Abstract
The octapeptide NAP has been shown to exert neuroprotective properties. Here, we investigated potential anti-inflammatory effects of NAP in an acute ileitis model. To address this, C57BL/6j mice were perorally infected with Toxoplasma gondii (day 0). Within 1 week postinfection (p.i.), placebo (PLC)-treated mice developed acute ileitis due to Th1-type immune responses. Mice that were subjected to intraperitoneal NAP treatment from day 1 until day 6 p.i., however, developed less distinct macroscopic and microscopic disease as indicated by less body weight loss, less distinct histopathological ileal changes, and lower ileal apoptotic, but higher proliferating cell numbers, less abundance of neutrophils, macrophages, monocytes, and T lymphocytes, but higher numbers of regulatory T cells in the ileal mucosa and lamina propria, and lower concentrations of pro-inflammatory mediators in the ilea as compared to PLC controls at day 7 p.i. Remarkably, NAP-mediated anti-inflammatory effects could also be observed in extra-intestinal compartments including liver and spleen. Strikingly, lower MCP-1, TNF, and IL-12p70 serum concentrations in NAP as compared to PLC-treated mice at day 7 p.i. indicate a pronounced systemic anti-inflammatory effect of NAP in acute ileitis. These findings provide first evidence for NAP as a potential novel treatment option in intestinal inflammation.
Keywords: NAP, ADNP, Toxoplasma gondii, acute ileitis, Th1-type immunopathology, anti-inflammatory effects, immunomodulatory properties, gut–brain axis
Introduction
Within 7 days following peroral infection with 100 cysts of Toxoplasma (T.) gondii ME49 strain, C57BL/6j mice develop nonself-limiting acute ileitis [1]. This fatal inflammatory scenario in the small intestinal tract is due to a Th1-type immunopathology orchestrated by intestinal epithelial cells, neutrophils, macrophages, monocytes, dendritic cells, and lymphocytes [2]. During the early course of T. gondii infection, parasitic interaction with antigen presenting cells results in activation of CD4+ T cells and subsequent over-production of pro-inflammatory mediators such as TNF, MCP-1, nitric oxide (NO), IL-6, IL-12, and IFN-γ [3–7]. The underlying immunopathogenesis, thus, mimics key features of chronic inflammatory bowel diseases (IBD) such as Crohn’s disease during the acute stage [2, 8].
The oligopeptide NAP consists of eight amino acids (namely NAPVSIPQ) and was initially identified as a biologically active fragment of the activity-dependent neuroprotective protein (ADNP) [9]. Besides the central nervous system, human ADNP expression could be detected in spleen, peripheral blood leukocytes, and macrophages [9–12]. The neuroprotective effects exerted by NAP have been investigated in vitro and in multiple in vivo models of neuronal injury including closed head injury, fetal alcohol syndrome, stroke, Alzheimer’s disease, and neonatal hypoxia–ischemia [9, 13–17]. Anti-oxidative as well as immunomodulatory actions have been proposed as potential underlying mechanisms of the beneficial effects exerted by NAP [18]. Moreover, NAP has been shown to suppress the production of pro-inflammatory cytokines including TNF, IL-6, and IL-12 [12] that are also involved in mediating acute T. gondii-induced ileitis [2]. This prompted us to investigate potential immunomodulatory properties of synthetic NAP in an acute murine model of small intestinal inflammation. We here demonstrate for the first time that the NAP oligopeptide is able to exert potent anti-inflammatory effects in the inflamed intestinal tract and diminishes extra-intestinal as well as systemic collateral damages of inflammation.
Methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) and approved by the local authorities. Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice, T. gondii infection, and treatment
Female C57BL/6j wildtype control mice were included into the experiments. For induction of acute ileitis, three-month-old mice were infected perorally with 100 T. gondii cysts (ME49 strain) derived from homogenized brains of intraperitoneally infected NMRI mice in a volume of 0.3 ml phosphate-buffered saline (PBS) by gavage, as described previously [19–21]. From day 1 until day 6 postinfection (p.i.), mice were either treated with synthetic NAP (1.0 mg per kg body weight per day) or PBS (placebo [PLC]) once daily via the intraperitoneal route. A potential antimicrobial effect of the NAP solution was excluded as described previously [22]. Naive mice served as uninfected and untreated (None) negative controls.
Sampling procedures
At day 7 postinfection (p.i.), mice were sacrificed by isofluran treatment (Abbott, Greifswald, Germany). Cardiac blood and tissue samples from spleen, liver, and ileum were removed under sterile conditions. Ileal ex vivo biopsies from each mouse were collected in parallel for histopathological, immunohistochemical, microbiological, and immunological analyses.
Histopathology and immunohistochemistry
Immunohistopathological changes were determined in ex vivo biopsies derived from the terminal ileum that were immediately fixed in 5% formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined by light microscopy (magnification 100× and 400×), and ileal histopathology was determined as described previously [19].
In situ immunohistochemical analysis of ileal paraffin sections was performed as described earlier [23–26]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), Ki67 (TEC3, Dako, Denmark, 1:100), myeloperoxidase (MPO-7, no. A0398, Dako, 1:500), F4/80 (no. 14-4801, clone BM8, eBioscience, San Diego, CA, USA, 1:50), CD3 (no. N1580, Dako, 1:10), and FOXP3 (FJK-16s, eBioscience, 1:100) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 0.287 mm2, 400× magnification) were determined microscopically by a double-blinded investigator.
Detection of ileal T. gondii DNA and cytokines
Ileal ex vivo biopsies were cut longitudinally and washed in PBS. In approximately 1 cm2 of homogenized ileal tissue, T. gondii DNA was measured as described previously [27]. Spleen, liver, or strips of approximately 1 cm2 ileal tissue were placed in 24-flat-bottom well culture plates (Nunc, Wiesbaden, Germany) containing 500 μl serum-free RPMI 1640 medium (Gibco, Life Technologies, Paisley, UK) supplemented with penicillin (100 U/ ml) and streptomycin (100 μg/ml; PAA Laboratories). After 18 h at 37 °C, culture supernatants and serum samples were tested for MCP-1, TNF, IL-6, and IL-12p70 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences) on a BD FACSCanto II flow cytometer (BD Biosciences). Nitric oxide (NO) was determined by Griess reaction as described earlier [19].
Statistical analysis
Medians and levels of significance were determined using Mann–Whitney U test (GraphPad Prism v5, La Jolla, CA, USA) as indicated. Two-sided probability (P) values ≤0.05 were considered significant.
Results
Clinical and histopathologic outcome in NAP-treated mice following acute ileitis induction
In order to induce acute ileitis, mice were perorally challenged with 100 cysts of T. gondii ME49 strain by gavage on day 0. From day 1 until day 6 p.i., infected mice were either treated with synthetic NAP or PLC. Whereas PLC control mice were suffering from wasting due to acute ileitis and a median weight loss of more than 15% at day 7 p.i., NAP-treated mice displayed better clinical conditions as indicated by a significantly less pronounced weight loss of approximately 10% (p < 0.0001 versus PLC; Fig. 1a). Less macroscopic disease following NAP treatment was accompanied by less distinct histopathological changes in the ileal mucosa as indicated by lower histopathological scores in NAP as compared to PLC-treated mice at day 7 following ileitis induction (p < 0.05; Fig. 1b). Notably, NAP did neither have a direct antimicrobial effect that could potentially affect the composition of the intestinal microbiota nor did it exert an anti-parasitic effect as indicated by ileal T. gondii DNA loads comparable to those detected in PLC mice (Fig. 1c). Given that apoptosis is a commonly used diagnostic marker in the histopathological evaluation and grading of intestinal disease [23] we quantitatively assessed numbers of caspase-3+ cells within the ileal mucosa of T. gondii-infected mice. During ileitis development apoptotic ileal epithelial cell numbers increased (p < 0.0001; Fig. 2a), whereas this increase, however, was less pronounced in NAP-treated mice (p < 0.0001; Fig. 2a).
Fig. 1.
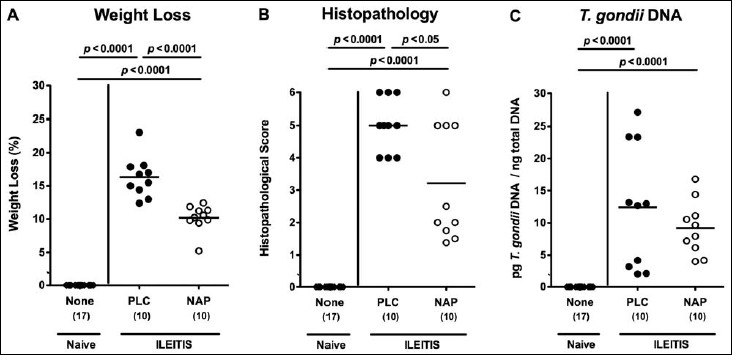
Clinical conditions and small intestinal histopathology in NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. (A) Relative loss of body weights between day 7 following ileitis induction and day 0 was determined (in %). (B) Histopathological mucosal changes were assessed in ileal H&E stained paraffin sections from respective mice 7 days following peroral T. gondii infection applying a standardized histopathological scoring system. (C) T. gondii DNA was determined in ex vivo ileal biopsies at day 7 p.i. Numbers of analyzed mice are given in parentheses. Medians (black bars) and significance levels (p-values) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Fig. 2.
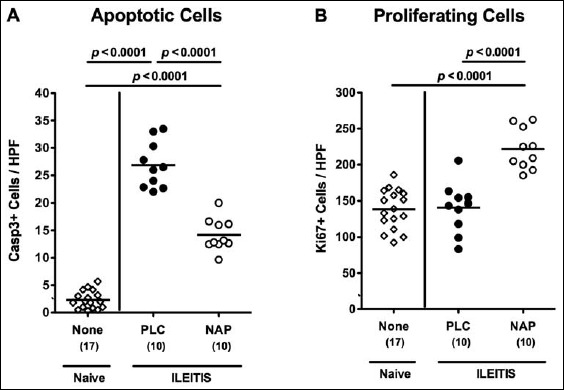
Apoptotic and proliferating cells in ileal samples of NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. The average numbers of apoptotic (positive for caspase-3, Casp3, panel A) and proliferating cells (positive for Ki67, panel B) from at least six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained ileal paraffin sections at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Since Ki67 comprizes a nuclear protein associated with and necessary for cellular proliferation [28], we also stained ileal paraffin sections with anti-Ki67 in order to determine proliferative measures of the small intestinal epithelium counteracting apoptosis following T. gondii infection. Only NAP-treated mice displayed elevated Ki67+ epithelial cell numbers in the ileal mucosa at day 7 p.i. as compared to naive mice (p < 0.0001 vs. naive and PLC; Fig. 2b).
Small intestinal immune cell responses in NAP-treated mice following acute ileitis induction
We next quantitatively assessed the influx of innate and adaptive immune as well as of effector cells into the ileal mucosa and lamina propria by in situ immunohistochemical staining of small intestinal paraffin sections. Seven days following ileitis induction, numbers of neutrophils, macrophages, and monocytes as well as of T lymphocytes, the major forces driving T. gondii-induced immunopathology [29], increased in small intestines of infected mice (p < 0.0001 vs. naive controls; Fig. 3a–c). These increases, however, were less distinct in NAP as compared to PLC-treated mice (p < 0.0005, p < 0.01, and p < 0.005, respectively; Fig. 3a–c). Remarkably, only following NAP treatment increased numbers of ileal FOXP3+ regulatory T cells could be observed (p < 0.05 vs. naive and PLC; Fig. 3d).
Fig. 3.
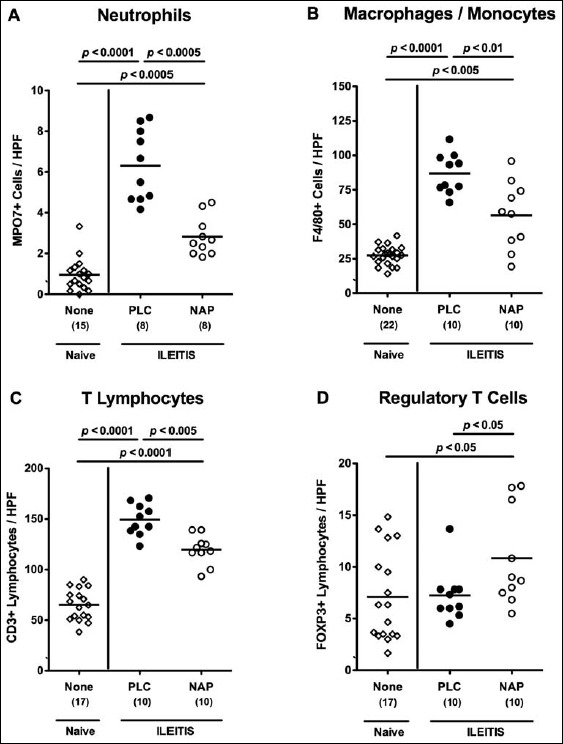
Ileal immune cell responses in NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. The average number of cells positive for (A) MPO7 (neutrophils), (B) F4/80 (macrophages and monocytes), (C) CD3 (T lymphocytes), and (D) FOXP3 (regulatory T cells) from at least six high power fields (HPF, 400× magnification) per animal was determined microscopically in immunohistochemically stained ileal paraffin sections derived from mice at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Ileal and extra-intestinal pro-inflammatory cytokine secretion in NAP-treated mice following acute ileitis induction
We next determined whether NAP treatment affected intestinal and extra-intestinal cytokine secretion in T. gondii-infected mice. Seven days following acute ileitis induction, increased pro-inflammatory mediators such as MCP-1, TNF, nitric oxide (NO), and IL-6 could be measured in supernatants of ileal ex vivo biopsies as compared to naive controls (p < 0.05–0.0001; Fig. 4). In NAP-treated mice, however, significantly lower ileal MCP-1 concentrations (p < 0.05; Fig. 4a) and a trend towards lower TNF and NO levels (n.s.; Fig. 4b and c) could be detected as compared to PLC mice at day 7 p.i. To address whether also extra- intestinal inflammatory sequelae of T. gondii infection could be ameliorated by NAP treament, we determined pro-inflammatory mediators in ex vivo liver biopsies before and after ileitis induction. Seven days following T. gondii infection, hepatic MCP-1, TNF, NO, and IL-6 concentrations increased multifold in PLC mice (p < 0.005–0.0001; Fig. 5). In livers of NAP-treated mice, however, significantly lower hepatic concentrations of respective mediators could be detected (p < 0.05, p < 0005, p < 0.05, and p < 0.005, respectively; Fig. 5). Notably, hepatic MCP-1 and NO concentration were comparable in NAP-treated and naive mice (Fig. 5a and c).
Fig. 4.
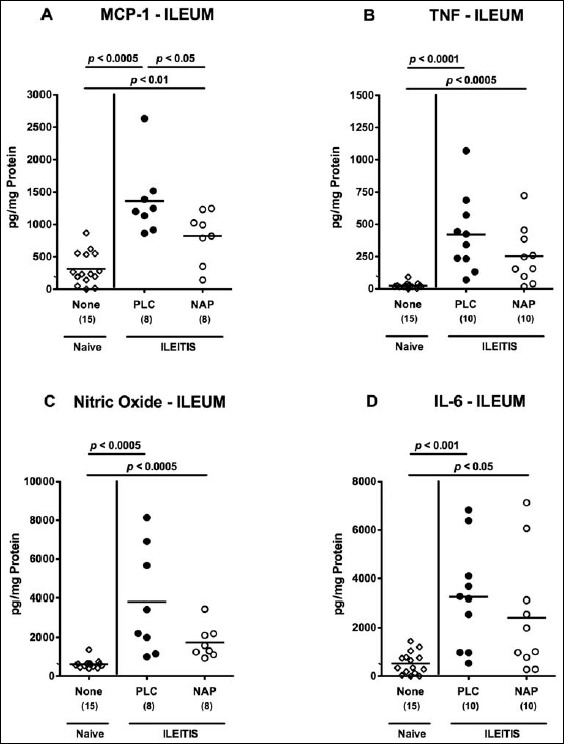
Ileal pro-inflammatory cytokine secretion in NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. (A) MCP-1, (B) TNF, (C) nitric oxide, and (D) IL-6 concentrations were determined in ex vivo biopsies taken from the terminal ileum at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Fig. 5.
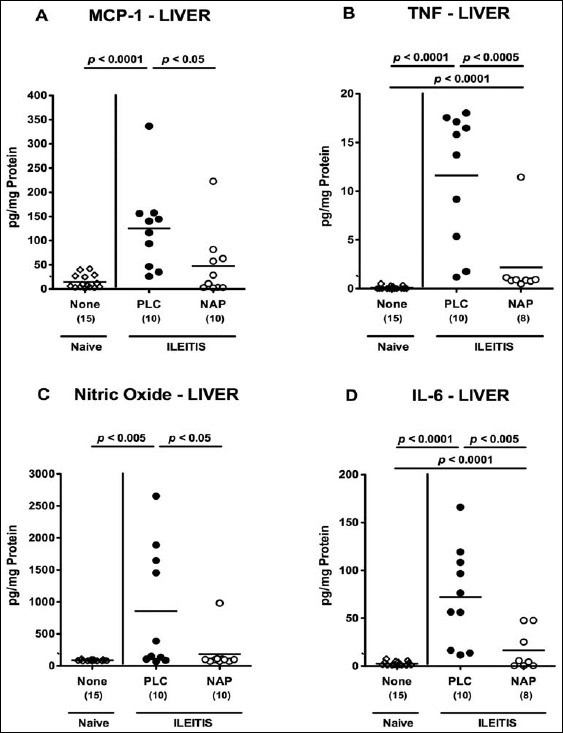
Pro-inflammatory cytokine secretion in livers of NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. (A) MCP-1, (B) TNF, (C) nitric oxide, and (D) IL-6 concentrations were determined in ex vivo biopsies taken from livers at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Systemic pro-inflammatory cytokine secretion in NAP-treated mice following acute ileitis induction
For the assessment of potential systemic anti-inflammatory effects of NAP treatment in T. gondii-infected mice, we measured pro-inflammatory mediators in spleen and serum. Seven days following ileitis induction, MCP-1, TNF, and NO, but not IL-6 levels, were increased in spleens of PLC control mice (Fig. 6). Remarkably, NAP-treated animals exhibited lower splenic MCP-1 and TNF concentrations as compared to PLC mice at day 7 p.i. (p < 0.05 and p < 0.05, respectively; Fig. 6a and b), whereas MCP-1 levels were comparable in NAP-treated and naive mice (Fig. 6a). Furthermore, serum MCP-1, TNF, IL-12p70, and IL-6 concentrations increased multifold in T. gondii-infected mice of either group (Fig. 7). Strikingly, serum concentrations of the former three cytokines were lower in NAP as compared to PLC-treated mice at day 7 p.i. (p < 0.05; Fig. 7a–c).
Fig. 6.
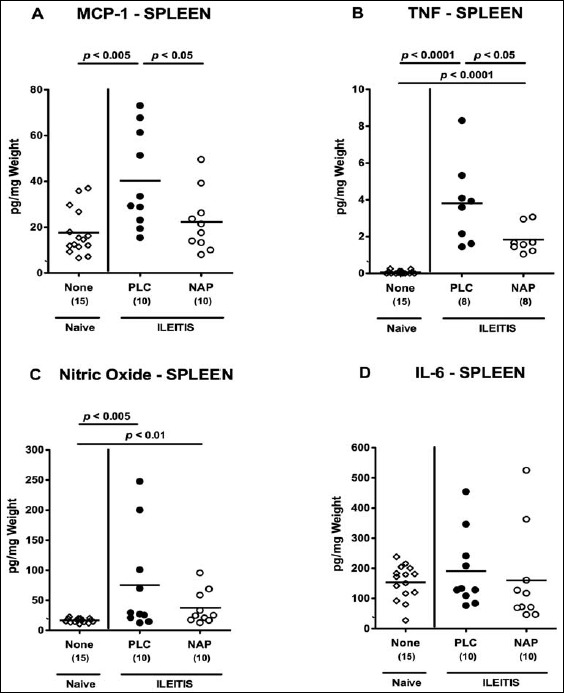
Pro-inflammatory cytokine secretion in spleens of NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. (A) MCP-1, (B) TNF, (C) nitric oxide, and (D) IL-6 concentrations were determined in ex vivo biopsies taken from spleens at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Fig. 7.
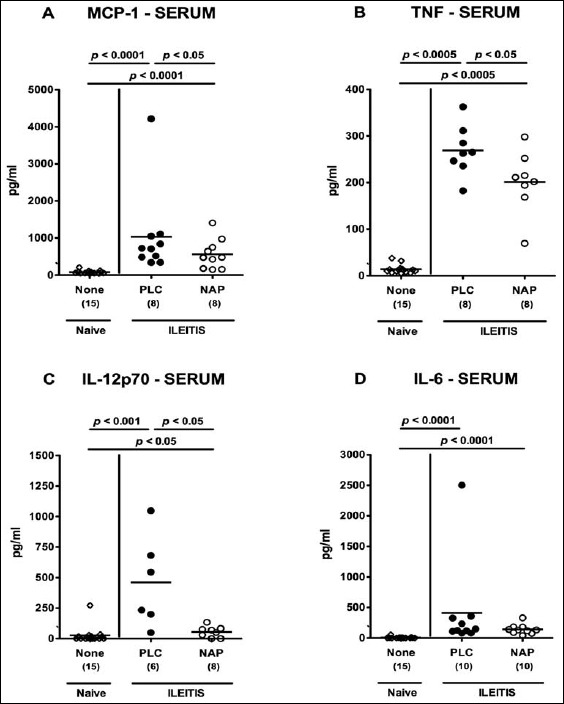
Systemic pro-inflammatory cytokine secretion in NAP-treated mice following acute ileitis induction. In order to induce acute ileitis, C57BL/6j wildtype mice were perorally infected with 100 cysts of T. gondii ME49 strain on day 0. Mice were treated either with synthetic NAP (open circles) or placebo (PLC; filled circles). Naive, uninfected, and untreated (None; open diamonds) mice served as negative controls. (A) MCP-1, (B) TNF, (C) IL-12p70, and (D) IL-6 concentrations were determined in serum samples at day 7 p.i. Numbers of analyzed animals are given in parenthesis. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Taken together, NAP exerts not only potent intestinal but also extra-intestinal and systemic anti-inflammatory effects in T. gondii-infected mice.
Discussion
Several in vitro and in vivo studies revealed that the octapeptide NAP exerts neuroprotective effects by anti-oxidative and immunomodulatory mechanisms [18]. For the first time, we investigated here the potential beneficial actions of NAP in an acute small intestinal inflammation model. Results demonstrate that NAP exerted potent anti-inflammatory effects in acute T. gondii-induced ileitis of mice as indicated by 1) less macroscopic and microscopic disease, 2) reduced ileal apoptosis but increased mucosal cell proliferation, 3) less influx of pro-inflammatory immune cells into the ileal mucosa and lamina propria, 4) less secretion of pro-inflammatory mediators in the ileum and extra-intestinal compartment such as the liver, and 5) diminished systemic inflammatory responses following NAP treatment. Notably, NAP did not interfere with the T. gondii infection given that comparable parasitic DNA loads could be detected in small intestines of NAP and PLC-treated mice. Moreover, treatment was initiated 24 h after T. gondii infection in order to avoid direct parasite– NAP interaction. Given that the distinct intestinal microbiota composition plays a critical role in the initiation and perpetuation of immunopathological conditions [30–34], we ruled out that NAP exerted any anti-bacterial effects in vitro.
In our study, NAP-treated mice did not only exhibit less distinct small intestinal histopathological changes including necrosis but also less pronounced apoptosis as indicated by lower numbers of caspase-3+ cells within the ileal mucosa. In line with this, recent studies revealed that the cell survival promoting activity of NAP in the brain was due to prevention of caspase-3 activation and, hence, of apoptotic cell death [35, 36]. Conversely, numbers of Ki67+ cells within the ileal mucosa were increased upon NAP application here, indicative for proliferative measures of the small intestinal epithelium thereby counteracting apoptosis.
In NAP-treated mice, lower numbers of innate and adaptive as well as of effector immune cells including neutrophils exerting oxidative stress to the intestinal epithelium in the ileal mucosa and lamina propria could be observed. Braitch et al. showed that monocytes, T and B cells, but not regulatory T cells (Tregs) derived from peripheral blood mononuclear cells (of healthy blood donors) expressed ADNP [18]. The better outcome in NAP-treated mice was due to a dampened Th1-type immunopathology as indicated by reduced accumulation of T lymphocytes, the major driving forces of T. gondii-induced ileitis [2], which were significantly reduced upon NAP application in our study. This is supported by Braitch and colleagues demonstrating that NAP suppressed proliferation and activation of T cells in vitro leading to a decrease in pro-inflammatory cytokine secretion including IFN-γ [18]. In NAP-treated mice, decreased concentrations of pro-inflammatory mediators could be observed not only in intestinal, but also in extra-intestinal compartments such as liver and spleen. This is well in line with in vitro data showing that NAP suppresses the production of pro-inflammatory cytokines such as TNF, IL-6, and IL-12 from murine macrophages [12] and of NO in cerebral cortical cultures [14]. The pronounced systemic anti-inflammatory responses observed in serum samples derived from NAP-treated mice in our study is remarkable. ADNP is hypothesized to be a secreted protein [10, 37] and NAP to exert its immunoregulatory properties in the systemic circulation in a cytokine-like (apocrine or paracrine) fashion, but without a distinct surface receptor [11].
We have very recently investigated the immunomodulatory properties of another neuropeptide, namely, pituitary adenylate cyclase-activating polypeptide (PACAP) in our acute ileitis model. As in the present study, synthetic PACAP was able to ameliorate acute small intestinal inflammation as well as extraintestinal and systemic sequelae by down-regulating Th-1 type immunopathology, reducing oxidative stress and upregulating anti-inflammatory cytokine responses [38].
In summary, we show here for the first time that the neuropeptide NAP exerts potent anti-inflammatory effects in acute ileitis that are not restricted to the intestinal tract but also diminishes extra-intestinal and systemic inflammatory collateral damages. Until now, synthetic NAP (generic name davunetide) has entered phase 3 clinical trials for the treatment of progressive supranuclear palsy [39].
We conclude that these findings provide novel treatment options of intestinal inflammatory diseases including IBD.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Ulrike Hagen, Alexandra Bittroff-Leben, Ines Puschendorf, Gernot Reifenberger, and Uwe Lohmann for excellent technical assistance. We are grateful to Simone Spieckermann for immunohistochemical staining of paraffin sections.
References
- 1.Liesenfeld O, Kosek J, Remington JS, Suzuki Y: Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med 1184(2), 597–607 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz M, Liesenfeld O, Heimesaat MM: Immunology of Toxoplasma gondii. Immunol Rev 240(1), 269–285 (2011) [DOI] [PubMed] [Google Scholar]
- 3.Khan IA, Schwartzman JD, Matsuura T, Kasper LH: A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci U S A 94(25), 13955–13960 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liesenfeld O Kang H Park D Nguyen TA Parkhe CV Watanabe H et al.: TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol 21(7), 365–376 (1999) [DOI] [PubMed] [Google Scholar]
- 5.Mennechet FJ, Kasper LH, Rachinel N, Li W, Vandewalle A, Buzoni-Gatel D: Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol 168(6), 2988–2996 (2002) [DOI] [PubMed] [Google Scholar]
- 6.Vossenkamper A Struck D Alvarado-Esquivel C Went T Takeda K Akira S et al.: Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol 34(11), 3197–3207 (2004) [DOI] [PubMed] [Google Scholar]
- 7.Buzoni-Gatel D, Schulthess J, Menard LC, Kasper LH: Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell Microbiol 8(4), 535–544 (2006) [DOI] [PubMed] [Google Scholar]
- 8.Liesenfeld O: Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis 185(Suppl 1), S96–101 (2002) [DOI] [PubMed] [Google Scholar]
- 9.Bassan M Zamostiano R Davidson A Pinhasov A Giladi E Perl O et al.: Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 72(3), 1283–1293 (1999) [DOI] [PubMed] [Google Scholar]
- 10.Zamostiano R Pinhasov A Gelber E Steingart RA Seroussi E Giladi E et al.: Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem 276(1), 708–714 (2001) [DOI] [PubMed] [Google Scholar]
- 11.Gozes I, Divinski I: The femtomolar-acting NAP interacts with microtubules: novel aspects of astrocyte protection. J Alzheimers Dis 6(6 Suppl), S37–41 (2004) [DOI] [PubMed] [Google Scholar]
- 12.Quintana FJ Zaltzman R Fernandez-Montesinos R Herrera JL Gozes I Cohen IR et al.: NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann N Y Acad Sci 1070, 500–506 (2006) [DOI] [PubMed] [Google Scholar]
- 13.Zemlyak I, Furman S, Brenneman DE, Gozes I: A novel peptide prevents death in enriched neuronal cultures. Regul Pept 96(1–2), 39–43 (2000) [DOI] [PubMed] [Google Scholar]
- 14.Ashur-Fabian O Segal-Ruder Y Skutelsky E Brenneman DE Steingart RA Giladi E et al.: The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides 24(9), 1413–1423 (2003) [DOI] [PubMed] [Google Scholar]
- 15.Beni-Adani L Gozes I Cohen Y Assaf Y Steingart RA Brenneman DE et al.: A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther 296(1), 57–63 (2001) [PubMed] [Google Scholar]
- 16.Rotstein M, Bassan H, Kariv N, Speiser Z, Harel S, Gozes I: NAP enhances neurodevelopment of newborn apolipoprotein E-deficient mice subjected to hypoxia. J Pharmacol Exp Ther 319(1), 332–339 (2006) [DOI] [PubMed] [Google Scholar]
- 17.Greggio S de Paula S de Oliveira IM Trindade C Rosa RM Henriques JA et al.: NAP prevents acute cerebral oxidative stress and protects against long-term brain injury and cognitive impairment in a model of neonatal hypoxiaischemia. Neurobiol Dis 44(1), 152–159 (2011) [DOI] [PubMed] [Google Scholar]
- 18.Braitch M Kawabe K Nyirenda M Gilles LJ Robins RA Gran B et al.: Expression of activity-dependent neuroprotective protein in the immune system: possible functions and relevance to multiple sclerosis. Neuroimmunomodulation 17(2), 120–125 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimesaat MM Bereswill S Fischer A Fuchs D Struck D Niebergall J et al.: Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177(12), 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 20.Heimesaat MM Fischer A Jahn HK Niebergall J Freudenberg M Blaut M et al.: Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 56(7), 941–948 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Plickert R, Fischer A, Göbel UB, Bereswill S: Can microbiota transplantation abrogate murine colonization resistance against Campylobacter jejuni? Eur J Microbiol Immunol (Bp) 3(1), 36–43 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, et al. : Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One 5(12), e15099 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, et al. : Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6(6), e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, et al. : Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS One 7(7), e40761 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, et al. : Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One 7(5), e35988 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimesaat MM Alutis M Grundmann U Fischer A Tegtmeyer N Böhm M et al.: The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol 4, 77 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz M Heimesaat MM Danker K Struck D Lohmann U Plickert R et al.: Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med 206(13), 3047–3059 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3), 311–322 (2000) [DOI] [PubMed] [Google Scholar]
- 29.Bereswill S Kühl AA Alutis M Fischer A Möhle L Struck D et al.: The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog 6, 19 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartor RB: Microbial influences in inflammatory bowel diseases. Gastroenterol 134(2), 577–594 (2008) [DOI] [PubMed] [Google Scholar]
- 31.Sartor RB: Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterol 139(6), 1816–1819 (2010) [DOI] [PubMed] [Google Scholar]
- 32.Thoene-Reineke C, Fischer A, Friese C, Briesemeister D, Göbel UB, Kammertoens T, et al. : Composition of intestinal microbiota in immune-deficient mice kept in three different housing conditions. PLoS One 9(11), e113406 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimesaat MM Fischer A Alutis M Grundmann U Boehm M Tegtmeyer N et al.: The impact of serine protease HtrA in apoptosis, intestinal immune responses and extra-intestinal histopathology during Campylobacter jejuni infection of infant mice. Gut Pathog 6, 16 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimesaat MM, Dunay IR, Alutis M, Fischer A, Möhle L, Göbel UB, et al. : Nucleotide-oligomerization-domain-2 affects commensal gut microbiota composition and intra-cerebral immunopathology in acute Toxoplasma gondii-induced murine ileitis. PLoS One 9(8), e105120 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leker RR Teichner A Grigoriadis N Ovadia H Brenneman DE Fridkin M et al.: NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke 33(4), 1085–1092 (2002) [DOI] [PubMed] [Google Scholar]
- 36.Idan-Feldman A, Ostritsky R, Gozes I: Tau and caspase 3 as targets for neuroprotection. Int J Alzheimers Dis 2012, 493670 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I: Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol 1(3), 193–199 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heimesaat MM, Dunay IR, Schulze S, Fischer A, Grundmann U, Alutis M, et al. : Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS One 9(9), e108389 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boxer AL Lang AE Grossman M Knopman DS Miller BL Schneider LS et al.: Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol 13(7), 676–685 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]


