Abstract
Background. An effective vaccine is urgently needed against the H7N9 avian influenza virus. We evaluated the immunogenicity and protective efficacy of a split-virion H7N9 vaccine with or without the oil-in-water adjuvants in ferrets.
Methods. Ferrets were vaccinated with 2 doses of unadjuvanted, MF59 or AS03-adjuvanted A/Shanghai/2/2013 (H7N9) vaccine, and the induction of antibodies to hemagglutinin (HA) or neuraminidase proteins was evaluated. Ferrets were then challenged with wild-type H7N9 virus to assess the vaccine's protective efficacy. The vaccine composition and integrity was also evaluated in vitro.
Results. Adjuvanted vaccines stimulated robust serum antibody titers against HA and neuraminidase compared with the unadjuvanted vaccines. Although there was a difference in adjuvanticity between AS03 and MF59 at a lower dose (3.75 µg of HA), both adjuvants induced comparable antibody responses after 2 doses of 15 µg. On challenge, ferrets that received adjuvanted vaccines showed lower viral burden than the control or unadjuvanted vaccine group. In vitro examinations revealed that the vaccine contained visible split-virus particles and retained the native conformation of HA recognizable by polyclonal and monoclonal antibodies.
Conclusions. The adjuvanted H7N9 vaccines demonstrated superior immunogenicity and protective efficacy against H7N9 infection in ferrets and hold potential as a vaccination regimen.
Keywords: H7N9, influenza, adjuvants, vaccine, ferrets
Since its emergence in China, the avian H7N9 influenza virus continues to spread and cause human infections with a high case fatality rate [1, 2]. Infection control is difficult because the virus causes only mild infections in poultry and is now widespread in China [3]. An effective vaccine is therefore needed to protect against infection and to limit virus spread. However, development of an effective avian influenza vaccine has been hampered by the poor immunogenicity reported in various human clinical trials for candidate H5N1, H7N7, and H7N1 vaccines [4–7]. Furthermore, elderly persons, who have been disproportionately affected by H7N9 [2], respond poorly to influenza vaccines [8]. One strategy to improve the immunogenicity of vaccines is with the use of adjuvants. Particularly successful adjuvants are the squalene oil-in-water emulsion adjuvants, MF59 (Novartis Vaccines) and AS03 (GlaxoSmithKline).
In the current study, we evaluated the immunogenicity and protective efficacy of a split-virion H7N9 vaccine, administered with or without the MF59 or AS03 adjuvants, in the ferret model. We also evaluated the composition of the H7N9 vaccine in a series of in vitro assays, as done previously [9]. We undertook this study to mirror that of the recently completed National Institute of Allergy and Infectious Diseases–sponsored human phase II clinical trials (http://clinicaltrials.gov; NCT 01942265 and 01938742), with the aim that our data will complement the findings of the human study.
MATERIALS AND METHODS
Vaccines and Adjuvants
The H7N9 vaccine candidate, MF59 and AS03-adjuvants, the A/Vietnam/1203/2004 (H5N1) (Sanofi Pasteur), and the 2011 trivalent influenza vaccine (TIV) (Fluzone; Sanofi Pasteur) were received through the Office of Biomedical Advanced Research and Development Authority. The H7N9 vaccine was a split-virion vaccine derived from A/Shanghai/2/2013 (H7N9) virus, manufactured by Sanofi Pasteur, the same lot used for the clinical trials (NCT 01942265 and 01938742).
Viruses and Cells
The virus A/Anhui/1/2013 (H7N9) was used for the ferret challenge experiment and is antigenically similar to the vaccine strain [10]. The reverse-genetics (rg)–derived, attenuated virus strains used for serological assays were generated with the hemagglutinin (HA) and neuraminidase (NA) from A/Anhui/1/2013 and with the 6 internal genes from A/Puerto Rico/8/1934 (PR8).
Virus stocks was prepared by propagation in 10-day-old embryonated chicken eggs at 35°C for 36 hours, aliquoted, and stored at −70°C. Marin–Darby canine kidney cells used for virus titration were propagated in minimal essential medium supplemented with 10% fetal calf serum, vitamins, l-glutamine, and antibiotics in a humidified 5% carbon dioxide environment.
Immunization and Challenge
Specific-pathogen–free male ferrets, 4–6 months old, were purchased from Triple F Farms. Ferrets in 3 groups (6 per group) received 3.75, 15, or 45 µg of unadjuvanted vaccine, and ferrets in another 4 groups received 3.75 or 15 µg of vaccine adjuvanted with MF59 or AS03. Doses were calculated based on the HA concentration, as specified by the manufacturer. Vaccine was diluted in saline to the desired dose and mixed 1:1 with the adjuvant. A control group received saline only. All ferrets received 2 doses of vaccine, 3 weeks apart and administered in 0.5 mL volume, by intramuscular injection. Serum samples were collected at 21 and 16 days after the first and second doses respectively.
The challenge experiment was performed 19 days after the second dose. Ferrets were anesthetized with isoflurane and inoculated intranasally with 1 mL of 105 egg-infectious dose-50 (EID50) of wild-type A/Anhui/1/2013 (H7N9) virus. Signs of infection, weight, and temperature were monitored for up to 12 days after the challenge. All animal experiments were performed according guidelines approved by the Institutional Animal Care and Use Committee in an enhanced animal biosafety level 3+ containment facility.
Nasal Wash and Tissue Samples
On days 3, 5, and 7 after virus inoculation, ferrets were anesthesized with ketamine, and nasal wash samples were collected in 1 mL of phosphate-buffered saline (PBS). On day 5 after inoculation, 3 ferrets from each group were euthanized, and lung tissues were collected from each lobe, pooled, and titrated on Marin–Darby canine kidney cells. After 3 days, a HA assay was performed and the virus titer was determined using the Reed and Muench method [11]. The limit of virus detection was <1 log10 tissue culture infectious dose-50 per milliliter.
Serologic Assays
Serum samples were treated with receptor-destroying enzyme (Denka Seiken), heat inactivated at 56°C for 30 minutes, and tested in an hemagglutination inhibition (HI) assay with 0.5% chicken red blood cells [12]. Virus neutralization (VN) assays were performed according to World Health Organization guidelines [13]. To test the cross-reactivity of the ferret serum samples, β-propiolactone (BPL)–inactivated viruses were used in the HI assay. BPL inactivation was performed by adding 0.1% (vol/vol) of BPL to egg-grown virus stock and incubated at 4°C for 3 days before storage.
NA-specific antibodies were determined using the enzyme-linked lectin assay, as described elsewhere [14]. Recombinant rg-H6N9 virus comprising the NA from A/Anhui/1/2013 and a mismatched HA from A/Teal/Hong Kong/W312/1997 (H6N1) was used as antigen. Serum samples were tested at a starting dilution of 1:20.
Enzyme-Linked Immunosorbent Assay
Plates were coated overnight with 0.1 µg/mL of vaccine as antigen. Negative control wells were coated with buffer only. Plates were washed 3 times with PBS containing 0.05% Tween-20, blocked and added with serially diluted samples (starting at 1:200) for 2 hours at 37°C. Plates were washed and incubated with horseradish peroxidase–conjugated ferret immunoglobulin (Ig) G (Novus Biologicals) before reaction was developed with 3,3′,5,5′-tetramethylbenzidine substrate. Absorbance was read at 450 nm, and the last serum dilution that gave a positive/negative optical density readout ratio of >2 was determined as the end-point titer. For determination of the epitope integrity, enzyme-linked immunosorbent assay (ELISA) plates were coated with native or denatured vaccines and probed with polyclonal ferret serum samples raised against rg-A/Anhui/1/2013 (rg-H7N9), rg-A/Vietnam/1203/2004 (rg-H5N1) or A/California/04/2009 (H1N1) or monoclonal antibodies, as listed in the following section on
sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
SDS-PAGE Analysis
Vaccine (1 µg) was added to Laemmli buffer (250 mmol/L Tris-hydrochloride, 10% SDS, 30% glycerol, 5% β-mercaptoethanol, and 0.02% bromophenol blue) and boiled (denatured) for 5 minutes. In the native preparation, the β-mercaptoethanol and boiling were omitted. Samples were electrophoresed on a 4%–15% gradient SDS-PAGE gel and transferred onto a nitrocellulose membrane. Membranes were probed with mouse monoclonal antibodies against influenza A H1 (monoclonal antibody CA09-9; gift from Elena Govorkova, St Jude Children's Research Hospital), H5 (clone 8D2; Abcam), or H7 (clone 1H11; Abcam; derived from A/seal/Massachusetts/1980 [H7N7]), followed by anti-mouse IgG–horseradish peroxidase conjugate (Cell Signaling) and developed with enhanced chemiluminescent substrate.
Transmission Electron Microscopy
3 µL of sample was deposited on a thin carbon film supported on a Holey Formvar grid and allowed to stand for 3 minutes. 3 µL of 2% phosphotungstic acid or 1% uranyl acetate was added to negatively stain the sample for 1 minute. Air-dried samples were subjected to image acquisition at 80 or 200 kV with an FEI TF20 transmission electron microscope.
Statistical Tests
Data were analyzed using software from GraphPad Prism (version 5.03) and SAS (version 9.3). Antibody titers were expressed as geometric mean titers (GMTs) with upper and lower 95% confidence intervals (CIs), and viral titers as mean titers with standard deviations (expressed as log10 tissue culture infectious dose per milliliter or per gram tissue). Vaccine immunogenicity was compared using log10-transformed antibody titers with analysis of variance. Bonferroni corrections were made for multiple comparisons. Antibody titers measured with the different assays were correlated using untransformed titers by calculating the Spearman correlation coefficient (rs).
The antibody correlate for protection (defined as 50% reduction of total virus shed or 50% inhibitory concentration [IC50]) was determined from nonlinear regression (4-parameter model, with variable slope function) of log-transformed antibody titers and total virus load, expressed as the area under the curve (AUC) from a virus shedding curve. To obtain the IC50, AUC values were normalized to percentages, with the minimum AUC (baseline) set to 0% and maximum AUC defined as the mean AUC of saline control group, set at 100%. Linear mixed model with Kenward–Roger corrections were applied to investigate log10 -transformed viral titers across various treatment groups for the virus challenge study. A weighted Fisher exact test was applied to evaluate cross-reactivity. Differences were considered statistically significant at P ≤ .05.
RESULTS
Immunogenicity of Unadjuvanted, MF59 and AS03-adjuvanted H7N9 Vaccine in Ferrets
To assess the immunogenicity of the different vaccine formulation, we measured the serum antibody responses against HA (by HI) and NA (by enzyme-linked lectin assay) as well as by VN assay and ELISA. All unadjuvanted vaccines failed to induce detectable HI titer even after 2 doses (Table 1). In contrast, with the exception of the 3.75-µg, MF59-adjuvanted group, most ferrets that received adjuvanted vaccines developed HI titers ≥40 (considered protective based on human seasonal influenza vaccine studies in adults [15]) after 1 dose. After boosting, however, almost all ferrets in the adjuvanted vaccine groups had HI titers ≥40. A single dose at 3.75 µg adjuvanted with AS03 was able to induce an antibody titer of ≥40 in all the ferrets (GMT, 71.3; 95% CI, 34.9–145.7), compared with only 1 ferret in the MF59-adjuvanted group (6 of 6 vs 1 of 6; P = .02). After 2 doses, AS03-adjuvanted vaccines induced a 20-fold difference compared with MF59 in the 3.75-µg group (P < .001). However, with the 15-µg groups, the difference was not statistically significant. HI assay performed with horse red blood cells showed similar trends (Supplementary Table 2).
Table 1.
Serum Antibody Titers by Vaccine Group as Measured With HI and VN Assays
| Vaccine Group | Dose, µg | Serum Antibody Titer by HI Assay |
Serum Antibody Titer by VN Assay |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Titer for 1st Dose, GMT (95% CI) | Ferrets With Titer ≥40, No.a | Titer for 2nd Dose, GMT (95% CI) | Ferrets With Titer ≥40, No.a | 1st Dose, GMT (95% CI) | Ferrets With Titer ≥40, No.a | 2nd Dose, GMT (95% CI) | Ferrets With Titer ≥40, No.a | ||
| Saline | 0 | <20 | 0 | <20 | 0 | <20 | 0 | <20 | 0 |
| Unadjuvanted | 3.75 | <20 | 0 | <20 | 0 | 12.6 (4.3–37.7) | 2 | 11.2 (4.3–29.5) | 1 |
| 15 | <20 | 0 | <20 | 0 | 8.9 (3.4–23.4) | 1 | 6.3 (3.5–11.4) | 0 | |
| 45 | <20 | 0 | <20 | 0 | 10 (4.5–22.2) | 0 | 8.9 (3.4–23.4) | 1 | |
| MF59 | 3.75 | 11.2 (4–29.5) | 1 | 44.9 (10.9–184.3) | 5 | 63.5 (35.1–115.0) | 6 | 201.6 (83.5–486.5)b | 6 |
| 15 | 40.0 (11.8–135.1) | 5 | 320.0 (19.4–5256)b | 5 | 100.8 (47.5–213.7) | 6 | 905 (200.2–4092)b | 6 | |
| AS03 | 3.75 | 71.2 (34.9–145.7) | 6 | 905.1 (100.8–8125)b,d | 6 | 179.6 (103.9–310.6) | 6 | 1280 (414.7–3951)b,d | 6 |
| 15 | 127.0 (59.9–269) | 6 | 1016 (243.0–4248)b,c | 6 | 201.6 (138.5–293.5) | 6 | 1810 (984.9–3327)b,c | 6 | |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HI, hemagglutination inhibition; VN, virus neutralization.
a There were 6 ferrets per group. Titers <20 were arbitrarily set at 5. Statistical significance was determined by analysis of variance using log-transformed antibody titer, with Bonferroni adjustment for multiple comparisons.
b P < .001 for comparisons with saline control and unadjuvanted vaccine groups.
c P < .01 for comparison with MF59-adjuvanted, 3.75-µg vaccine group.
d P < .05 for comparison with MF59-adjuvanted, 3.75-µg vaccine group.
Because neutralization assays have greater sensitivity in detecting antibodies against avian influenza viruses [16–18], we tested the serum samples in a VN assay. The VN assay was more sensitive than the HI assay (Table 1) in detecting influenza antibodies, a difference that was most evident in the lower titer range. VN antibodies at titers >40 were detected in some ferrets that did not show HI titers ≥40 in the unadjuvanted vaccine and 3.75-µg, MF59 groups. Overall, after 2 doses of vaccines, VN titers correlated strongly with HI titers (rs = 0.92; P < .001) (Supplementary Figure 1A). This statistical significance was weaker when analysis was stratified to individual groups, probably owing to the smaller sample size. No meaningful correlations were detected for the unadjuvanted group because they had low or no detectable titers.
Adjuvanted vaccines also stimulated robust NA-inhibiting (NI) titers after 2 doses (GMT, 200–1796), compared with unadjuvanted vaccines (GMT, 7–13) (Table 2). AS03 induced approximately 4-5-fold higher NI titer compared with MF59 at the same doses, but this difference was not statistically significant. Overall, after 2 doses, there was a strong correlation between NI titers with VN (rs = 0.88; P < .001) (Supplementary Figure 1B) and HI (rs = 0.87; P < .001) (Supplementary Figure 1C) titers, suggesting that there is a concurrent induction of HA and NA antibodies that is associated with VN activities. When results were stratified by the individual vaccine group, significant HI-NI titer correlation was observed only in the MF59-adjuvanted 3.75-µg group (rs = 0.82; P = .06). Notably in the AS03-adjuvanted 15-µg group, there seemed a trend toward an inverse correlation between HI-NI titer (rs = −0.71; P = .13), and VN-NI titers (rs = −0.66; P = .18).
Table 2.
Serum NA-Inhibiting Antibody Titers by Vaccine Group as Measured With Enzyme-Linked Lectin Assay
| Vaccine Group | Dose, µg | NA-Inhibiting Antibody Titer |
|||
|---|---|---|---|---|---|
| Titer for 1st Dose, GMT (95% CI) | Ferrets With Titer ≥20, No.a | Titer for 2nd Dose, GMT (95% CI) | Ferrets With Titer ≥20, No.a | ||
| Saline | 0 | <20 | 0 | <20 | 0 |
| Unadjuvanted | 3.75 | <20 | 0 | 10 (4.5–22.2) | 1 |
| 15 | <20 | 0 | 6.3 (3.5–11.4) | 1 | |
| 45 | <20 | 0 | 15.8 (6.6–38.3) | 3 | |
| MF59 | 3.75 | 7.9 (3.7–16.8) | 2 | 200.0 (64.8–617.3)b | 5 |
| 15 | 17.8 (2.7–115.0) | 2 | 356.4 (80.7–1573)b | 5 | |
| AS03 | 3.75 | 35.6 (11.1–114.3) | 5 | 800.0 (285.9–2238)b | 6 |
| 15 | 35.6 (12.2–104.0) | 5 | 1796 (615.5–5241)b,c | 6 | |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; NA, neuraminidase.
a There were 6 ferrets per group. The starting dilution was 1:20, with titers <20 arbitrarily set at 5.
b P < .05 by analysis of variance for comparison with saline control and unadjuvanted vaccine group.
c P < .05 for comparison with MF59-adjuvanted, 3.75-µg vaccine group.
To determine whether the low titers detected in the unadjuvanted group were due to poor immunogenicity of the H7N9 vaccine or selective failure to induce functional antibodies, we measured total HA-specific IgG in serum samples. After 2 doses, unadjuvanted vaccine groups did not produce significantly higher titers compared with the saline group (Table 3). In contrast, all ferrets that received adjuvanted vaccines showed at least 50–100-fold higher influenza HA-specific IgG titer (mean GMT, 11 404–25 600). There was also a significant correlation between HA-specific IgG-titers and VN titers (rs = 0.91; P < .001) overall, but no significant correlation was detected with stratification by individual vaccine groups (Supplementary Figure 1D). Taken together, the H7N9 vaccine was poorly immunogenic overall, generating low antigen-specific antibody responses.
Table 3.
Serum Influenza HA-Specific IgG Titers by Vaccine Group as Measured With ELISA
| Vaccine Group | Dose, µg | Serum IgG Titer |
|||
|---|---|---|---|---|---|
| Titer for 1st Dose, GMT (95% CI) | Ferrets With Titer Higher Than Baseline, No.a | Titer for 2nd Dose, GMT (95% CI) | Ferrets With Titer Higher Than Baseline, No.a | ||
| Saline | 0 | 200 (200.0–200.0) | 0 | 200 (200.0–200.0) | 0 |
| Unadjuvanted | 3.5 | 224.5 (166.8–302.1) | 1 | 224.5 (166.8–302.1) | 1 |
| 15 | 252 (139.1–456.4) | 1 | 317.5 (175.3–575.0) | 3 | |
| 45 | 224.5 (166.8–302.1) | 1 | 449.0 (191.8–1051) | 4 | |
| MF59 | 3.5 | 635 (436.1–924.5) | 6 | 10 159 (4209–24 520)b | 6 |
| 15 | 3592 (2077–6211) | 6 | 36 204 (13 281–98 692)b,c | 6 | |
| AS03 | 3.5 | 4032 (1902–8547) | 6 | 28 735 (21 351–38 673)b | 6 |
| 15 | 12 800 (6677–24 537) | 6 | 36 204 (16 880–77 650)b,c | 6 | |
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer; HA, hemagglutinin; IgG, immunoglobulin G.
a There were 6 ferrets per group. Baseline is the maximum titer (200) in the saline control group that was positive.
b P < .001 by analysis of variance for comparison with saline control and unadjuvanted vaccine group.
c P < .05 for comparison with MF59-adjuvanted 3.75-µg vaccine group.
Protection Against Virus Challenge
To evaluate the protective efficacy of the vaccines, we subsequently challenged the ferrets with 105 EID50 of the wild-type A/Anhui/1/3013 (H7N9) virus. At challenge, no overt symptoms were observed in any ferrets although, inexplicably, ferrets in the 45-µg group seemed to lose more weight than the saline-treated group (Supplementary Figure 2).
With the exception of the 3.75-µg, MF59 group, ferrets in the adjuvanted vaccine groups shed less virus in the nasal wash samples than did the unadjuvanted and saline groups on days 3 and 5 (Figure 1) (.0003 ≤ P < .05). There was an earlier viral clearance in all ferrets in the adjuvanted vaccine and the 45-µg groups but not in the saline and unadjuvanted 3.75-µg and 15-µg groups. There was a trend toward reduced viral shedding in the AS03 group compared with the MF59 group, but this difference was not statistically significant. In the lungs, 1 ferret each in the saline control and 45-µg groups and 2 ferrets in the 15-µg group had detectable virus titer; no ferrets in the unadjuvanted 3.75-µg and the adjuvanted vaccine groups had detectable virus titers in the lungs (Supplementary Table 1).
Figure 1.
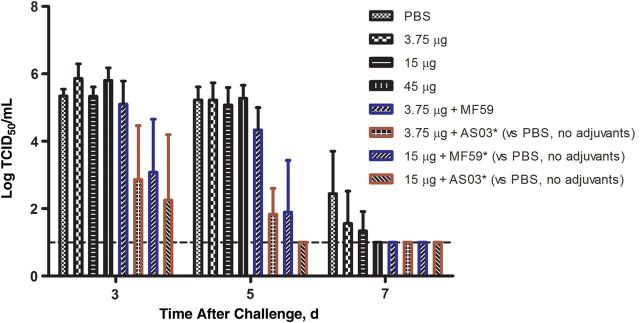
Viral titers in the respiratory tracts of vaccinated and saline control ferrets after challenge with A/Anhui/1/2013 (H7N9) virus. Virus titers in the upper respiratory tract were determined from nasal wash samples collected on days 3, 5, and 7 after challenge. Viral titers are presented as means and standard deviations. Linear mixed model with Kenward–Roger corrections were applied to investigate log10 -transformed viral titers across various groups, and differences were considered statistically significant at P ≤ .05. Abbreviations: PBS, phosphate-buffered saline; TCID50, tissue culture infectious dose-50.
Correlate of Protection
To obtain a measure of correlate of protection based on antibody titers in the ferret model, we performed nonlinear regression of serum HI, VN, NA, and HA-IgG titer with the cumulative viral load shed by the individual ferrets. All 4 antibody titers showed a good correlation with the cumulative virus load, with HI titers showing the best correlation (R2 values as follows: HI, 0.89; VN, 0.82; NI, 0.73; and HA-IgG, 0.63) (Figure 2). Based on the curve fitted onto the data, the antibody titer that correlates to a 50% reduction in cumulative viral load, or IC50, was 115 (95% CI, 75–176) for HI, 365 (235–568) for VN, 164 (91–298) for NA, and 9730 (4185–22 618) for HA-IgG. The large CI for the IC50 for NI, and HA-IgG titers suggests that these assays may not be as robust in predicting infection outcome.
Figure 2.
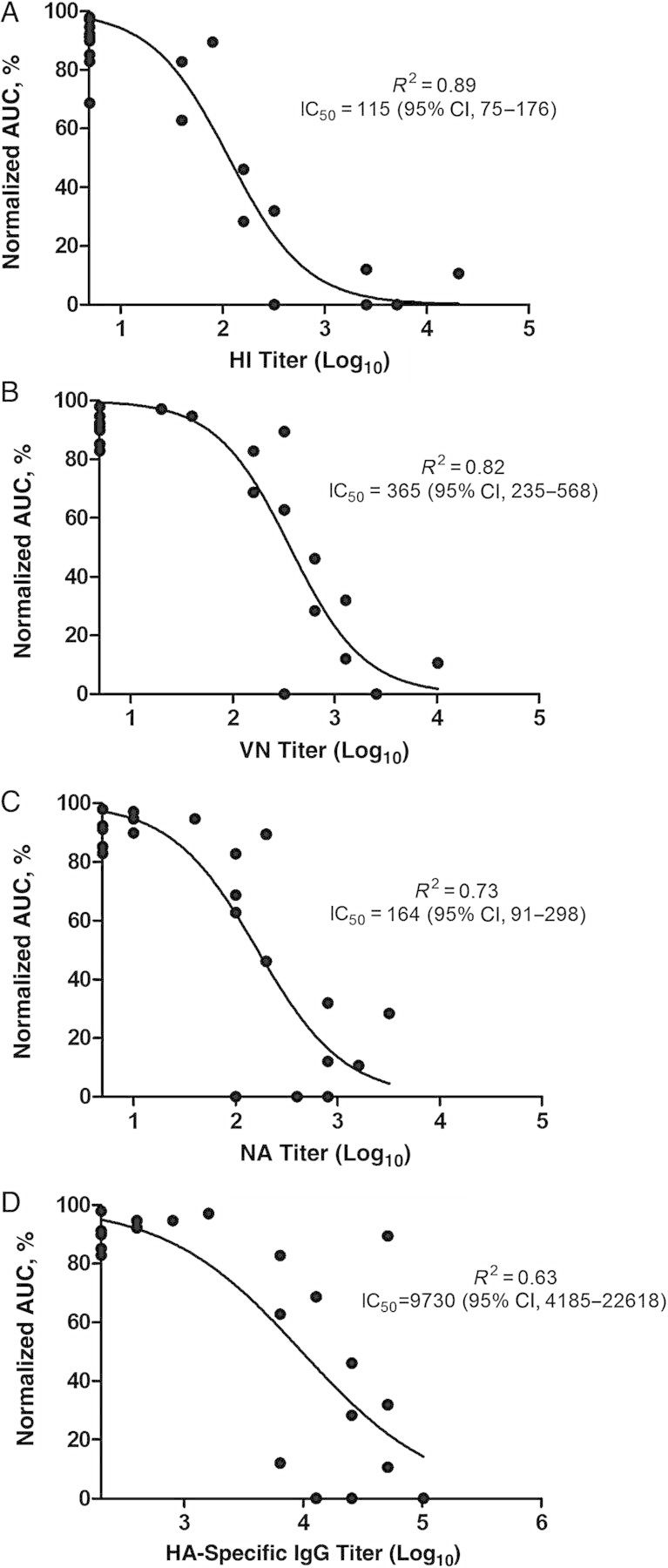
Relationship between hemagglutination inhibition (HI) (A), virus neutralization (VN) (B), neuraminidase (NA) (C), and hemagglutinin (HA)–immunoglobulin (Ig) G antibody (D) titers with total viral load. The x-axes represent log-transformed antibody titers; the y-axes, the area under the curve (AUC) of a viral shedding curve as a measure of cumulative viral load throughout the study period. The AUC values here have been normalized to a percentage, with the minimum and maximum AUCs set to 0% and 100%, respectively. The R2 value indicates the goodness of fit, and the antibody titer that correlates to 50% reduction in total viral load is expressed as the 50% inhibitory concentration (IC50). Abbreviation: CI, confidence interval.
Cross-reactivity of the H7N9 Vaccine
To test the cross-reactivity of the antibodies, we tested the serum samples after 2 doses in an HI assay against 2 H7 strains that have caused human infections, that is, H7N7 (A/Netherlands/219/03), and H7N3 (rg-A/Canada/rv444/04). Two prototypical H7 strains, A/equine/Prague/2/56 (H7N7) and A/ruddy turnstone/New Jersey/65/85 (H7N3), were included as references. Netherlands/219, equine/Prague, and the H7N9 viruses represent the Eurasian genetic lineage, which are genetically and antigenically dissimilar from the North American lineage viruses (Canada/rv444 and ruddy turnstone/NJ) (Supplementary Table 3).
The unadjuvanted vaccine group did not have detectable HI titer to any of the viruses tested (data not shown), and ferrets in the 3.75-µg, MF59-adjuvanted group showed no or low cross-reactive activity. HI titers were detected in ferrets that mounted a robust homologous HI antibody response (Table 4). Titers were higher against Netherlands/219 (H7N7) than against Canada/rv444 (H7N3) in the 15-µg, MF59-adjuvanted group (P < .01). Interestingly, despite the higher homologous GMT in the AS03, 15-µg group, the magnitude, number of seropositive ferrets, and breadth of cross-reactivity were lower than in the lower-dose (3.75-µg) group (P < .001). This observation was still valid even after the high-responder ferret, ARC12, was excluded from analysis (P < .001).
Table 4.
Cross-reactivity of Vaccinated Ferret Serum to Heterologous H7 Avian Influenza Viruses in an HI Assay
| Vaccine Group (Dose, µg) by Ferret ID | HI-Titer by Virus Straina |
||||
|---|---|---|---|---|---|
| rg-A/Netherlands/219/03 (H7N7) | rg-A/Canada/rv444/04 (H7N3) | A/Equine/Prague/56 (H7N7) | A/Ruddy Turnstone/NJ/65/85 (H7N3) | A/Anhui/1/2013 (H7N9)b | |
| MF59 (3.75) | |||||
| ARC1 | 5 | 5 | 5 | 5 | 40 |
| ARC2 | 5 | 5 | 5 | 5 | 40 |
| ARC3 | 5 | 5 | 20 | 5 | 80 |
| ARC4 | 5 | 5 | 5 | 5 | 5 |
| ARC5 | 5 | 5 | 20 | 40 | 320 |
| ARC6 | 5 | 5 | 5 | 5 | 40 |
| GMT (95% CI) | 5 (5) | 5 (5) | 7.9 (3.7–16.83) | 7.1 (2.9–17.2) | 44.9 (10.9–184.3) |
| AS03 (3.75) | |||||
| ARC7 | 5 | 5 | 40 | 40 | 320 |
| ARC8 | 5 | 5 | 5 | 40 | 160 |
| ARC9 | 40 | 40 | 80 | 160 | 2560 |
| ARC10 | 5 | 5 | 20 | 40 | 320 |
| ARC12 | 80 | 40 | 320 | 320 | 40 960 |
| ARC13 | 40 | 20 | 40 | 80 | 320 |
| GMT (95% CI) | 15.9 (4.1–61.5) | 12.6 (4.2–37.7) | 40 (9.4–171.4) | 80 (31.9–200.8) | 905.1 (100.8–8125) |
| MF59 (15) | |||||
| ARC23 | 20 | 5 | 5 | 40 | 320 |
| ARC24 | 40 | 20 | 80 | 80 | 20 480 |
| ARC25 | 40 | 5 | 5 | 10 | 5 |
| ARC26 | 20 | 20 | 20 | 40 | 640 |
| ARC27 | 40 | 5 | 5 | 40 | 160 |
| ARC28 | 40 | 10 | 20 | 40 | 320 |
| GMT (95% CI) | 31.7 (21.8–46.2) | 8.9 (4.3–18.2) | 12.6 (3.8–41.3) | 35.6 (17.4–72.9) | 320.0 (19.4–5256) |
| AS03 (15) | |||||
| ARC29 | 5 | 5 | 5 | 5 | 160 |
| ARC30 | 160 | 80 | 160 | 80 | 2560 |
| ARC31 | 5 | 5 | 10 | 20 | 5120 |
| ARC32 | 5 | 5 | 20 | 20 | 640 |
| ARC34 | 5 | 5 | 5 | 40 | 320 |
| ARC35 | 5 | 5 | 160 | 40 | 2560 |
| GMT (95% CI) | 8.9 (2.0–39.4) | 7.9 (2.5–26.0) | 22.4 (4.2–121.1) | 25.2 (9.3–68.1) | 1016 (243.0–4248) |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HI, hemagglutination inhibition.
a Titers less than the starting dilution of 1:20 were arbitrarily set at 5.
b Homologous titers.
In Vitro Correlate of Immunogenicity
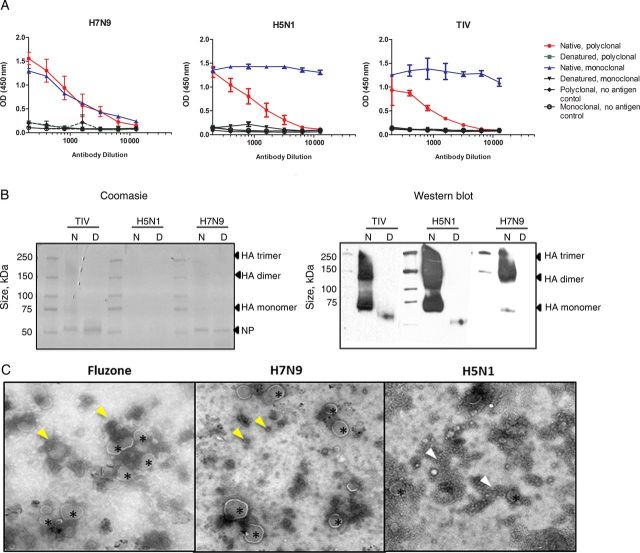
To better understand the in vitro correlates of immunogenicity, we undertook an investigation similar to that done by Couch et al [9], comparing the current H7N9 vaccine with the human seasonal and the H5N1 vaccines. We used ELISAs and Western blot analyses with native and denatured vaccine preparations to evaluate the integrity of the vaccine epitopes (Figure 3A). With ELISAs, reactivity was higher with native vaccine in both ferret polyclonal serum samples and monoclonal antibodies for all 3 vaccines. In a Coomasie-stained gel, bands corresponding to sizes of nucleoprotein, HA-monomer (75 kDa), and larger protein complexes were visible in the native samples for TIV and H7 (150–250 kDa) (Figure 3B), but not the H5 vaccine. In the Western blot analysis, blot bands were detected in the native lanes at high molecular weights corresponding to HA trimer, dimer, and monomers in all 3 vaccines (Figure 3B). Cleaved HA bands were also detected for TIV and H5 vaccine under nonreduced conditions (data not shown). A band corresponding to HA-monomer was detected in denatured samples in the TIV and H5 samples but not in the denatured H7 samples. This could likely due to the mismatched H7 monoclonal antibody used as the primary detection antibody. These results suggest that the H7N9 vaccine contained HA complexes with intact immune epitopes.
Figure 3.
Conformation and morphological evaluation of the vaccines. A, Reactivity of immune serum samples to native and denatured H7N9 vaccine, rg-A/Vietnam/1203/2004 (H5N1) vaccine, and seasonal trivalent influenza vaccine (TIV; Fluzone) by enzyme-linked immunosorbent assay. The polyclonal serum samples used against the respective vaccine types were ferret polyclonal serum samples raised against rg-A/Anhui/1/2013 (H7N9), rg-A/Vietnam/1203/04 (H5N1), and A/California/04/2009 (pdmH1N1), and the monoclonal antibodies used were H7 (clone 1H11; Abcam), H5 (clone 8D2; Abcam), and H1 specific. Wells coated with buffer only served as background control. B, Coomasie-stained polyacrylamide gel electrophoresis gel and Western blot analysis of native (N) and denatured (D) preparation of the TIV, H7N9, and H5N1 vaccines. The blot was allowed to be overexposed to visualize the less abundant hemagglutinin (HA) monomer in the reduced samples. C, Electron micrograph of the vaccines. Small spherical structures (arrowheads) and large spherical structures with glycoproteinlike projections emanating from the convex face (asterisks) are seen. Images were captured at ×29 000 magnification for TIV and H7N9 and ×50 000 magnifications for H5N1 vaccines. Abbreviations: NP, nucleoprotein; OD, optical density.
Electron micrographs showed that all 3 vaccines contained numerous small, punctate structures (10–30 nm; yellow arrowheads in Figure 3C), which tended to aggregate in the H5 vaccine (white arrowhead). Larger spherical structures with glycoproteinlike projections, corresponding to those described by Couch et al [9] as intact or split-virus particles, were observed with H7N9 and TIV vaccine preparations but were mostly absent with the H5N1 preparation.
DISCUSSION
The results of a phase II trial (NTC01938742) of the same H7N9 vaccine used in our study were published in 2014 [19]. Our findings mirrors the clinical findings in 2 ways: (1) adjuvanted vaccines induced more robust antibody titers than unadjuvanted vaccines and (2) neutralization titers were generally 2–3-fold higher than HI titers. In contrast to our results, Mulligan et al [19] did not see improved antibody titers with increasing HA dose beyond the 3.75-µg HA-MF59 adjuvanted dose. Although this is speculative, perhaps the saturated response is due to the interaction with preexisting immunity to seasonal influenza in adult humans, as reported in that study [19], compared with the naive ferrets that we used. In some situations however, antigen dose can influence immunogenicity, as was observed in the MF59-adjuvanted H5N1 clinical trial [20].
Adjuvant use has circumvented the issue of poor immunogenicity for influenza vaccines in many situations [21–25]. Both MF59 and AS03 are squalene-based emulsions that act by stimulating a local inflammatory environment, and by promoting the recruitment and activation of immune cells. AS03 has an added component of α-tocopherol (vitamin E), an immunostimulant [26] that was important for its potent adjuvant effect [27]. Despite the better immunogenicity of AS03-adjuvanted vaccines, α-tocopherol can also be associated with higher reactogenicity. Thus, recent formulations of AS03 with less α-tocopherol have shown a better reactogenicity-immunogenicity balance [28, 29]. In our study, when AS03 was administered to ferrets in equivalent doses, we found that it was more potent than MF59, because it raised higher antibody titers at a lower antigen dose. However, at a 15-µg dose, there were essentially no differences between the adjuvants. Because Focetria and Pandemrix (MF59- and AS03-adjuvanted monovalent 2009 pandemic H1N1 vaccines, licensed for use in Europe), are formulated in 7.5- and 3.75-µg doses, respectively, we expect that the eventual MF59-adjuvanted H7N9 vaccine will be formulated with a higher dose of HA than AS03, and will thus be likely to show comparable immunogenicity.
In our study, ferrets that had a robust antibody response also mounted cross-reactive antibodies to other H7 strains. This may already be sufficient to protect against heterologous H7 virus infection, [30] or prime the adaptive immune arm against subsequent H7 virus infections. Interestingly, with an increased antigen dose, AS03 seemed to have a more HA-dominant and specific antibody repertoire, as evidenced by the inverse correlation in HI-NI titer and limited cross-reactive titers. Because the availability of antigen can influence the resulting antibody repertoire in an adjuvanted vaccine response [31], it is likely that the higher abundance of HA may have dominated and perhaps even narrowed the specificity of the antibody response.
Although VN assays were more sensitive than HI assays, HI titers were a better antibody correlate of protection, at least in the ferret model. Based on the predicted IC50, a single dose of AS03-adjuvanted vaccine at 15 µg may already be sufficient to reduce the viral load by 50% in the ferret. Nevertheless, though this correlate of protection is in the context of the ferret model and does not represent a similar correlate in humans, it does demonstrate that antibody-mediated protection is real. This protection is also not due to nonspecific effects mediated by the adjuvants since immunization with the adjuvants alone has been shown not to impact viral burden [32]. Although preexisting HI titers were well correlated to reduced viral load, the absence of an HI titer does not preclude the absence of protection. Observations from other studies [33, 34], as well as our own finding of early viral clearance in the unadjuvanted 45-µg group, suggest that other immune mechanism, such as cellular immunity, may be important in limiting virus infection after vaccination.
A limitation to our study is that we were unable to determine the vaccine's effects in preventing infection in the lungs because we observed inconsistent results in the lung viral titers. Although the replication is efficient in the upper respiratory tract, replication in the lungs is less uniformly achieved with H7N9 in the ferret model [35–39].
In conclusion, we find that adjuvanted H7N9 vaccines stimulated robust antibody response and decreased the viral load in infected ferrets. Given the dismal performance of unadjuvanted formulations in human clinical trials [4, 5, 40], we believe that adjuvanted H7N9 vaccine would be sufficiently immunogenic and able to reduce the H7N9 disease burden in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Office of Biomedical Advanced Research and Development Authority for the vaccine and adjuvants used in this study. We also thank Beth Little and David Carey from the Animal Resource Center, and Sharon Frase and Richard Gursky in the Cell and Tissue Imaging core facility for excellent technical assistance and Yulong Shu, PhD from the Chinese Center for Disease Control, Beijing for providing the A/Anhui/1/2013 (H7N9) virus.
Financial support. This work was supported by the American Lebanese Syrian Associated Charities and by the National Institute of Allergy and Infectious Disease's program Centers of Excellence for Influenza Research and Surveillance (Contract Number HHSN266200700005C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Human infections with avian influenza A (H7N9) virus 2014. http://www.who.int/influenza/human_animal_interface/RiskAssessment_H7N9_21Jan14.pdf Accessed 3 March 2014. [PubMed]
- 2.Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013; 368:2277–85. [DOI] [PubMed] [Google Scholar]
- 3.Lam TT, Wang J, Shen Y, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013; 502:241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–51. [DOI] [PubMed] [Google Scholar]
- 5.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One 2012; 7:e49704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresson JL, Perronne C, Launay O, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006; 367:1657–64. [DOI] [PubMed] [Google Scholar]
- 7.Cox RJ, Madhun AS, Hauge S, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889–97. [DOI] [PubMed] [Google Scholar]
- 8.Reber AJ, Chirkova T, Kim JH, et al. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis 2012; 3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 9.Couch RB, Decker WK, Utama B, et al. Evaluations for in vitro correlates of immunogenicity of inactivated influenza a H5, H7 and H9 vaccines in humans. PLoS One 2012; 7:e50830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO provisional recommendation on influenza A(H7N9) vaccine virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ProvisionalRecommendation_H7N9_31May13.pdf Accessed 21 January 2014.
- 11.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol 1938; 27:493–7. [Google Scholar]
- 12.World Health Organization Global Influenza Surveillance Network. Serological diagnosis of influenza by hemagglutination inhibition testing: manual for the laboratory diagnosis and virological surveillance of influenza. Geneva: WHO; Press, 2011:59–62. [Google Scholar]
- 13.World Health Organization Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva: WHO Press, 2011. [Google Scholar]
- 14.Sandbulte MR, Gao J, Straight TM, Eichelberger MC. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza Other Respir Viruses 2009; 3:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood JM, Levandowski RA. The influenza vaccine licensing process. Vaccine 2003; 21:1786–8. [DOI] [PubMed] [Google Scholar]
- 16.Kida H, Ito T, Yasuda J, et al. Potential for transmission of avian influenza viruses to pigs. J Gen Virol 1994; 75(pt 9):2183–8. [DOI] [PubMed] [Google Scholar]
- 17.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001; 19:1732–7. [DOI] [PubMed] [Google Scholar]
- 18.Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis 2006; 194:159–67. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan MJ, Bernstein DI, Winokur P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1409–19. [DOI] [PubMed] [Google Scholar]
- 20.Belshe RB, Frey SE, Graham IL, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1420–8. [DOI] [PubMed] [Google Scholar]
- 21.Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–16. [DOI] [PubMed] [Google Scholar]
- 22.Gasparini R, Pozzi T, Montomoli E, et al. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol 2001; 17:135–40. [DOI] [PubMed] [Google Scholar]
- 23.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 2010; 201:1644–53. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937–43. [DOI] [PubMed] [Google Scholar]
- 25.Langley JM, Risi G, Caldwell M, et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis 2011; 203:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giudice GD, Rappuoli R. Inactivated and adjuvanted influenza vaccines. In: Compans RW, Oldstone MBA, eds. Influenza pathogenesis and control—volume II. Series: Current Topics in Microbiology and Immunology, Vol 386 Cham, Switzerland: Springer International Publishing, 2015:151–80. [DOI] [PubMed] [Google Scholar]
- 27.Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011; 29:2461–73. [DOI] [PubMed] [Google Scholar]
- 28.Meier S, Bel M, L'Huillier A, et al. Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine 2011; 29:3548–57. [DOI] [PubMed] [Google Scholar]
- 29.Rumke HC, Richardus JH, Rombo L, et al. Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial. BMC Infect Dis 2013; 13:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer F, Albrecht RA, Tan GS, et al. Divergent H7 immunogens offer protection from H7N9 challenge. J Virol 2014; 88:3976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baras B, Stittelaar KJ, Simon JH, et al. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One 2008; 3:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrest HL, Khalenkov AM, Govorkova EA, Kim JK, Del Giudice G, Webster RG. Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine 2009; 27:4187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis 2006; 194:1040–3. [DOI] [PubMed] [Google Scholar]
- 35.Belser J, Tumpey T. Mammalian models for the study of H7 virus pathogenesis and transmission. In: Compans RW, Oldstone MBA, eds. Influenza pathogenesis and control—volume I. Vol 385 Springer International Publishing, 2014:275–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belser JA, Gustin KM, Pearce MB, et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 2013; 501:556–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, Kiso M, Fukuyama S, et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature 2013; 501:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Shi J, Deng G, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 2013; 341:410–4. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Wang D, Kelvin DJ, et al. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science 2013; 341:183–6. [DOI] [PubMed] [Google Scholar]
- 40.Atmar RL, Keitel WA, Patel SM, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis 2006; 43:1135–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.