Abstract
We investigated neural correlates when attending to a movement that could be made automatically in healthy subjects and Parkinson's disease (PD) patients. Subjects practiced a visuomotor association task until they could perform it automatically, and then directed their attention back to the automated task. Functional MRI was obtained during the early-learning, automatic stage, and when re-attending. In controls, attention to automatic movement induced more activation in the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex, and rostral supplementary motor area. The motor cortex received more influence from the cortical motor association regions. In contrast, the pattern of the activity and connectivity of the striatum remained at the level of the automatic stage. In PD patients, attention enhanced activity in the DLPFC, premotor cortex, and cerebellum, but the connectivity from the putamen to the motor cortex decreased. Our findings demonstrate that, in controls, when a movement achieves the automatic stage, attention can influence the attentional networks and cortical motor association areas, but has no apparent effect on the striatum. In PD patients, attention induces a shift from the automatic mode back to the controlled pattern within the striatum. The shifting between controlled and automatic behaviors relies in part on striatal function.
Keywords: attentional networks, controlled pattern, dopamine depletion, neural correlates, putamen
Introduction
A general characteristic of the motor system is that people can perform some learned movements automatically. Automatic movements are executed without attention directed towards the details of the movement. Automaticity is common, particularly for movements that require low levels of precision or for movements that are frequently made (Bernstein 1967). Indeed, many of our daily behaviors are carried out automatically.
However, it is also common that people in some situations choose to pay attention to the details of the movements that already can be automatically performed. For example, walking is automatic in adults; but, in some situations, like walking in a cluttered environment, people may need to think about each step. While the processing of automaticity is accompanied by improved performance, it has been recognized that attention can disrupt the performance of well-practiced skills (Wulf and Prinz 2001). For example, after extensive practice, gymnasts are able to perform some complex skills automatically. However, more attention directed to the performance, such as during a competition, may disrupt the performance of a well-practiced skill.
Previous studies found that when a movement becomes automatic, neural efficiency is increased, which appeared as a reduction of neural activity (Wu et al. 2004; Lehéricy et al. 2005; Poldrack et al. 2005; Balsters and Ramnani 2011), and a more tight connection of some task-related neural networks (Wu et al. 2008). Moreover, automated motor skills are likely stored in or facilitated by the sensorimotor territory of the striatum (Lehéricy et al. 2005).
In contrast to automatic processing, the behavioral and neural modulations when attending to movements that can already be automatically performed have never been investigated. In an early study, Jueptner, Stephan et al. (1997) showed more activity in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) when attending to an overlearned motor sequence. However, it is unclear whether the subjects achieved automaticity in that study. Improvement on a task after extensive practice does not guarantee that it is automatic (Lang and Bastian 2002).
Previous studies have commonly reported increased activity in cortical regions during attention to motor tasks (Jueptner, Stephan et al. 1997; Rowe et al. 2002); thus, we expect that attention to movements that could be made automatically would be accompanied by increased activation in attentional networks and cortical motor association regions. What we focused on in the current study was the effects of attention on the striatum. As the striatum has been suggested to be important in both automatic and controlled processing (Poldrack et al. 1999; Beauchamp et al. 2003; Debaere et al. 2004), attention might result in a shift from the automatic mode back to the controlled pattern within the striatum. However, Jueptner, Stephan et al.'s study (1997) only showed a trend of more activation in the caudate nucleus when attending to learned movements when a very low threshold was applied (P < 0.01, uncorrected). Therefore, it is possible that the activity pattern in the striatum may remain at the automatic stage, and is not modulated by attention.
We further investigated whether the storage of an automatic pattern relies on a functionally intact striatum. An ideal model to investigate this problem is Parkinson's disease (PD). It has been recognized that PD patients have difficulty in achieving and performing movement automatically (Wu and Hallett 2005); in contrast, attention towards the motor task may improve movements (Oliveira et al. 1997). Attention to motor action has different influences on cortical networks in PD patients compared with healthy subjects, like less augmentation of activation in the supplementary motor area (SMA), and a disconnection between the prefrontal cortex, premotor cortex (PMC), and SMA (Rowe et al. 2002). We assume that PD patients must use different strategies to store and execute automatic programs, as dopamine is significantly depleted mostly in the posterior putamen, a part of the sensorimotor striatum (Brooks et al. 1990); this automatic pattern may not be stable and might be modulated by attention.
In the current study, we used functional MRI (fMRI) to investigate the underlying neural changes at both local activity and network levels when attending to movements that could be made automatically in PD patients and healthy subjects. This study promotes understanding about the role of the striatum in shifting, execution, and storage of automatic movements.
Materials and Methods
Subjects
Thirty PD patients were involved in this study. The diagnosis of PD was based on the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes et al. 1992). Akinesia/rigidity was the predominant symptom and was more severe on the right side in every patient. To avoid disturbance of the fMRI signal, all patients were chosen to have at most a mild tremor. Patients were assessed with the UPDRS (Unified Parkinson's Disease Rating Scale; Lang and Fahn 1989), the Hoehn and Yahr disability scale (Hoehn and Yahr 1967) and Mini-Mental State Examination while off their medications. After extensive practice, 25 patients achieved automaticity. Three patients were excluded because of excessive head motion during the fMRI acquisition. The remaining 22 patients were on average 55.86 years old (ranged from 52 to 61 years old, 7 females, 15 males). The clinical data are shown in Table 1.
Table 1.
Clinical details of patients with Parkinson's disease (mean ± SD)
| Age (years) | 55.86 ± 3.21 |
| Sex | 7 females, 15 males |
| Disease duration (years) | 2.86 ± 1.17 |
| UPDRS Motor score (off medication) | 16.77 ± 4.34 |
| Hoehn and Yahr staging (off medication) | 1.52 ± 0.39 |
| Mini-Mental State Examination | 29.14 ± 0.99 |
| l-Dopa dose (mg/day) | 306.82 ± 74.48 |
Twenty-two age and gender matched healthy subjects were involved as the control group (average 55.54 years old, ranged from 52 to 61 years old, 7 females, 15 males). All PD and control subjects were right-handed according to the Edinburgh Inventory (Oldfield 1971). The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects gave their written informed consent for the study.
Task
All subjects were instructed to press a button with different right hand fingers according to the visual signals being presented (visuomotor association task). They used the index finger when a red circle or a yellow square was shown, and used the middle finger when a green circle or a black square was shown, and used the ring finger when a yellow circle or a green square was presented. Each visual signal was presented for 300 ms. Intervals between the signals were irregular (from 3 to 4 s). The paradigm and an example sequence of the visuomotor task are shown in Supplementary Figure 1. As a first priority, we asked the subjects to perform the task correctly, and then a second priority, to press as quickly as possible.
fMRI and Training Procedure
Patients were studied only after their medication had been withdrawn for at least 12 h. Three fMRI scanning sessions were conducted in 2 days. fMRIs only contained the visuomotor task, and were performed on a 3-T MR scanner (Trio system; Siemens Magnetom scanner, Erlangen, Germany). A standard head coil was used with foam padding to restrict head motion. High-resolution axial T1- and T2- weighted images were obtained in every participant to detect clinically silent lesions. High-resolution anatomical images were acquired with 3D-MPRAGE sequence (TR = 2530 ms, TE = 3.39 ms, 128 axial slices, 1.33-mm thickness, field of view [FOV] = 256 mm). Blood oxygen level–dependent (BOLD) data were acquired with gradient-echo echo-planar sequences (TR = 2000 ms, TE = 30 ms, 33 axial slices, 3.5-mm thickness, Flip angle = 90°, FOV = 220 mm, matrix size = 64 × 64). Each fMRI trial was 10 min. All trials were block-designed and contained 2 conditions, which were defined as the “rest” and “active” condition, respectively. Each condition lasted 30 s and was repeated 10 times. During the active condition, the subjects performed the visuomotor association task. The visual signals were presented on a screen in front of the subjects. In the rest condition, subjects were asked to relax and focus on the screen. A MRI compatible electrical response device with 3 buttons was fixed to each subject's right hand and was used to record finger presses during fMRI scanning. Feedback was presented at the end of each scan, but not during the scan, to tell the subjects whether their performance was correct.
The first scanning session was done on the first day of training, just as the subjects could perform the task in one trial correctly (early-learning stage). After this scanning session, the subjects had more training. Each training trial lasted 3 min, the intertrial interval was 2 min, and 10 trials composed a training session. There were 4 training sessions each day, and the subjects had about 15 min rest between training sessions. Training was conducted on consecutive days. The accuracy of finger press and reaction times (RTs) in each trial were recorded. No feedback was provided during training trials to tell subjects whether their finger movements were correct or incorrect. However, after each trial, we told the subjects if they made errors during the trial.
The second fMRI session was done when the subjects could perform the task automatically (automatic stage). The evidence that a task has become automatic can be proven by the fact that a secondary task can be performed without obvious interference (Passingham 1996; Wu et al. 2004). In the present study, we used a dual-task paradigm, which contained an auditory counting task to be performed simultaneously with the visuomotor task, to evaluate whether automaticity was achieved (Wu et al. 2004). For the auditory counting task, a random series of the numbers 1, 2, 3, and 4 were read and subjects were asked to identify the number of times they heard a specified target number. The numbers were presented at the same time as the visual signals. The paradigm is similar to that employed in previous studies (Lang and Bastian 2002; Wu et al. 2004; Wu and Hallett 2005). Similar to the visuomotor task trial, a dual-task trial also lasted 3 min, and intervals between the tasks were irregular (from 3 to 4 s). The average RT of the visuomotor task was assessed, and, at the end of the trial, subjects reported the number of target letters. Only when the subjects could perform the dual-task correctly (without error for either visuomotor and auditory counting task), and the RT of visuomotor task in the dual-task trial had no significant difference from that in the single visuomotor task trial, would their performance on the visuomotor task be considered automatic. If after 5 days' practice (20 training trials), the subjects still could not perform the task automatically, they were considered as having difficulty in achieving automaticity. Then, their training was stopped and their data were removed.
After the second scan, we told the subjects that there were errors in performance, even if their performance was correct, but did not show them which presses were incorrect. Then we asked them to perform the third scanning trial immediately, and emphasized that they must perform the task correctly with first priority (attention stage). We did not simply require the subjects to pay more attention to the task, because otherwise they would have difficulty to know how much attention they should use on the task, and which component of the task they should attend to, as they already could perform the task automatically. However, since we told them there were errors, they would have to increase their attention to the accuracy of the task to ensure that their performance was correct. After this session, all subjects reported that they paid attention to the accuracy of the task to ensure the task was correctly performed.
Data Analysis
Behavioral Data Analysis
For training or scanning trials of the visuomotor task, the accuracy and RT of finger presses were recorded. The button presses that did not correspond to the target visual signals were considered errors. For the dual-task, both the accuracy and RT of finger presses, and the numbers being reported were recorded. The RT of visuomotor task in the dual-task trial was compared with that in the single visuomotor task trial in each subject to examine whether the performance of visuomotor task achieved automatic stage (two-sample t-test, P > 0.05).
We used a mixed-effects model with random block effect and fixed effects of group and stages. The study groups (PD patients and controls) and stages (early-learning, automatic, attention), and group-stage interaction were considered as fixed effects and intercept term as random effect. Post hoc tests of between stages or groups were investigated using polynominal contrasts and Tukey test was applied with correction of family-wise error (FWE). All tests were two-sided, with a P value of 0.05 considered statistically significant. Statistical analyses were performed with SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA).
Imaging Data Analysis
Data Preprocessing
Image analysis was performed with SPM8 software (Wellcome Institute of Cognitive Neurology, London, UK). fMRI data were slice-time corrected and aligned to the first image of each run for motion correction. Functional images were co-registered to high-resolution anatomical images. After spatial normalization, all images were resampled into voxels that were 3 × 3 × 3 mm in size, and smoothed with a 6-mm Gaussian smoothing kernel. Each participant's movement parameters were examined. Three patients had excessive head motion (>1.5 mm maximum translation in x, y, or z, or 1.5° of maximum angular rotation about each axis), and their datasets were discarded.
Brain Activity Analysis
Data were analyzed for each single participant separately on a voxel-by-voxel basis using the general linear model approach for the time series. We defined a model using a fixed-effect boxcar design convolved with a hemodynamic response function for analysis of task-dependent activation. We added the 6 head motion parameters as regressors to optimally control for the motion effects. A contrast representing the effect of the active condition compared with the rest condition was calculated in each participant. These contrast images were used in the second level for random-effects analyses.
At the second level, first, a one-sample t-test model was used to identify the brain activity in each stage in each group (P < 0.05, FWE corrected). We performed a repeated-measures analysis of variance (ANOVA) to determine the interaction between group (PD patients and healthy controls) and experimental condition (early-learning, automatic, and attention). The contrast images from the first-level analysis were modeled using flexible factorial design. Then, post hoc t-tests were further performed to explore the differences between the automatic and early-learning stage, and between the attention and automatic stage in each group; as well as the differences between the 2 groups in each stage (P < 0.05, FWE corrected). Extent threshold was 10 voxels.
Effective Connectivity Analysis
We further investigated the neural network changes following attention to the automatic movement. As the DLPFC is critical in attentional networks, and the right DLPFC was activated before automaticity in our study, we chose the right DLPFC as a region of interest (ROI) to measure the influences from the attentional network on other task-related regions. We selected the primary motor cortex (M1) as another ROI to measure the influences that the motor execution network received from other brain regions in each condition. Because the task was a right hand movement, we chose the left M1 as a ROI. We used Granger causality analysis method (Granger 1969) to measure effective connectivity in the form of signed path coefficients between these ROIs and other brain regions, following Chen's model (Chen et al. 2009; Hamilton et al. 2011; Palaniyappan et al. 2013).
The ROIs were centered at the voxels showing the maximum magnitude of activation in each condition (early-learning, automatic, and attention) within the selected areas, with a radius of 5 mm. We only chose data in active conditions for connectivity analysis. The Granger causality analysis was performed using the REST-GCA toolkit (http://www.restfmri.net). Eight nuisance covariates were regressed, including the white matter signal, cerebrospinal fluid signal, and 6 head motion parameters. We measured the signed path coefficients between the ROIs and other task-related areas in each subject, and in each condition, based on our results of activations. For the DLPFC, its connectivity to the left M1, rostral SMA (pre-SMA), caudal SMA (SMA-proper), bilateral PMC, right ACC, left superior parietal lobule, right inferior parietal lobule, left caudate nucleus, left anterior and posterior putamen, and bilateral cerebellum (posterior lobe, tuber) were calculated. For the M1, its connectivity from the right DLPFC, pre-SMA, SMA-proper, bilateral PMC, right ACC, left superior parietal lobule, right inferior parietal lobule, left caudate nucleus, bilateral anterior and posterior putamen, and bilateral cerebellum (posterior lobe, tuber) were measured. The resulting path coefficients characterized the strength and direction of the temporal relation between the DLPFC or M1 and other task-related areas. A two-sample t-test was used to evaluate whether the path coefficients between the ROIs and other regions were significantly different from zero (P < 0.001; Hamilton et al. 2011).
We used a mixed-effects model to examine the interaction between group (PD patients and healthy controls) and experimental condition (early-learning, automatic, and attention). Post hoc comparisons were applied to compare the effective connectivity between the attention and automatic stage, and between the automatic and early-learning stage within each group, as well as between 2 groups in each condition (P < 0.001). In the current study, positive/negative results of connectivity indicate that the increased activity in the DLPFC predicts subsequent increasing/decreasing activity in the corresponding areas, or the M1 receives excitatory/inhibitory influences from the corresponding areas.
Results
Task Performance
In the early-learning stage, the subjects in both groups had few errors in performing the visuomotor task. After practice (13.73 ± 1.39 sessions), all controls could perform the task automatically. In contrast, after 5 days' practice (20 training sessions), 5 patients still could not achieve the automatic stage. Data from these 5 patients were excluded. The average practice time in 25 PD patients who achieved automaticity was 16.92 ± 1.19 sessions. At the automatic stage, the subjects could perform the task without any error, and the RT could not be further shortened during a training session. Most importantly, the subjects had no error in performing the dual-task, and the RT in the dual-task had no significant difference compared with that in the single visuomotor task (two-sample t-test, P > 0.05). As described previously, 3 patients were excluded because of excessive head motion during fMRI scanning. The behavioral data in the remaining 22 patients and controls are shown in Table 2.
Table 2.
Task performance in each stage in Parkinson's disease and control group
| Accuracy (percentage of errors) |
Reaction time (ms) |
|||
|---|---|---|---|---|
| Control group | Patient group | Control group | Patient group | |
| Early-learning stage | 3.84 ± 2.92 | 4.62 ± 2.66 | 1028.27 ± 148.24 | 1059.18 ± 175.49 |
| Automatic stage | 0 | 0 | 316.32 ± 30.25 | 337.68 ± 45.31 |
| Attention stage | 0 | 0 | 575.42 ± 77.20 | 601.78 ± 86.22 |
| Dual-task | ||||
| Visuomotor | 0 | 0 | 325.39 ± 34.87 | 348.57 ± 40.28 |
| Counting | 0 | 0 | ||
Note: Values are given as mean ± SD for percentage of errors, or reaction times. The results of the dual-task are given as errors of movements and errors of number counting.
Analysis with the mixed-effect model showed that the main effect of stage (early-learning, automatic, and attention) was statistically significant (P < 0.0001, FWE corrected). In contrast, the main effect of group (PD patients and controls) and interaction between group and stage was not significant (P = 0.41 and P = 0.84, respectively). Post hoc comparisons showed that the RTs between the automatic and early-learning stage, as well as between the attention and automatic stage were significant in both groups (P < 0.0001, FWE corrected). The RTs in patients were longer than that in controls in each stage; however, the differences were not significant (P = 0.39). There were no errors in the automatic and attention stages.
Brain Activation Results
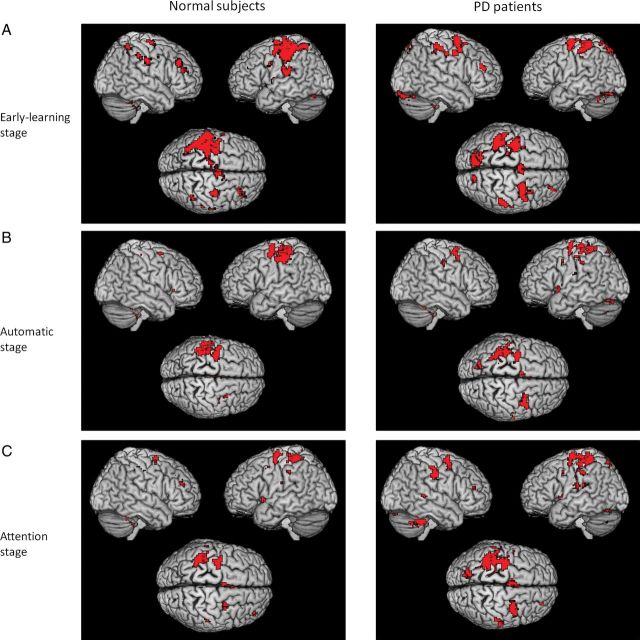
In both groups, performance of visuomotor task was associated with activations in the M1, PMC, parietal cortex, cerebellum, putamen, and thalamus bilaterally, and pre-SMA, SMA-proper, right DLPFC, left caudate nucleus, and right ACC in the early-learning stage (one-sample t-test, P < 0.05, FWE corrected; Fig. 1A). In the automatic stage, the PMC, parietal cortex, putamen, and thalamus bilaterally, and left M1, SMA-proper were activated in both groups, while the right DLPFC was additionally activated in patients (Fig. 1B). In the attention stage, there was activation of the PMC, parietal cortex, putamen, thalamus, and cerebellum bilaterally, and left M1, right DLPFC, and SMA-proper in both groups; additionally, the pre-SMA and right ACC were activated in controls (Fig. 1C).
Figure 1.
Brain activity during the process of developing automatic movements and in the attention stage. Brain regions activated in early-learning (A), automatic (B), and attention (C) stages in healthy subjects (left column), and Parkinson's disease patients (right column) while performing a visuomotor association task (one-sample t-test, P < 0.05, FWE corrected). L, left; R, right.
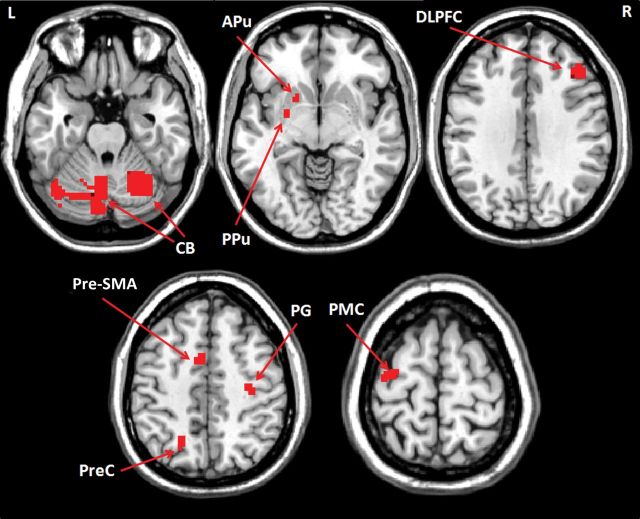
There was significant interaction between group and experimental condition in the left anterior and posterior putamen, bilateral cerebellum, right DLPFC, left pre-SMA, right precentral gyrus, left precuneus, and left PMC (repeated-measures ANOVA, P < 0.05, FWE corrected; Fig. 2 and Table 3). The interaction between group and condition means that the effect of group on brain activity is dependent on experimental condition (and vice versa). In both groups, the automatic stage had decreased activity in the bilateral PMC, pre-SMA, right ACC, right DLPFC, left superior parietal lobule, right inferior parietal lobule, left caudate nucleus, and bilateral cerebellum (posterior lobe, tuber) compared with the early-learning stage (post hoc t-test, P < 0.05, FWE corrected; Fig. 3A and Supplementary Table 1). In controls, bilateral anterior putamen was less activated, while the bilateral posterior putamen was more activated in the automatic stage compared with the early-learning stage (post hoc t-test, P < 0.05, FWE corrected; Fig. 3B, Supplementary Table 1). In contrast, there were no regions more activated in the automatic stage than in the early-learning stage in the patients. At automatic stage, patients had more activity in the PMC, parietal cortex, cerebellum, and anterior putamen bilaterally, and right DLPFC, but had less activity in the bilateral posterior putamen compared with controls (post hoc t-test, P < 0.05, FWE corrected; Fig. 4 and Supplementary Table 2).
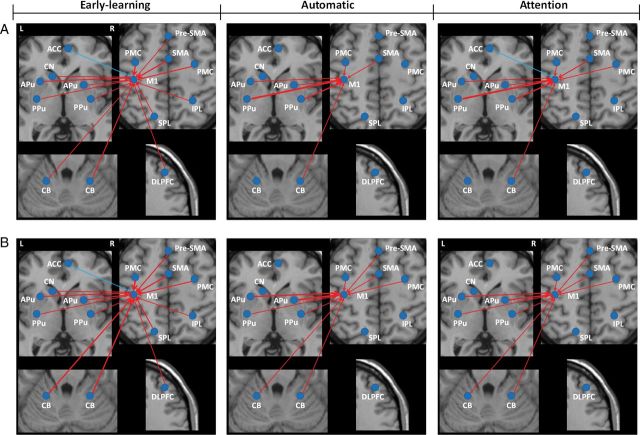
Figure 2.
The interaction between group and experimental conditions on brain activation. The activations show the interaction between experimental conditions (early-learning, automatic, and attention) and group (patients and controls); repeated-measures ANOVA, P < 0.05, FWE corrected, Table 3). L, left; R, right; APu, anterior putamen; CB, cerebellum; DLPFC, dorsolateral prefrontal cortex; PG, precentral gyrus; PMC, premotor cortex; PPu, posterior putamen; PreC, precuneus; pre-SMA, rostral supplementary motor area.
Table 3.
The interaction between groups and experimental conditions
| Brain region | Brodmann area | MNI coordinates |
F-value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R Precentral gyrus | 4 | 34 | −19 | 48 | 34.07 | 513 |
| L PMC | 6 | −33 | −12 | 66 | 36.23 | 891 |
| L Pre-SMA | 6 | −8 | 4 | 54 | 37.06 | 1026 |
| L Precuneus | 7 | −18 | −63 | 48 | 33.30 | 648 |
| R DLPFC | 9 | 42 | 30 | 33 | 35.55 | 1188 |
| L Anterior putamen | −15 | 9 | −9 | 31.06 | 378 | |
| L Posterior putamen | −24 | −3 | −6 | 30.65 | 459 | |
| L Cerebellum, posterior lobe, declive | −42 | −66 | −21 | 41.85 | 4320 | |
| R Cerebellum, posterior lobe, declive | 30 | −63 | −24 | 34.26 | 1377 | |
Note: The results are the areas showing the interaction between conditions (early-learning, automatic, and attention) and group (PD patients and controls). Repeated-measures ANOVA, P < 0.05, FWE corrected.
L, left; R, right; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex; pre-SMA, rostral supplementary motor area.
Figure 3.
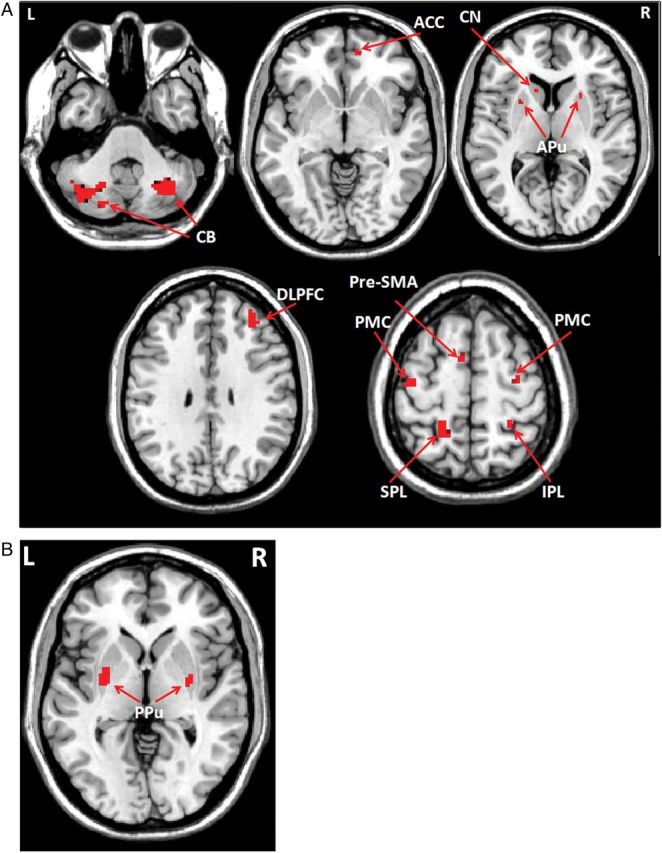
Differences of brain activation between automatic and early-learning stages in controls. Brain regions more activated in the early-learning than in the automatic stage (A), and more activated in the automatic than in the early-learning stage (B) while performing the visuomotor association task (post hoc t-test, P < 0.05, FWE corrected, Supplementary Table 1). L, left; R, right; ACC, anterior cingulate cortex; APu, anterior putamen; CB, cerebellum; CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area; SPL, superior parietal lobule.
Figure 4.
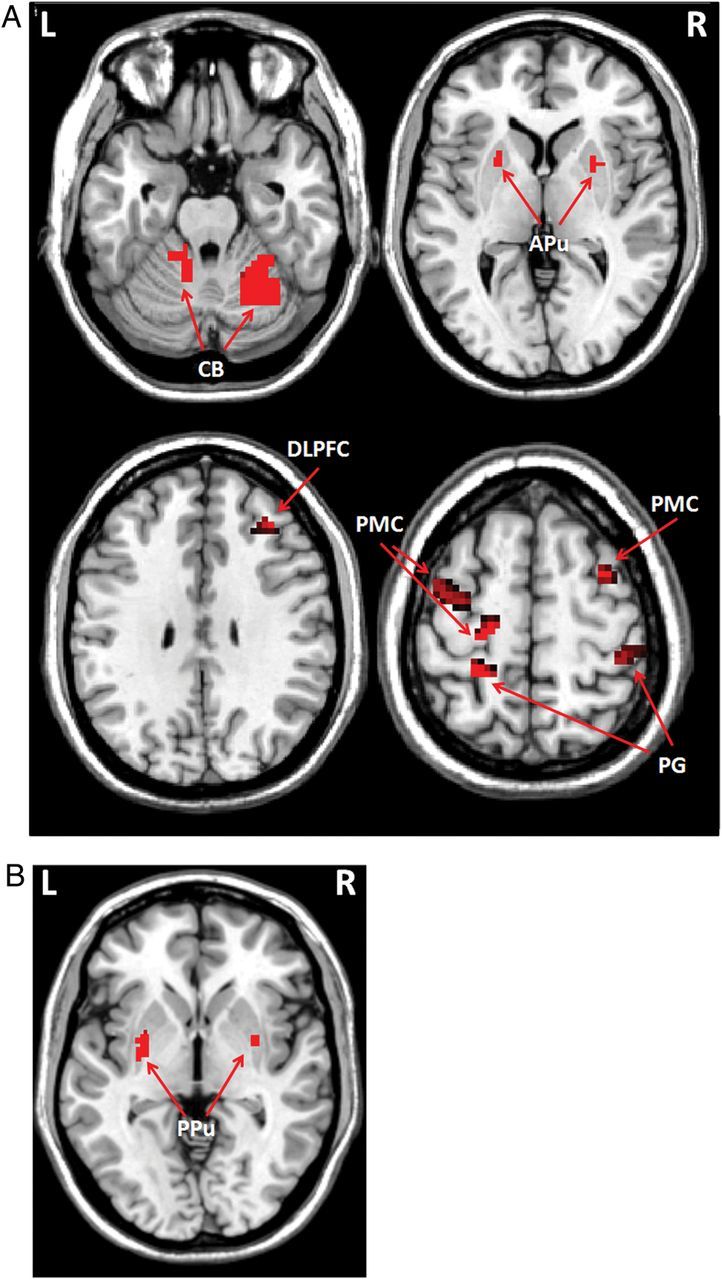
Differences of brain activation between 2 groups at the automatic stage. Brain regions more activated in the Parkinson's disease patients than in controls (A), and more activated in control than in patients (B) at the automatic stage (post hoc t-test, P < 0.05, FWE corrected, Supplementary Table 2). L, left; R, right; APu, anterior putamen; CB, cerebellum; DLPFC, dorsolateral prefrontal cortex; PG, precentral gyrus; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area.
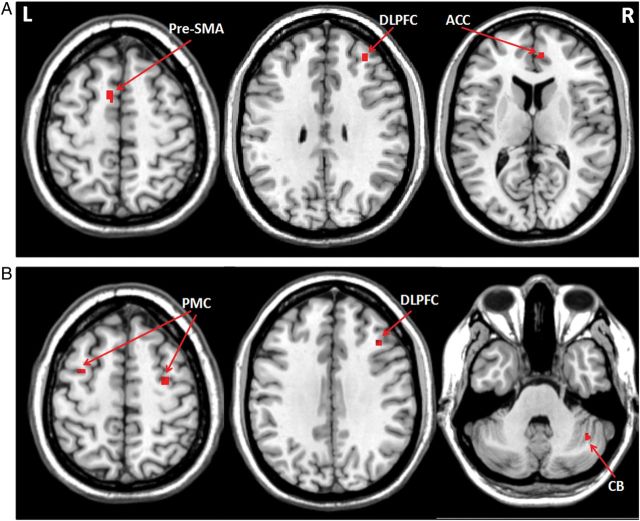
Attention to the automatic task increased activations in the right DLPFC, right ACC, and left pre-SMA in controls (Fig. 5A and Table 4), and enhanced activity in the right DLPFC, bilateral PMC, and right cerebellum in patients compared with the automatic stage (Fig. 5B and Table 4). During attention to automatic movement, patients had more activity in the PMC, parietal cortex, cerebellum, and anterior putamen bilaterally, but had less activity in the left pre-SMA and bilateral posterior putamen compared with controls (Supplementary Table 3).
Figure 5.
Brain regions more activated when attending to an automatic movement. Brain regions more activated in attention than in automatic stage in controls (A), and Parkinson's disease patients (B) when performing the visuomotor association task (post hoc t-test, P < 0.05, FWE corrected). Attention to the automatic task increased activations in the right DLPFC (MNI coordinates 34, 43, 32), right ACC (6, 46, 11), and left pre-SMA (−10, 6, 55) in controls, and enhanced activity in the right DLPFC (42, 25, 34), bilateral PMC (−32, 2, 52, and 32, −6, 55), and right cerebellum (40, −55, −35) in patients compared with the automatic stage (Table 4). L, left; R, right; ACC, anterior cingulate cortex; CB, cerebellum; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex; pre-SMA, rostral supplementary motor area.
Table 4.
The difference of brain activity between attention and automatic stages in each group
| Group | Brain region | Brodmann area | MNI coordinates |
t-value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controls | L Pre-SMA | 6 | −10 | 6 | 55 | 8.06 | 864 |
| R DLPFC | 9 | 34 | 43 | 32 | 7.42 | 486 | |
| R ACC | 32 | 6 | 46 | 11 | 6.78 | 540 | |
| PD patients | L PMC | 6 | −32 | 2 | 52 | 6.62 | 297 |
| R PMC | 6 | 32 | −6 | 55 | 7.51 | 405 | |
| R DLPFC | 9 | 42 | 25 | 34 | 6.65 | 378 | |
| R Cerebellum, anterior lobe, culmen | 40 | −55 | −35 | 7.13 | 594 | ||
Note: The results are the areas more activated in the attentional stage compared with automatic stage in controls and PD patients.
L, left; R, right; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex; pre-SMA, rostral supplementary motor area.
Network Connectivity Results
The connections between the DLPFC and all other task-related regions, and between the M1 and SMA-proper, right DLPFC, left superior parietal lobule, right inferior parietal lobule, right ACC, left and right anterior putamen, left and right posterior putamen, and left cerebellum showed significant interaction between group and experimental conditions (mixed-effect model, P < 0.001, Supplementary Table 4).
At early-learning stage, the right DLPFC had significantly positive connections with several task-related areas in both groups (Table 5). However, these connections were weaker in patients compared with controls (post hoc comparison, P < 0.001). These connections were reduced with automaticity. At the automatic stage, the DLPFC did not show significant connection to these task-related areas in controls, but still had significant connections to the pre-SMA and bilateral PMC in patients.
Table 5.
Effective connectivity from the DLPFC to other brain regions in PD and control groups in each condition
| Brain regions | Normal subjects |
PD patients |
||||
|---|---|---|---|---|---|---|
| Early-learning | Automatic | Attention | Early-learning | Automatic | Attention | |
| L M1 | 0.127 ± 0.053a | 0.033 ± 0.006 | 0.036 ± 0.005 | 0.077 ± 0.025 | 0.040 ± 0.006 | 0.038 ± 0.004 |
| Pre-SMA | 0.170 ± 0.072a | 0.032 ± 0.004 | 0.075 ± 0.022a | 0.088 ± 0.021 | 0.055 ± 0.008a | 0.060 ± 0.005 |
| SMA-proper | 0.132 ± 0.035a | 0.022 ± 0.006 | 0.058 ± 0.013a | 0.040 ± 0.014 | 0.028 ± 0.011 | 0.036 ± 0.003 |
| L PMC | 0.158 ± 0.037a | 0.030 ± 0.006 | 0.072 ± 0.020a | 0.067 ± 0.019 | 0.049 ± 0.008a | 0.053 ± 0.015 |
| R PMC | 0.142 ± 0.018a | 0.036 ± 0.006 | 0.068 ± 0.014a | 0.074 ± 0.013 | 0.054 ± 0.008a | 0.053 ± 0.009 |
| L SPL | 0.091 ± 0.010a | 0.022 ± 0.007 | 0.030 ± 0.006 | 0.034 ± 0.010 | 0.024 ± 0.006 | 0.022 ± 0.006 |
| R IPL | 0.102 ± 0.027a | 0.019 ± 0.004 | 0.030 ± 0.008 | 0.054 ± 0.015 | 0.025 ± 0.006 | 0.029 ± 0.006 |
| R ACC | 0.101 ± 0.024a | 0.022 ± 0.004 | 0.026 ± 0.008 | 0.067 ± 0.016 | 0.023 ± 0.004 | 0.032 ± 0.005 |
| L CN | 0.130 ± 0.020a | 0.033 ± 0.009 | 0.031 ± 0.007 | 0.052 ± 0.012 | 0.031 ± 0.007 | 0.031 ± 0.004 |
| L APu | 0.106 ± 0.026a | 0.027 ± 0.006 | 0.034 ± 0.006 | 0.054 ± 0.017 | 0.031 ± 0.006 | 0.029 ± 0.006 |
| R APu | 0.087 ± 0.022a | 0.030 ± 0.006 | 0.033 ± 0.006 | 0.051 ± 0.012 | 0.025 ± 0.003 | 0.028 ± 0.004 |
| L PPu | 0.089 ± 0.022a | 0.027 ± 0.005 | 0.032 ± 0.006 | 0.063 ± 0.018 | 0.027 ± 0.004 | 0.028 ± 0.004 |
| R PPu | 0.079 ± 0.013a | 0.032 ± 0.007 | 0.034 ± 0.006 | 0.063 ± 0.013 | 0.036 ± 0.007 | 0.035 ± 0.005 |
| L CB | 0.121 ± 0.020a | 0.030 ± 0.006 | 0.031 ± 0.007 | 0.077 ± 0.012 | 0.028 ± 0.006 | 0.068 ± 0.017a |
| R CB | 0.138 ± 0.031a | 0.039 ± 0.006 | 0.034 ± 0.004 | 0.085 ± 0.012 | 0.034 ± 0.008 | 0.074 ± 0.010a |
Note: Values are group mean path coefficients from the right DLPFC to other task-related areas. Values are given as mean ± SD. Group means in bold are significantly different from zero (two-sample t-test, P < 0.001).
L, left; R, right; PD, Parkinson's disease; ACC, anterior cingulate cortex; APu, anterior putamen; CB, cerebellum; CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; M1, primary motor cortex; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area; SMA-proper, caudal supplementary motor area; SPL, superior parietal lobule.
aSignificant between-group difference.
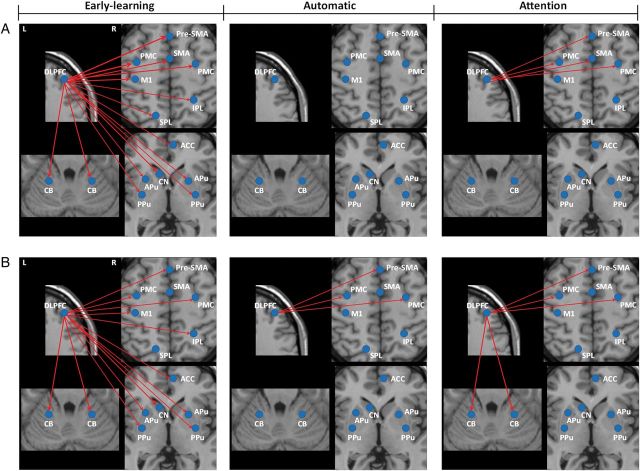
In controls, attention induced increased connections from the DLPFC to the pre-SMA, SMA-proper, and bilateral PMC, but did not change the connections to the M1, striatum, or cerebellum compared with the automatic stage (post hoc comparison, P < 0.001; Fig. 6A). In patients, the DLPFC had significant connections to the pre-SMA, bilateral PMC, and bilateral cerebellum during attention (Fig. 6B). However, the connections from the DLPFC to the pre-SMA and PMC were not significantly changed compared with that at the automatic stage (post hoc comparison, P > 0.05). The DLPFC had stronger connectivity to the bilateral cerebellum, but had weaker connectivity to the bilateral PMC, pre-SMA, and SMA-proper in patients compared with controls.
Figure 6.
The effective connections in the DLPFC in each stage in each group. The influences from the right DLPFC on other task-related areas in the early-learning, automatic, and attention stages in controls (A) and Parkinson's disease patients (B). The results shown are the path coefficients between the ROIs and other regions that are significantly different from zero (two-sample t-test, P < 0.001, Table 5). The red lines indicate that the DLPFC had positive influences on other task-related areas. The thicker lines indicate stronger connections. L, left; R, right; ACC, anterior cingulate cortex; APu, anterior putamen; CB, cerebellum; CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; M1, primary motor cortex; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area; SMA, caudal supplementary motor area; SPL, superior parietal lobule.
In both groups, the left M1 received positive connections from extensive task-related areas, but received negative connectivity from the ACC at early-learning stage (Table 6). The connectivities from the pre-SMA, bilateral PMC, parietal cortex, and cerebellum to the M1 were significantly stronger, whereas the connections from the DLPFC and posterior putamen to the M1 were weaker in patients than that in controls (post hoc comparison, P < 0.001). During automatic processing, most of these connections were weakened, whereas the connection from the bilateral posterior putamen to the M1 was strengthened in controls, and the connections from the bilateral anterior putamen and right posterior putamen to the M1 were strengthened in patients (post hoc comparison, P < 0.001). The connections from the bilateral anterior putamen, pre-SMA, bilateral PMC, and bilateral cerebellum to the M1 were stronger, whereas the connections from the bilateral posterior putamen were weaker in patients than in controls at automatic stage (post hoc comparison, P < 0.001).
Table 6.
Effective connectivity the M1 received from other brain regions in PD and control groups in each condition
| Brain regions | Normal subjects |
PD patients |
||||
|---|---|---|---|---|---|---|
| Early-learning | Automatic | Attention | Early-learning | Automatic | Attention | |
| Pre-SMA | 0.084 ± 0.016 | 0.023 ± 0.006 | 0.031 ± 0.006 | 0.132 ± 0.019a | 0.071 ± 0.013a | 0.076 ± 0.013a |
| SMA-proper | 0.182 ± 0.045 | 0.082 ± 0.010 | 0.121 ± 0.024a | 0.193 ± 0.047 | 0.086 ± 0.016 | 0.085 ± 0.014 |
| L PMC | 0.161 ± 0.035 | 0.060 ± 0.016 | 0.088 ± 0.013 | 0.205 ± 0.044a | 0.085 ± 0.013a | 0.117 ± 0.022a |
| R PMC | 0.132 ± 0.030 | 0.033 ± 0.007 | 0.063 ± 0.012 | 0.181 ± 0.053a | 0.069 ± 0.015a | 0.097 ± 0.010a |
| R DLPFC | 0.116 ± 0.038a | 0.026 ± 0.005 | 0.034 ± 0.004 | 0.072 ± 0.016 | 0.020 ± 0.008 | 0.023 ± 0.006 |
| L SPL | 0.085 ± 0.008 | 0.022 ± 0.007 | 0.028 ± 0.006 | 0.120 ± 0.017a | 0.032 ± 0.005 | 0.036 ± 0.003 |
| R IPL | 0.081 ± 0.009 | 0.024 ± 0.005 | 0.029 ± 0.006 | 0.116 ± 0.024a | 0.028 ± 0.006 | 0.030 ± 0.007 |
| R ACC | −0.091 ± 0.016 | −0.026 ± 0.006 | −0.054 ± 0.013a | −0.078 ± 0.007 | −0.031 ± 0.005 | −0.035 ± 0.005 |
| L CN | 0.062 ± 0.012 | 0.020 ± 0.006 | 0.021 ± 0.005 | 0.054 ± 0.010 | 0.018 ± 0.004 | 0.019 ± 0.006 |
| L APu | 0.132 ± 0.019 | 0.071 ± 0.013 | 0.069 ± 0.010 | 0.128 ± 0.026 | 0.182 ± 0.035a | 0.132 ± 0.025a |
| R APu | 0.118 ± 0.022 | 0.069 ± 0.014 | 0.068 ± 0.008 | 0.124 ± 0.018 | 0.222 ± 0.028a | 0.159 ± 0.029a |
| L PPu | 0.112 ± 0.016a | 0.247 ± 0.037a | 0.240 ± 0.030a | 0.063 ± 0.018 | 0.065 ± 0.014 | 0.068 ± 0.012 |
| R PPu | 0.086 ± 0.011a | 0.168 ± 0.033a | 0.165 ± 0.026a | 0.065 ± 0.012 | 0.102 ± 0.017 | 0.075 ± 0.010 |
| L CB | 0.124 ± 0.020 | 0.033 ± 0.008 | 0.030 ± 0.007 | 0.163 ± 0.023a | 0.071 ± 0.011a | 0.106 ± 0.018a |
| R CB | 0.148 ± 0.024 | 0.072 ± 0.011 | 0.076 ± 0.010 | 0.187 ± 0.030a | 0.090 ± 0.011a | 0.116 ± 0.014a |
Note: Values are group mean path coefficients that the left M1 received from other task-related areas. Values are given as mean ± SD. Group means in bold are significantly different from zero (two-sample t-test, P < 0.001).
L, left; R, right; PD, Parkinson's disease; ACC, anterior cingulate cortex; APu, anterior putamen; CB, cerebellum; CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; M1, primary motor cortex; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area; SMA-proper, caudal supplementary motor area; SPL, superior parietal lobule.
aSignificant between-group difference.
Attention to the task-strengthened connections from the SMA-proper, bilateral PMC, and ACC to the M1, but did not change the connections from the striatum to the M1 compared with that in the automatic stage in controls (post hoc comparison, P < 0.001; Fig. 7A). In patients, attention increased connections from the bilateral PMC and cerebellum to the M1, but decreased connectivity from the bilateral anterior putamen and right posterior putamen to the M1 compared with that in the automatic stage (post hoc comparison, P < 0.001; Fig. 7B). The connectivities from the bilateral anterior putamen, pre-SMA, bilateral PMC, and bilateral cerebellum to the M1 were stronger, whereas the connections from the bilateral posterior putamen, SMA-proper, and ACC were weaker in patients than in controls (post hoc comparison, P < 0.001). The overall activity and connectivity changes in the striatum across the experimental conditions in patients and controls are summarized in Supplementary Table 5. The effect sizes in the striatum in each condition in PD and control groups are shown in Supplementary Figure 2.
Figure 7.
The effective connections in the M1 in each stage in each group. The influences the left M1 received from other task-related areas in the early-learning, automatic, and attention stages in controls (A) and Parkinson's disease patients (B). The results shown are the path coefficients between the ROIs and other regions that are significantly different from zero (two-sample t-test, P < 0.001, Table 6). The red lines indicate that the M1 received positive influences from other task-related areas. The blue lines indicate that the ACC had negative influence on M1. The thicker lines indicate stronger connections. L, left; R, right; ACC, anterior cingulate cortex; APu, anterior putamen; CB, cerebellum; CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; M1, primary motor cortex; PMC, premotor cortex; PPu, posterior putamen; pre-SMA, rostral supplementary motor area; SMA, caudal supplementary motor area; SPL, superior parietal lobule.
Discussion
The present study is the first to demonstrate that there are correlative behavioral and neural changes while attending to a movement that could be made automatically. In normal subjects, attention to the automatic movement was with more activation in the DLPFC, ACC, and pre-SMA; the effective connections from the attentional networks to cortical motor association areas were strengthened. In contrast, attention had no influence on the striatum; the activation and connectivity patterns of the striatum remained at the level of the automatic stage. In PD patients, attention reduced connectivity from the striatum to motor execution networks, and enhanced activations in the DLPFC, PMC, and cerebellum. The attentional networks had more connectivity to the cerebellum, but not to the cortical motor association regions.
Automaticity-Related Neural Modifications
The RTs in our patients were longer than that in controls in each stage, although differences were not statistically significant. It has been long recognized that PD patients typically have longer RT than healthy people (Brown et al. 1993). Because the RTs in PD patients and controls were not very different, the motor performance should have no effect on the observed different brain activity or connectivity between groups.
Our findings in controls support the concept that when a movement becomes automatic, neural efficiency is increased (Wu et al. 2004, 2008; Poldrack et al. 2005). The patients had greater activations in the PMC, parietal cortex, and cerebellum compared with controls, which suggests that neural networks in PD are not as efficient as that in healthy people while performing automatic movements (Wu and Hallett 2005).
The DLPFC is important in generating new movements (Deiber et al. 1991; Jueptner, Stephan et al. 1997; Jueptner, Frith et al. 1997), in task rehearsal (Petrides et al. 1993), and in performance monitoring (Owen et al. 1996). The ACC may play roles in conflict monitoring (Botvinick et al. 1999) and attention/selection of action (Petersen et al. 1988; Isomura et al. 2003). These 2 regions are critical in the attentional networks. The ACC had negative influence on M1, which may indicate that the ACC has an inhibitory effect (Margulies et al. 2007). In controls, these 2 areas were no longer activated, and did not show obvious influence on other task-related areas, at the automatic stage (Figs 1, 6, and 7), which indicates that when a movement achieves automaticity, the attentional networks become much less necessary in healthy people (Wu et al. 2008). In contrast, although the activity or connectivity was decreased, the DLPFC remained activated, and exerted significant influences on some task-related areas in the automatic stage in PD, which indicates that the patients need to recruit attentional networks even at the automatic stage (Wu and Hallett 2005).
The automatic-related activity and connectivity in the striatum also showed different patterns in the 2 groups. In controls, the activity in the anterior putamen decreased while the activation in the posterior putamen increased in the automatic stage compared with the early-learning stage (Fig. 3), which is in agreement with Lehéricy's report (2005). The connection from the anterior putamen to the M1 was weakened, while the connectivity from the posterior putamen to the M1 was strengthened during automatic processing (Fig. 7 and Table 6). The anterior putamen is considered an association area, while the posterior putamen is a sensorimotor area. Studies with animal and human subjects have demonstrated that the associative striatum is more involved in acquisition of new motor skills and in regulating goal-directed behaviors, while the sensorimotor striatum is critical in performance of habitual or automatic movements (Miyachi et al. 1997, 2002; Lehéricy et al. 2005).
In PD patients, the activity and connectivity in the more affected (left side) posterior putamen were not increased as movements became automatic. Although the connectivity from the less affected (right side) posterior putamen to the M1 was increased during automatic processing, it was significantly weaker compared with that in controls. Thus, the dopamine depletion in the posterior putamen, which is part of the sensorimotor striatum, likely contributes to the difficulty of acquiring/executing automatic program/actions. In contrast, the connectivity from the anterior putamen to the M1 was strengthened during automatic processing, and was significantly stronger than that in controls (Table 6). This increased connectivity is more obvious in the less affected (right side) anterior putamen. Loss of dopaminergic neurons can trigger collateral sprouting of residual neurons (Finkelstein et al. 2000; Song and Haber 2000), and thus spared dopaminergic fibers in the anterior putamen may compensate for severe dopamine depletion in the posterior putamen (Mounayar et al. 2007) helping to store and execute automatic programs.
We did not detect such automatic-related changes in the striatum in our earlier studies (Wu et al. 2004; Wu and Hallett 2005). This difference may due to 2 reasons. First, the tasks being employed were not the same. In our previous studies, self-initiated sequential movements were used, while in the current study, a visuomotor task was studied. Second, the field strengths of MRI scanning were different. The field strength in our previous studies was 1.5 T, but in this study was 3 T. It has been demonstrated that activation volume can be significantly increased in the striatum at 3 T compared with that at 1.5 T when performing the same tasks (Krasnow et al. 2003).
Attention-Related Neural Modifications in Controls
During attending to the automated motor task, our subjects did not make any error, but had significant prolongation of their RTs. Our finding demonstrates that attention can worsen performance compared with that in the automatic stage. The prolonged RT is evidence that the task was not performed automatically, but became a controlled behavior.
In healthy controls, we did not find a shift of the automatic mode back to the controlled pattern within the striatum, specifically the activation from the posterior putamen back to the anterior putamen, during attention. Indeed, attention did not modulate the activity or connectivity pattern in the striatum. The activity in the striatum, and the influences from the striatum to the M1 were the same as that in the automatic stage. In addition, the attentional networks had no significant influence on the striatum. These results indicate that in healthy people, once automaticity is achieved, the automatic mode in the striatum is relatively stable, and is not further adjusted by the attentional networks even when the subjects return their attention to the task. It is very likely that the striatum continues to “send” the same message to the motor execution system. These findings may help to explain why once action sequences have become automatic it is often difficult to revert to controlled behaviors (Schneider and Chein 2003). Because our evaluation of the attention effects were performed shortly after the subjects achieved automaticity, it is unclear whether the automatic mode in the striatum can be stable for a long period, and that needs further investigation.
In healthy people, attention to the task increased the activity in the DLPFC, ACC, and pre-SMA compared with the automatic condition (Jueptner, Frith et al. 1997; Rowe et al. 2002). The increased activity in the pre-SMA may be a reflection of more effort in preparation for movement (Jenkins et al. 2000; Cunnington et al. 2002). The inhibitory connection from the ACC to the M1 was strengthened during attention. We did not detect increased connection from the DLPFC to the M1; however, the DLPFC had enhanced connections to the PMC and SMA-proper, and these regions in turn had more influence on M1 in attention. Thus, the DLPFC may exert influence on the motor execution networks by the way of cortical motor association areas. Our findings demonstrate that attention to automatic movements recruits attentional networks and cortical motor association regions. These areas have more influence on the motor execution networks, which may be a reason contributing to the worsened performance in the attention stage because of the increased cortical resources required.
Attention-Related Neural Modifications in Parkinson's Disease
Unlike that in the healthy controls, attention to automatic movement in PD patients modified the connectivity pattern of the striatum. The connections from the bilateral anterior putamen and right posterior putamen to the M1 were decreased compared with that in the automatic stage (Fig. 7 and Table 6). Thus, there is a trend shifting from the automatic mode back to the controlled pattern within the striatum in PD patients. Our results suggest that not only the shifting of a new motor skill to automatic, but also the storage of the automatic program relies on the striatum being functionally intact, especially the posterior (sensorimotor) striatum. Although PD patients at a relatively early stage can use different strategies, such as compensation from the anterior putamen, to achieve automaticity, this modified automatic mode is not stable and can be disrupted by attention.
A common deficit in PD patients is that even at an early stage, the ability to perform some previously achieved automatic movements, such as arm-swinging with walking, is impaired (Marsden 1982). Our findings provide a likely explanation for this problem: dopamine depletion in the sensorimotor striatum might destabilize or disrupt previously stored automatic programs.
Attention-evoked activations were also different in patients and controls. PD patients did not show more activity in the pre-SMA and ACC. The ACC has been proven to be interconnected with the striatum (Takada et al. 2001; Di Martino et al. 2008; Beckmann et al. 2009). The dysfunction of the striatum may result in underactivity in the ACC. The hypoactivation of SMA in motor tasks that need attention or preparation has been extensively reported in PD (Jahanshahi et al. 1995; Cunnington et al. 1997; Buhmann et al. 2003; Wu, Wang et al. 2010). It has been suggested that the dysfunction of the pre-SMA due to the deficit of nigrostriatal dopamine system is an important factor contributing to akinesia in PD (Grafton 2004).
PD patients had enhanced activity in the PMC and cerebellum when attending to the movement than when in automatic state (Fig. 5), and the activations in these regions were greater in patients than in controls (Supplementary Table 3). Several studies have reported the hyperactivation in the PMC or cerebellum while performing motor tasks in PD, and suggested that the overactivation in these regions is possibly a compensation for the dysfunction of basal ganglia (Rascol et al. 1997; Catalan et al. 1999; Haslinger et al. 2001; Wu and Hallett 2005).
Rowe et al. (2002) did not find any regions more activated when attending to a motor action in PD patients. We suppose this inconsistency may due to the symptomatic severity in patients. Our patients were at the relatively early stage of the disorder (duration 2.86 years, UPDRS motor score 16.77); whereas the patients in Rowe et al.'s study (2002) were at more advanced stage (duration 5.4 years, UPDRS motor score 33.7). It has been suggested that compensatory effects might gradually diminish as the disorder progresses (Jankovic 2005; Wu and Hallett 2013); thus, the enhanced activation in these regions may become less obvious in more advanced patients. Actually, although it was not significant, there was a trend for more activation in the PMC in PD patients than in controls in that study (Rowe et al. 2002).
The DLPFC did not exert more influence on cortical motor association areas compared with the automatic stage in patients. The disruption of effective connections of attentional networks in PD has been reported previously (Rowe et al. 2002; Wu, Chan et al. 2010). In contrast, the DLPFC had strengthened connectivity to the cerebellum during attention in PD, and the cerebellum had increased connectivity to the M1 (Figs 6 and 7). Thus, instead of exerting more influence on motor execution network directly, or through the cortical motor association areas, the DLPFC may exert more influence on motor execution network by the way of the cerebellum when attending to an automatic movement in PD.
Limitations
As electromyography (EMG) was not recorded during fMRI scanning, it is not clear if the amount of muscle activity or tremor between the conditions or the groups could have affected the results. Because the predominant symptom was akinesia/rigidity in all our patients, and they had at most a mild tremor, it is more likely that tremor did not have significant effect on our study. However, as EMG monitoring during fMRI scanning is helpful to regress out the potential influence of tremor or muscle activity (Helmich et al. 2011), it is reasonable to include EMG monitoring in future studies.
We did not compare the difference of neural activity at the rest condition. Our previous study showed that neural activity in the resting state is different between PD patients and controls (Wu et al. 2009). In addition, we did not assess for deactivations, which means more activity during rest condition than during active condition. These measurements may provide useful information. However, as we focused on motor-related neural activity and connectivity in the current study, the changes at rest condition and deactivations will need to be investigated in future studies.
In conclusion, our findings demonstrate that when achieving automaticity, the posterior putamen appears critical for the storage and running of automatic programs. Attention back to the movements evokes the attentional networks and modifies the activity of cortical motor association areas, but has no effect on the subcortical areas. The activity and connectivity pattern in the striatum remains at the automatic mode. The motor execution networks are controlled by the automatic processes of the striatum, as well as the cortical motor association networks. For PD patients, striatal dysfunction due to dopamine depletion results in their difficulty in achieving automaticity. Patients at early stage can achieve automaticity with compensatory efforts from other, relatively spared regions, such as the anterior putamen. However, this modified automatic mode is not stable, attention can induce a shift from the automatic mode back to the controlled pattern within the striatum. Therefore, not only shifting from controlled to automatic behaviors, but also shifting back from automatic to attended behaviors rely on the striatum being intact.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Science Foundation of China (grant numbers 81071012 and 81271429 to T.W.).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Balsters JH, Ramnani N. 2011. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 31:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JA, Doyon J. 2003. Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage. 20:1649–1660. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. 2009. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. 1967. The co-ordination and regulation of movements. London: Pergamon Press. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. 1999. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 402:179–181. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS. 1990. Differing patterns of striatal 18F-dopa uptake in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 28:547–555. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Schwarz U, Bowman EM, Fuhr P, Robinson DL, Hallett M. 1993. Dopamine dependent reaction time deficits in patients with Parkinson's disease are task specific. Neuropsychologia. 31:459–469. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. 2003. Pharmacologically modulated fMRI—cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 126:451–461. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. 1999. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain. 122:483–495. [DOI] [PubMed] [Google Scholar]
- Chen G, Hamilton JP, Thomason ME, Gotlib IH, Saad ZS, Cox RW. 2009. Granger causality via vector auto-regression tuned for fMRI data analysis. Proc Intl Soc Mag Reson Med. 17:1718. [Google Scholar]
- Cunnington R, Iansek R, Johnson KA, Bradshaw JL. 1997. Movement-related potentials in Parkinson's disease. Motor imagery and movement preparation. Brain. 120:1339–1353. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. 2004. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 21:1416–1427. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. 1991. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 84:393–402. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. 2008. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. 2000. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 97:99–112. [DOI] [PubMed] [Google Scholar]
- Grafton ST. 2004. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol. 14:715–719. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. 1969. Investigating causal relations by economic models and cross-spectral methods. Econometrica. 37:424–438. [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. 2011. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 16:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. 2001. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 124:558–570. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. 2011. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 69:269–281. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. 1967. Parkinsonism: onset, progression and mortality. Neurology. 17:427–442. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. 1992. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. 2003. Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J Neurosci. 23:8002–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. 1995. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 118:913–933. [DOI] [PubMed] [Google Scholar]
- Jankovic J. 2005. Progression of Parkinson disease: are we making progress in charting the course? Arch Neurol. 62:351–352. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. 2000. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 123:1216–1228. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE. 1997. Anatomy of motor learning. II. Subcortical structures and learning by trail and error. J Neurophysiol. 77:1325–1337. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ. 1997. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 77:1313–1324. [DOI] [PubMed] [Google Scholar]
- Krasnow B, Tamm L, Greicius MD, Yang TT, Glover GH, Reiss AL, Menon V. 2003. Comparison of fMRI activation at 3 and 1.5T during perceptual, cognitive, and affective processing. Neuroimage. 18:813–826. [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. 2002. Cerebellar damage impairs automaticity of a recently practiced movement. J Neurophysiol. 87:1336–1347. [DOI] [PubMed] [Google Scholar]
- Lang AE, Fahn S. 1989. Assessment of Parkinson's disease. In: Munsat TL, editor. Quantification of neurological deficit. Boston: Butterworths; p. 285–309. [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. 2005. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 102:12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2007. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 37:579–588. [DOI] [PubMed] [Google Scholar]
- Marsden CD. 1982. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 32:514–539. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu XF. 2002. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 146:122–126. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. 1997. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 115:1–5. [DOI] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Féger J, Savasta M, François C, Tremblay L. 2007. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain. 130:2898–2914. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. 1997. Micrographia in Parkinson's disease: the effect of providing external cues. J Neurol Neurosurg Psychiatry. 63:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. 1996. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 6:31–38. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. 2013. Neural primacy of the salience processing system in schizophrenia. Neuron. 79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE. 1996. Attention to action. Philos Trans R Soc Lond B Biol Sci. 351:1473–1479. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Poaner MI, Mintum M, Raichle ME. 1998. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 331:585–589. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. 1993. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA. 90:873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. 1999. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 13:564–574. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. 2005. The neural correlates of motor skill automaticity. J Neurosci. 25:5356–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F. 1997. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 120:103–110. [DOI] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. 2002. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain. 125:276–289. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. 2003. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cogn Sci. 27:525–559. [Google Scholar]
- Song DD, Haber SN. 2000. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 20:5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. 2001. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 14:1633–1650. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. 2010. Effective connectivity of neural networks in automatic movements in Parkinson's disease. Neuroimage. 49:2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. 2008. Modifications of the interactions in the motor network when a movement becomes automatic. J Physiol. 586:4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. 2005. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 128:2250–2259. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. 2013. The cerebellum in Parkinson's disease. Brain. 136:696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. 2004. How self-initiated memorized movements become automatic: a fMRI study. J Neurophysiol. 91:1690–1698. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. 2009. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp. 30:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. 2010. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain. 133:2394–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G, Prinz W. 2001. Directing attention to movement effects enhances learning: a review. Psychon Bull Rev. 8:648–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.