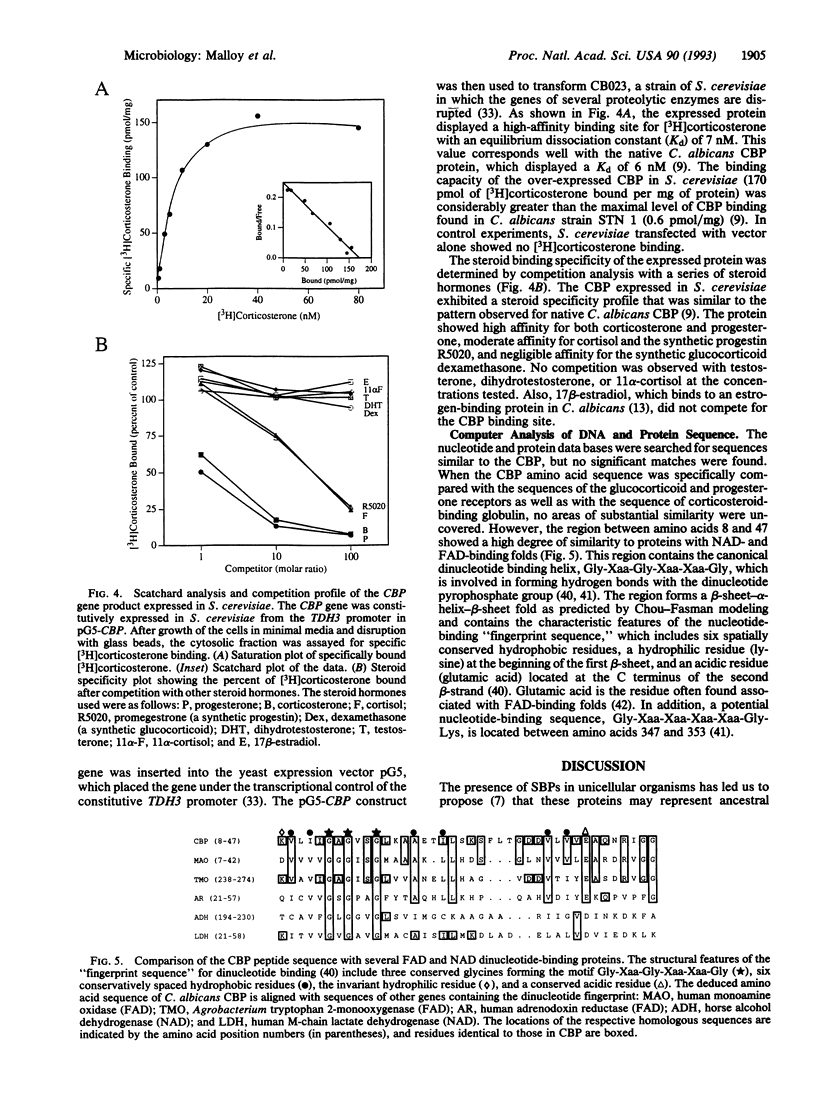
We have previously demonstrated the presence of a corticosteroid-binding protein (CBP) in Candida albicans and speculated on its homology to the glucocorticoid receptor. To explore this relationship further, we cloned the CBP gene. Our strategy employed sequencing enzymatically derived peptide fragments from purified CBP and using this information to synthesize degenerate oligonucleotide primers for use in the PCR. A 117-bp fragment amplified from C. albicans DNA was then used to screen a genomic library. Hybridizing clones were isolated, and DNA sequencing revealed an open reading frame of 1467 bp which encoded a protein with a molecular weight of 55,545. Southern analysis demonstrated that the gene was present at a unique locus within chromosome R of the Candida genome, while Northern analysis showed that the gene was expressed in C. albicans as a 1.8-kb transcript. CBP was over-expressed in Saccharomyces cerevisiae, and it exhibited an apparent dissociation constant (Kd) of 7 nM for [3H]corticosterone and displayed a steroid hormone binding profile comparable to that of the native protein. Searches of the data banks revealed little overall similarity to other cloned genes. However, the amino acid sequence contains a dinucleotide-binding fingerprint. In conclusion, we have cloned the gene encoding the CBP from C. albicans and have shown that the expressed protein has the properties of the native CBP. A comparison of the cloned gene to members of the steroid-thyroid-retinoic acid receptor gene superfamily showed that CBP is unrelated to these hormone receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- Brenner C., Fuller R. S. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):922–926. doi: 10.1073/pnas.89.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D. L., Dautricourt J. P., Maulik S., Relph J. Improved sensitivity of biological sequence database searches. Comput Appl Biosci. 1990 Jul;6(3):237–245. doi: 10.1093/bioinformatics/6.3.237. [DOI] [PubMed] [Google Scholar]
- Burshell A., Stathis P. A., Do Y., Miller S. C., Feldman D. Characterization of an estrogen-binding protein in the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Mar 25;259(6):3450–3456. [PubMed] [Google Scholar]
- Calaycay J., Rusnak M., Shively J. E. Microsequence analysis of peptides and proteins. IX. An improved, compact, automated instrument. Anal Biochem. 1991 Jan;192(1):23–31. doi: 10.1016/0003-2697(91)90177-u. [DOI] [PubMed] [Google Scholar]
- Clemons K. V., Feldman D., Stevens D. A. Influence of oestradiol on protein expression and methionine utilization during morphogenesis of Paracoccidioides brasiliensis. J Gen Microbiol. 1989 Jun;135(6):1607–1617. doi: 10.1099/00221287-135-6-1607. [DOI] [PubMed] [Google Scholar]
- Clemons K. V., Schär G., Stover E. P., Feldman D., Stevens D. A. Dermatophyte-hormone relationships: characterization of progesterone-binding specificity and growth inhibition in the genera Trichophyton and Microsporum. J Clin Microbiol. 1988 Oct;26(10):2110–2115. doi: 10.1128/jcm.26.10.2110-2115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons K. V., Stover E. P., Schär G., Stathis P. A., Chan K., Tökès L., Stevens D. A., Feldman D. Steroid metabolism as a mechanism of escape from progesterone-mediated growth inhibition in Trichophyton mentagrophytes. J Biol Chem. 1989 Jul 5;264(19):11186–11192. [PubMed] [Google Scholar]
- Crislip M. A., Edwards J. E., Jr Candidiasis. Infect Dis Clin North Am. 1989 Mar;3(1):103–133. [PubMed] [Google Scholar]
- Cutler J. E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- Denich K. T., Malloy P. J., Feldman D. Cloning and characterization of the gene encoding the ADP-ribosylation factor in Candida albicans. Gene. 1992 Jan 2;110(1):123–128. doi: 10.1016/0378-1119(92)90455-x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D. The growing problem of mycoses in patients infected with the human immunodeficiency virus. Rev Infect Dis. 1991 May-Jun;13(3):480–486. doi: 10.1093/clinids/13.3.480. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Do Y., Burshell A., Stathis P., Loose D. S. An estrogen-binding protein and endogenous ligand in Saccharomyces cerevisiae: possible hormone receptor system. Science. 1982 Oct 15;218(4569):297–298. doi: 10.1126/science.6289434. [DOI] [PubMed] [Google Scholar]
- Feldman D. Ketoconazole and other imidazole derivatives as inhibitors of steroidogenesis. Endocr Rev. 1986 Nov;7(4):409–420. doi: 10.1210/edrv-7-4-409. [DOI] [PubMed] [Google Scholar]
- Kalo A., Segal E. Interaction of Candida albicans with genital mucosa: effect of sex hormones on adherence of yeasts in vitro. Can J Microbiol. 1988 Mar;34(3):224–228. doi: 10.1139/m88-042. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Feldman D. Characterization of a unique corticosterone-binding protein in Candida albicans. J Biol Chem. 1982 May 10;257(9):4925–4930. [PubMed] [Google Scholar]
- Loose D. S., Schurman D. J., Feldman D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature. 1981 Oct 8;293(5832):477–479. doi: 10.1038/293477a0. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stevens D. A., Schurman D. J., Feldman D. Distribution of a corticosteroid-binding protein in Candida and other fungal genera. J Gen Microbiol. 1983 Aug;129(8):2379–2385. doi: 10.1099/00221287-129-8-2379. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stover E. P., Restrepo A., Stevens D. A., Feldman D. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Mechanisms of natural resistance to human pathogenic fungi. Annu Rev Microbiol. 1991;45:509–538. doi: 10.1146/annurev.mi.45.100191.002453. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Salazar M. E., Cano L. E., Stover E. P., Feldman D., Stevens D. A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984 Nov;46(2):346–353. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Salazar M. E., Restrepo A., Stevens D. A. Inhibition by estrogens of conidium-to-yeast conversion in the fungus Paracoccidioides brasiliensis. Infect Immun. 1988 Mar;56(3):711–713. doi: 10.1128/iai.56.3.711-713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saral R. Candida and Aspergillus infections in immunocompromised patients: an overview. Rev Infect Dis. 1991 May-Jun;13(3):487–492. doi: 10.1093/clinids/13.3.487. [DOI] [PubMed] [Google Scholar]
- Schär G., Stover E. P., Clemons K. V., Feldman D., Stevens D. A. Progesterone binding and inhibition of growth in Trichophyton mentagrophytes. Infect Immun. 1986 Jun;52(3):763–767. doi: 10.1128/iai.52.3.763-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski R., Feldman D. Characterization of an estrogen-binding protein in the yeast Candida albicans. Endocrinology. 1989 Apr;124(4):1965–1972. doi: 10.1210/endo-124-4-1965. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stover E. P., Loose D. S., Stevens D. A., Feldman D. Ketoconazole binds to the intracellular corticosteroid-binding protein in Candida albicans. Biochem Biophys Res Commun. 1983 Nov 30;117(1):43–50. doi: 10.1016/0006-291x(83)91538-3. [DOI] [PubMed] [Google Scholar]
- Stover E. P., Schär G., Clemons K. V., Stevens D. A., Feldman D. Estradiol-binding proteins from mycelial and yeast-form cultures of Paracoccidioides brasiliensis. Infect Immun. 1986 Jan;51(1):199–203. doi: 10.1128/iai.51.1.199-203.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Hasegawa S., Hishinuma F., Kurata S. Estrogen can regulate the cell cycle in the early G1 phase of yeast by increasing the amount of adenylate cyclase mRNA. Cell. 1989 May 19;57(4):675–681. doi: 10.1016/0092-8674(89)90136-0. [DOI] [PubMed] [Google Scholar]
- Virca G. D., Northemann W., Shiels B. R., Widera G., Broome S. Simplified northern blot hybridization using 5% sodium dodecyl sulfate. Biotechniques. 1990 Apr;8(4):370–371. [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]




