Abstract
The peptide trefoil factor family 3 (TFF3) is a major constituent of the intestinal mucus, playing an important role in the repair of epithelial surfaces. To further understand the role of TFF3 in the protection of intestinal epithelium, we tested the influence of TFF3 in a murine Toxoplasma gondii-induced ileitis model. Surprisingly, TFF3KO mice showed a reduced immune response in the ileum when compared to wild-type animals. Interleukin-12 and interferon-γ expression levels as well as the number of CD4+ lymphocytes were reduced in the infected TFF3KO mice. These effects were in line with the trend of elevated parasite levels in the ileum. Moreover, TFF1 expression was upregulated in the spleen of infected mice. These initial results indicate that TFF3 is involved in the immune pathology of T. gondii infection-induced intestinal inflammation. Thus far, the mechanisms of how TFF3 influences the immune response are not fully understood. Further studies should identify if TFF3 affects mucus sensing of dendritic cells and how TFF3 is involved in regulating the immune response as an intrinsic secretory peptide of immune cells.
Keywords: TFF3, TFF1, TFF peptide, trefoil factor, Toxoplasma gondii, intestinal inflammation, FCGBP
Introduction
The peptide TFF3 (formerly intestinal trefoil factor, P1.B) is a member of the trefoil factor family (TFF) and is a typical secretory product of mucous epithelia (reviews: [1–3]). The predominant expression occurs in intestinal goblet cells together with the mucin Muc2 [4, 5]. However, TFF3 and Muc2 expression are not coordinately regulated [6]. From cDNA cloning, the size of the mature peptide was predicted to be 59 amino acid residues including seven cysteine residues [5]. Surprisingly, mature intestinal TFF3 exists predominantly as a disulfide-linked heteromer with the mucus-associated IgG Fc binding protein (FCGBP, Mr: >300.000); about 15–20% of TFF3 occurs in a monomeric form and only very little as a homo-dimer [7]. Other mucous epithelia with exocrine TFF3 synthesis are the salivary glands [8], the esophagus [9], the gastric cardia and antrum [9, 10], the Vater’s ampulla [11], the respiratory tract [12], the uterus [13], the vagina [14], the urinary tract [15], the conjunctiva [16], and the efferent tear ducts [17]. In addition, TFF3 is also secreted by the endocrine pancreas [18], the central nervous system [19–21], and by certain cell subsets of the immune system [20, 22].
TFF3 is considered to play a key role in intestinal protection and repair mechanisms in vivo after damages by various noxes (compilation: [3]). For example, the intraperitoneal application of recombinant TFF3 significantly reduced the inflammatory index in a trinitrobenzene sulfonic acid (TNBS)-induced murine colitis model which was paralleled by downregulated toll-like receptor 4 (TLR4) and nuclear factor κB (NF-κB) expression [23]. Interestingly, in two rat colitis models, only the direct luminal application of recombinant TFF3 homo-dimer by a catheter was protective [24], whereas systemically administered TFF3 aggravated the colitis scores, particularly the TFF3 monomer [24]. Furthermore, the ectopic expression of TFF3 in the jejunum of transgenic mice resulted in a reduced sensitivity to indomethacin [25] as well as active delivery of TFF3 by genetically modified Lactococcus lactis protected against dextran sulfate sodium (DSS)-induced acute colitis [26]. In contrast, TFF3-deficient (TFF3KO) mice were markedly more sensitive in a DSS colitis model (50% of the animals died in contrast to 5% of the wild-type animals) [27]. TFF3KO mice were also more susceptible to chemotherapy- and radiation-induced mucositis, and oral recombinant TFF3 was able to reduce the severity of the mucositis [28].
In vitro, a relatively weak motogenic effect (compilation: [3]) due to chemotaxis [29] as well as both pro- and anti-apoptotic effects have been reported for TFF3 [30, 31]. Thus far, all attempts have failed to characterize specific TFF3 receptors. Chemokine receptors might be candidates [32]. However, recombinant dimeric TFF3 has been reported to bind DMBT1gp340 in a Ca2+ dependent manner [33]. Interestingly, TFF3 – similar to TFF1 [34] and TFF2 [35, 36] – has also been reported to act as a lectin [34]. This would enable TFF3 to bind to a plethora of transmembrane glycoproteins modulating different biological effects.
TFF3 is typically upregulated in chronically inflamed epithelia, particularly in the ulcer-associated cell lineage (UACL) [2, 5, 37]. The TFF3 regulatory regions are complex (review: [3]); a hallmark being the hypoxia-inducible factor-1 (HIF-1) [38]. Of note, strong immediate induction of TFF3 expression has been observed after TLR2 activation, whereas the ulcerative colitis-associated TLR2-R753Q variant is functionally deficient in the ability to induce TFF3 synthesis [39]. Furthermore, TFF3 was also induced in the injured rat jejunum after treatment with indomethacin possibly due to increased nitric oxide (NO) synthesis and stabilization of hypoxia-inducible factor-1 (HIF-1) [40]. Generally, inflammatory bowel disease (IBD) is characterized by increased TLR4/NF-κB signaling which triggers the upregulation of pro-inflammatory cytokines and contributes to the development of ulceration. In a recent study, the application of recombinant TFF3 promoted a protective effect against colitis and was followed by downregulated TNF levels in the colon. Thus, TFF3 inhibition of TLR4/NF-κB expression is a potential therapeutic in treatment of IBD [23].
TFF3–FCGBP and MUC2 are the major components of intestinal mucus, and they both colocalize in the gut [7, 41, 42]. When TFFs and mucin were combined, they were more effective in protecting the barrier function of epithelial cells [43]. Only within the last years did it become evident that intestinal mucus is not just a static barrier but directly communicates with the gut microbiota and immune cells of the gut. The mucus layer, which overlays the epithelium, forms an important barrier covering the entire gastrointestinal tract [44, 45]. This shield serves as a barrier to protect the host tissue from pathogens [46]. Recent studies indicate that mucus secretion by intestinal goblet cells involves crosstalk with the gut microbiota and food components via the process of autophagy [47, 48]. Both autophagy and endosome formation are required to trigger secretory granule accumulation and efficient mucus secretion via the generation of reactive oxygen species (ROS) [49]. Additionally, mucus also influences the function of antigen presenting cells by educating them to develop tolerance towards food and commensal antigens. Dendritic cells (DCs) are exposed to the mucus, and MUC2 in particular suppresses the responses of DCs to microbe-derived signals and promotes their capacity to stimulate the production of regulatory T (Treg) cells [50, 51]. Thus, it is not too surprising that aberrant mucus production is often associated with inflammatory responses and has profound consequences on tissue homeostasis [51] because mucus is part of a complex feedback loop regulating both invasion of microbes as well as oral tolerance.
In a further attempt to investigate the potential protective role of TFF3 for the intestinal epithelium, we tested the influence of TFF3 on the intestinal inflammation caused by Toxoplasma gondii in a low-dose model [52]. The acute phase of infection followed by oral infection of mice with the parasite is associated with intestinal inflammation, and the high dose infection is a well-established model for acute ileitis [53]. Both acute and chronic stages of the infection are controlled by the pro-inflammatory cytokine interferon (IFN)-γ. Interleukin (IL)-12, mainly produced by DCs, macrophages, and polymorphonuclear neutrophils (PMNs), further drives the production of IFN-γ [54–57]. As a consequence, inflammatory monocytes are recruited to the ileum in a Ccr2- and Ccl2-dependent manner and act as a first line of defense expressing antimicrobial activities during T. gondii infection [52, 55, 58].
TFF2, another member of the trefoil factor family, has been previously reported to antagonize IL-12 release by macrophages and DCs after T. gondii infection [59]. Thus, TFF2 deficiency was associated with elevated IL-12 production and increased T-cell recruitment in naive mice. Infected TFF2KO mice displayed lower parasite numbers and reduced gut immunopathology [59]. In contrast, TFF2 positively regulates type 2 immunity and IL-33 production, e.g., after infection with the hookworm parasite Nippostrongylus brasiliensis [60].
Materials and methods
Murine oral T. gondii infection model
TFF3KO mice [27] were originally obtained from Prof. D.K. Podolsky (Harvard Medical School). These animals were then backcrossed to 129/Sv and C57BL/6 mice leading to a mixed background. As described previously, TFF3 homozygous sister lines were established (now crossings for more than ten generations) representing a TFF3-deficient genotype (TFF3KO) and a wild-type (WT, TFF3+/+) line, respectively [61–63].
Animal care and experimental procedures were performed according to legal regulations, and T. gondii infection experiments were approved by the state authorities (Landesverwaltungsamt Sachsen–Anhalt, Halle). The animals were kept in standard cages under specific-pathogen free (spf) conditions at the animal facility of the Medical Faculty, maintained on laboratory food and tap water ad libitum in a regular 12 h dark–light cycle with a temperature of 22 °C.
To obtain T. gondii cysts, NMRI mice (Harlan-Winkelmann, Borchen, Germany) were orally infected with ten cysts of a type II strain (ME49) 5–6 months previously, and the tissue cysts in the brain homogenates were counted as described previously [57]. Experimental mice (age: 2–3 months) were orally infected with a brain inoculum equivalent to three cysts per mouse. Seven days post-infection, animals were anesthetized and transcardially perfused with 50 ml 0.9% NaCl, and tissue samples were collected for reverse transcription-polymerase chain reaction (RT-PCR) analysis and histological studies, respectively.
DNA and RNA extraction, PCR analysis
For genotyping the animals, genomic DNA was isolated from tail clippings taken at weaning and purified with Invisorb® spin tissue mini kit (1032100 300, STRATEC Molecular GmbH, Berlin, Germany) following the manufacturer’s instruction. One percent of the DNA was used for PCR analysis (primer pairs: TFF3 MB1871/98, neomycin resistance gene/Neo MB1920/1921).
Total RNA/DNA of tissues was isolated and purified using TRIzol® reagent (Life Technologies GmbH, Darmstadt, Germany) according to the manufacturer’s protocol. One microgram DNA isolated from the ileum was used for PCR analysis to test infection with T. gondii (primer pair MB2342/2343). Alternatively, RNA was isolated with the Isolate RNA mini Kit (BIO-52073, Bioline GmbH, Luckenwalde, Germany).
Prior to reverse transcription, RNA preparations were digested with RNAse-free DNAse I (Thermo Fisher Scientific, Fermentas Walldorf, Germany) as described previously [64]. The concentration and purity of the RNA were estimated with a Nanodrop ND-1000 spectrophotometer (Peqlab Biotechnologie GmbH, Erlangen, Germany). First strand complementary DNA (cDNA) synthesis was performed with 1.0 μg RNA primed with oligo(dT)18 using RevertAid H Minus Reverse Transcriptase (Thermo Fisher Scientific, Fermentas Walldorf, Germany). The relative expression level of selected genes was monitored by RT-PCR analysis including semiquantitative evaluation as described previously [64]. Generally, the highest intensity for each gene was set to 100, and then the relative intensities were normalized against the corresponding relative β-actin intensities. As a control for the integrity of the cDNA preparations, β-actin transcripts were amplified in parallel reactions. The cDNA was also checked for contaminating chromosomal DNA by amplification of a promoter sequence from the β-actin gene (primer pair MB1783/1784). The specific primer pairs used in this study are listed in Table 1 or have been published previously [65].
Table 1.
Oligonucleotides used for (RT)-PCR analysis and calculated size of the products. Actb, β-actin; Il-12a, Il-12p35 transcript variant 2, Iba1/Aif1, ionized calcium binding adapter molecule 1
| Genes | Accession No. | Primer No. | Primer Pairs | Nucleotide Positions | Tm (°C) | Size (bp) | Intron Spanning |
|---|---|---|---|---|---|---|---|
| Actb | NM_007393.4 | MB1912 MB1913 |
CCCTCACGCCATCCTGCGTC ACGCAGCTCAGTAACAGTCCGC |
622-641 1259-1238 |
60 | 638 | yes |
| Actb | NM_0Q7393.4 | MB2166 MB2167 |
AGTACCCCATTGAACATGGC GTAAAACGCAGCTCAGTAACAG |
312-331 1264-1243 |
60 | 953 | yes |
| Iba1/Aif1 | NM_019467 2 | MB1727 MB1728 |
GGATTTGCAGGGAGGAAAAG GCCACTGGACACCTCTCTAA |
329-348 602-533 |
60 | 274 | yes |
| Ifny | NM_008337.3 | MB2054 MB2055 |
TCCTCCTGCGGCCTAGCTCTG TGGCGCTGGACCTGTGGGTT |
83-103 494-475 |
60 | 412 | yes |
| II-1β | NM_008361.3 | MB2038 MB2039 |
GTGGCTGTGGAGAAGCTGTGGC CAGGGTGGGTGTGCCGTCTT |
270-291 659-640 |
60 | 390 | yes |
| II-10 | NM_010548 2 | MB2154 MB2155 |
CTGCTCTTACTGACTGGCAT GGAGTCGGTTAGCAGTATG |
95-114 274-255 |
60 | 180 | yes |
| ll-12a | NM_008351 3 | MB2133 MB2134 |
CACAGTCCTGGGAAAGTCCTG TAGCCAGGCAACTCTCGTTC |
9-29 410-391 |
60 | 402 | yes |
| TFF1 | NM_009362 2 | MD7 MD8 |
AAACATGTATCATGGCCC GAATTCGAGGACTAAAAGTCTG |
123-145 450-9 |
57 | 323 | yes |
| TFF2 | NM_009363.3 | MD5 MD6 |
TTCCACCCACTTCCAAAC AATGCTGTGTCTAGCCACTG |
242-259 551-532 |
57 | 310 | yes |
| TFF3 | NM_011575.2 | MB1847 MB1848 |
TCTGGCTAATGCTGTTGGTG TCAGATCAGCCTTGTGTTGG |
52-71 443-424 |
60 | 392 | yes |
| Tnfa | NM_013693.3 | MB2052 MB2053 |
GCAGCCAACAGGCAGGTTCT ACGTAGTCGGGGCAGCCTTGT |
94-114 622-602 |
60 | 529 | yes |
| Actb/promoter | NC_000071.6 | MB1783 MB1784 |
GATGCTGACCCTCATCCACT ATGAAGAGTTTTGGCGATGG |
142907148-129 142906954-973 |
60 | 195 | no |
| Asl | NC_000071.6 | TCTTCGTTAGCGGCAAGTCACCT ATGACCCAGCAGCTAAGCAGATCA |
130022680-657 130022575-598 |
60 | 106 | ||
| Neo | AM235741.1 | MB1920 MB1921 |
TGCTCTGATGCCGCCGTGTT GCACGAGGAAGCGGTCAGCC |
1073-1092 1709-1690 |
60 | 637 | |
| TFF3 | AJ271004.1 | MB1871 MB98 |
CTGTCACATCGGAGCAGTGT TGACCCTGTGTCATCACCCT |
4943-4962 5233-5214 |
60 | 291 | e2-i2 |
| T. gondii B1 | KC607827.1 | MB2342 MB2343 |
TCCCCTCTGCTGGCGAAAAGT AGCGTTCGTGGTCAACTATCGATTG |
113-133 210-186 |
60 | 98 |
Toxoplasma real-time PCR
Semiquantitative real-time PCR analyses were performed to determine parasite loads in brains as described previously [66]. FastStart Essential DNA Green Master (Roche, Grenzach-Wyhlen, Germany) was used with 90 ng genomic DNA in a reaction volume of 20 μL. Triplicate reactions were developed in a LightCycler 480 Instrument II (Roche, Grenzach-Wyhlen, Germany). After an initial activation step (95 °C for 10 min), 45 amplification cycles were run, comprising of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s, and elongation at 72 °C for 15 s. The primers manufactured by Tib MolBiol (Berlin, Germany) were used at a final concentration of 0.3 μM. The specific primer pairs are listed in Table 1 (T. gondii B1 gene, murine argininosuccinate lyase/Asl as a reference). For normalization against Asl, target–reference ratios were calculated with the LightCycler 480 Software release 1.5.0 (Roche, Grenzach-Wyhlen, Germany). The resulting data were further normalized against a control group, i.e., the ileum of WT animals.
Histological studies
Tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin, and 5 μm sections were stained with hematoxylin and eosin (H&E) as described previously [52]. Immunohistochemistry was performed using a rabbit monoclonal anti-CD4 antibody (1:50; Epitomics, Burlingame/CA, USA) and a Benchmark XT automated slide staining system (Ventana, Tucson, AZ, USA) according to the manufacturer’s recommendations.
Microscopic findings were evaluated using a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany) with Olympus DP26 digital camera and CellSens Entry software (Olympus, Tokyo, Japan). The height of the mucosa was measured in triplicate, and a mean height value was calculated for each specimen. The number of CD4+ lymphocytes in the mucosa was counted in three different high power fields (HPF), and a mean value of CD4+ lymphocytes per HPF was calculated.
Statistical analysis
Student’s t-test was performed using the Excel 2003 software package (Microsoft, USA). The error bars in Figs 1–4 represent ±SEM. Significant differences between the mean values of the different experimental groups are indicated by asterisks (P ≤ 0.05: significant, *; P ≤ 0.01: highly significant, **; P ≤ 0.001: extremely highly significant, ***).
Fig. 1.
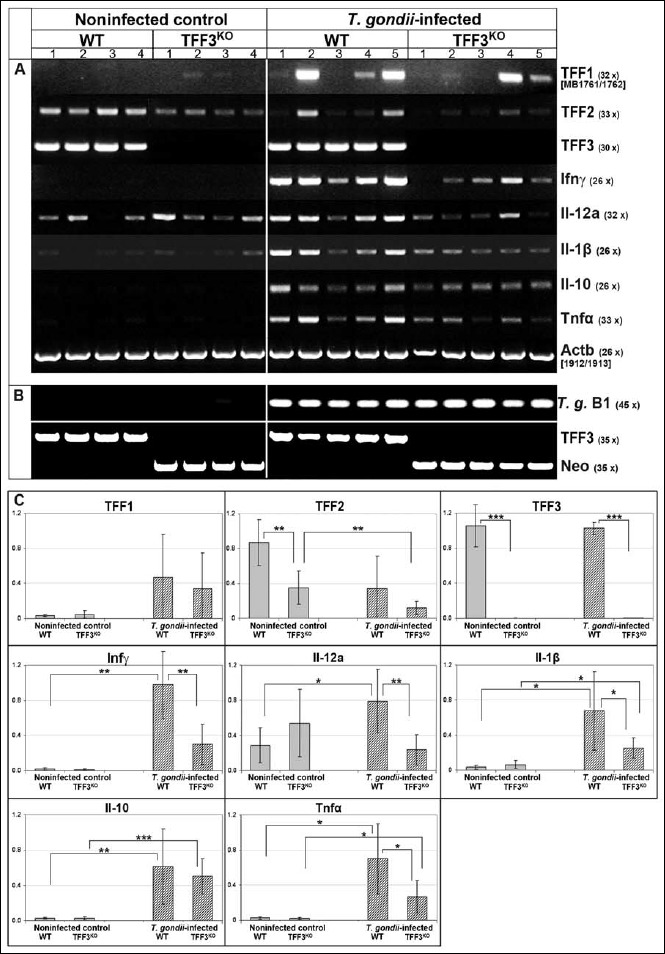
(RT)-PCR analysis of the ileum. (A) TFF1, TFF2, TFF3, interferon-γ (Ifnγ), interleukin-12a (Il-12a), Il-1β, Il-10, and tumor necrosis factor α (Tnfα) expression were monitored in the ileum of noninfected control animals (4 WT and 4 TFF3KO mice, respectively) and orally T. gondii-infected animals (5 WT and 5 TFF3KO mice, respectively). The number of amplification cycles is given in parentheses. (B) Results of the T. gondii infection test (PCR analysis of DNA from the ileum for the T. gondii gene B1) and the genotyping (PCR analysis of genomic DNA from tail clippings for TFF3 and the neomycin resistance gene/Neo). (C) Results of the semiquantitative RT-PCR analyses (relative gene expression levels normalized against β-actin). Significant differences are indicated by asterisks
Fig. 2.
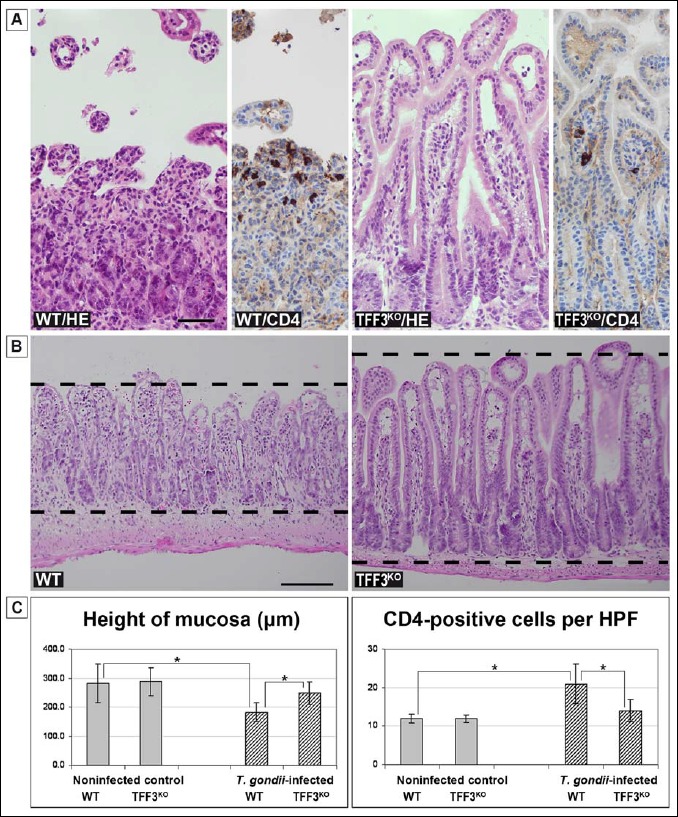
Histological analysis of the murine ileum. (A) Representative hematoxylin and eosin (H&E) stained sections and CD4 immunohistochemistry, respectively, of the ileum of T. gondii-infected animals (WT and TFF3KO mouse, respectively). Scale bar: 50 μm. (B) Representative H&E stained sections of the ileum of T. gondii-infected animals (WT and TFF3KO mouse, respectively). The dashed lines indicate the height of the mucosa. Scale bar: 50 μm. (C) Height of the mucosa (left) and the number of CD4+ lymphocytes per high power field (HPF; right) of the different animal groups (the infected animals investigated here are the same as in Fig. 1, whereas the noninfected control animals originate from a different series as those in Fig. 1). The significance of the differences between the different groups is indicated by asterisks
Fig. 3.
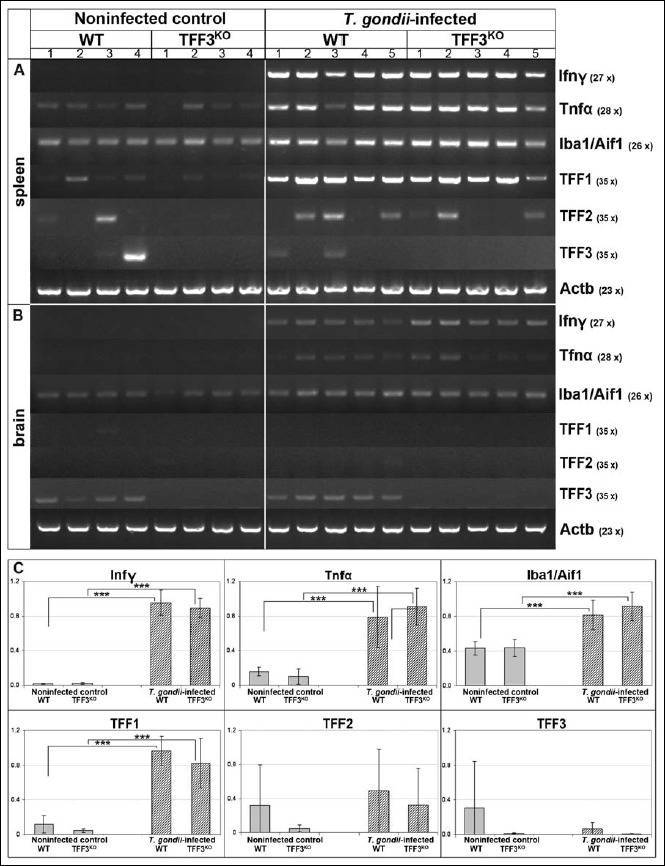
RT-PCR analysis of spleen and brain. Interferon-γ (Ifnγ), tumor necrosis factor α (Tnfα), ionized calcium binding adapter molecule 1 (Iba1/Aif1), and TFF1 expression were monitored in the spleen (A) as well as the brain (B) of noninfected control animals and orally T. gondii-infected animals (same animals as in Fig. 1). The number of amplification cycles is given in parentheses. The integrity of the cDNAs was tested by monitoring the transcript level of β-actin. (C) Results of the semiquantitative RT-PCR analyses of the spleen (relative gene expression levels normalized against β-actin). Significant differences are indicated by asterisks
Fig. 4.
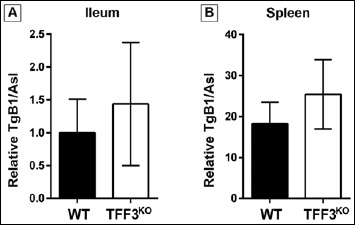
Semiquantitative real-time PCR analysis of the T. gondii burden in the ileum and spleen. T. gondii B1 (TgB1) load was measured in the ileum (A) as well as in the spleen (B) of orally T. gondii-infected animals. Relative parasite DNA levels were normalized against murine argininosuccinate lyase (Asl)
Results
Altered immune response in the ileum of TFF3KO and WT mice upon T. gondii infection
Clearly, the expression profile of the noninfected control ileum (4 WT and 4 TFF3KO mice, respectively) is altered when compared to the T. gondii-infected samples (5 WT and 5 TFF3KO mice, respectively) concerning eight selected genes as well as β-actin as a control (Fig. 1). Figure 1c shows a semiquantitative analysis of these eight genes. Furthermore, the infection with T. gondii was controlled by amplifying the B1 gene (Fig. 1b). WT and TFF3KO mice were affirmed by amplifying the TFF3 or the neomycin resistance gene, respectively (Fig. 1b).
As anticipated, T. gondii infection is followed by the elevated expression level of several inflammatory markers (Fig. 1a and 1c). Notably, the expression of Ifnγ, IL-12, IL-1β, and Tnfα genes is significantly diminished in T. gondii-infected TFF3KO animals when compared with T. gondii-infected WT animals (Fig. 1c).
Of note, TFF2 expression is downregulated in the ileum of noninfected TFF3KO mice when compared with the noninfected WT mice (Fig. 1c). A reduced TFF2 expression trend can also be observed upon T. gondii infection (Fig. 1c). In contrast, TFF1 expression of WT or TFF3KO mice is not altered (Fig. 1c). Furthermore, there is a tendency to increased TFF1 expression in T. gondii-infected mice (this is not significant because of relatively large individual variations; Fig. 1c).
Based on the observation that the differently expressed inflammatory genes differ significantly in the ileum of WT and TFF3KO mice after oral T. gondii infection, the ileum of these mice was subject to a brief pathological review (Fig. 2). In the mucosa of T. gondii-infected WT mice, typical inflammatory changes including increased cellularity and necrosis with a loss of villi were observed (Fig. 2a). Furthermore, the height of the mucosa (Fig. 2b) and the number of CD4-positive lymphocytes per HPF were measured (Fig. 2a). In particular, the height of the mucosa of T. gondii-infected animals was significantly shorter in the WT mice when compared with the TFF3KO mice, whereas the number of CD4-positive lymphocytes was significantly increased in the WT mice (Fig. 2c).
Taken together, WT mice – in comparison to TFF3KO mice – showed a significantly increased inflammatory response (RT-PCR analysis) along with microscopic inflammatory changes including necrosis, a reduced height of the mucosa, and an increased number of CD4+ lymphocytes in the ileum after T. gondii infection. Thus, TFF3KO mice seem to be partially protected from low-dose oral infection with T. gondii.
Gene expression profiling in the spleen and brain after oral T. gondii infection
In order to get a rough estimate on how far oral T. gondii infection affected other organs, expression profiling of the spleen and brain was performed concerning inflammatory genes (Ifnγ and Tnfα), the ionized calcium binding adapter molecule 1 (Iba1/Aif1) as an established marker for activated microglial cells, and the TFF genes (Fig. 3).
In the spleen (Fig. 3a), expression of Ifnγ and Tnfα was significantly upregulated in the T. gondii-infected animals (Fig. 3c). No difference was detectable between WT and TFF3KO mice. Of note, TFF1 was significantly upregulated in the T. gondii-infected animals (Fig. 3c).
Furthermore, also the T. gondii burden was estimated in the infected animals by semiquantitative real time PCR (Fig. 4). In both the spleen and the ileum, the T. gondii B1 levels were slightly elevated in the TFF3KO mice when compared to the WT animals. However, these results were not significant.
In the brain, Ifnγ and Tnfα expression was slightly induced in T. gondii-infected mice, whereas Iba1 expression was hardly changed at this early time point (Fig. 3b). In contrast to spleen, TFF1 expression was hardly detectable in the brain (Fig. 3b).
Discussion
The mechanisms regulating the mucosal barrier and inflammatory response in the intestine are complex and incompletely understood thus far. In particular, the involvement of TFF peptides is still enigmatic. Based on the fact that TFF3–Fcgbp is a major component of the intestinal mucus [7], one may expect that TFF3KO mice, which are markedly more sensitive in a DSS colitis model [27], are also more susceptible to T. gondii infection because of the defective mucus barrier. Thus, it is surprising that TFF3KO mice appear to be partially protected from T. gondii infection-induced inflammation and disparate to the report that TFF2KO mice show reduced immunopathology after oral low-dose T. gondii infection [59]. In the latter study, TFF2 was shown to negatively regulate IL-12 levels, and thus, TFF2KO mice had an increased baseline intestinal inflammation, which is protective. In this report, infected TFF2KO mice displayed lower parasite rates compared to WT controls. In contrast, in the current experiments presented in Fig. 4, slightly elevated T. gondii levels were detected in the ileum and the spleen of TFF3KO mice. The reduced proinflammatory cytokine response in the TFF3KO mice could explain the tendency of the elevated parasite numbers in these animals, because insufficient immune cells and response are available for parasite control.
As shown in Fig. 1c, in the T. gondii-infected animals, the IL-12a expression is significantly reduced in the TFF3KO animals when compared with the WT indicating a diminished inflammatory response in TFF3KO mice. This is in line with the reduced expression of most other inflammatory marker genes (Fig. 1c), which is further supported by the histological examination (Fig. 2). This reduced inflammatory response could be a consequence of the increased IL-12a expression level tendency in the noninfected TFF3KO, compared to WT animals (Fig. 1c). Elevated IL-12 levels may protect TFF3KO mice from initial T. gondii infection. Of note, the TFF2 expression is significantly lower in the noninfected TFF3KO mice, compared to those in WT animals (Fig. 1c). Thus, the situation in the TFF3KO mice shows some similarity with that of the TFF2KO mice, both having diminished TFF2 expression levels in comparison to WT animals.
Thus far, it is not known how TFF3 influences the immune response following T. gondii infection. Considering that TFF3 is a major mucus constituent, the recently described mucus function to educate DCs in developing tolerance against common gut content and to influence Treg cell production [50, 51] could be altered in the absence of TFF3. Also, Fcgbp could possibly be involved in this process. To confirm this theory, further experiments are needed to evaluate DC and Treg dynamics and function. Another possible hypothesis is that TFF3 is directly involved in regulating the immune response as an intrinsic secretory product of immune cells. This assumption is based on the report that TFF3 (and TFF2) is synthesized in lymphoid tissues, such as the spleen (see also Fig. 3), thymus, lymph nodes, or bone marrow and it stimulates migration of monocytes [22]. TFF3 is predominately synthesized in the mesenterial lymph nodes (Stürmer, Znalesniak, and Hoffmann, unpublished results). TFF3 is also synthesized by activated microglial cells in vitro, which represent the primary immune cells of the central nervous system [20]. For TFF2, a direct function in regulating the immune response has already been demonstrated [67, 68].
In agreement with a previous report [22], TFF3 and TFF2 are also expressed in the spleen of both noninfected and T. gondii-infected animals (Fig. 3). There are relatively large individual variations, and TFF2 expression levels might be elevated in the T. gondii-infected animals.
In contrast, TFF1 expression is clearly increased in the T. gondii-infected animals with no difference between the WT and the TFF3KO mice. This is the first report that, in the spleen, TFF1 expression is specifically induced during an inflammatory response. Currently, the consequences are not understood and are subject to detailed analysis. Based on results on epithelial cells [64], TFF1 can be expected to act as a motogen/chemokine in the immune system. Of note, a comparable induction of TFF1 expression has been observed in the brain of a murine Toxoplasma encephalitis model following intraperitoneal infection with T. gondii [63]. Thus, TFF1 seems to be generally involved in inflammatory responses. However, TFF1 expression was not induced in the brain 7 days after oral T. gondii infection (Fig. 3) because, at that early time point, the brain is virtually unaffected.
These first outcomes suggest that TFF3 is engaged in the immune pathology of intestinal inflammation followed by T. gondii infection. However, further experiments should endorse and scrutinize how TFF3 is involved in the pathological processes, immune cell recruitment and function in the T. gondii infection-induced inflammation model. The potential targets of TFF peptides in the immune system are still not identified, though interesting relations with chemokine receptors such as CXCR4 were previously described [32]. Importantly, recent developments point toward the potential role of TFF peptides in binding to a plethora of transmembrane glycoproteins due to their different lectin activities modulating different signal transduction processes, particularly in the immune system [45].
Acknowledgements
The authors thank Prof. D.K. Podolsky (Harvard Medical School) for providing the TFF3KO mice and D. Lorenz for her help with the art work.
References
- 1.Hoffmann W. (2013): TFF Peptides. In: Handbook of Biologically Active Peptides (2nd edition), ed. Kastin AJ, Elsevier, San Diego, pp. 1338–1345 [Google Scholar]
- 2.Kjellev S: The trefoil factor family – small peptides with multiple functionalities. Cell Mol Life Sci 66, 1350–1369 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann W, Jagla W: Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int Rev Cytol 213, 147–181 (2002) [DOI] [PubMed] [Google Scholar]
- 4.Suemori S, Lynch-Devaney K, Podolsky DK: Identification and characterization of rat intestinal trefoil factor: Tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A 88, 11017–110721 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser F, Poulsom R, Chinery R, Rogers LA, Hanby AM, Wright NA, Hoffmann W: hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci U S A 90, 6961–6965 (1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka Y, Pascall JC, Brown KD: Quantitative analysis reveals differential expression of mucin (MUC2) and intestinal trefoil factor mRNAs along the longitudinal axis of rat intestine. Biochim Biophys Acta 1489, 336–344 (1999) [DOI] [PubMed] [Google Scholar]
- 7.Albert TK, Laubinger W, Müller S, Hanisch FG, Kalinski T, Meyer F, Hoffmann W: Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res 9, 3108–3117 (2010) [DOI] [PubMed] [Google Scholar]
- 8.Kouznetsova I, Gerlach KL, Zahl C, Hoffmann W: Expression analysis of human salivary glands by laser microdis-section: Differences between submandibular and labial glands. Cell Physiol Biochem 26, 375–382 (2010) [DOI] [PubMed] [Google Scholar]
- 9.Kouznetsova I, Kalinski T, Peitz U, Mönkemüller KE, Kalbacher H, Vieth M, Meyer F, Roessner A, Malfertheiner P, Lippert H, Hoffmann W: Localization of TFF3 peptide in human esophageal submucosal glands and gastric cardia: Differentiation of two types of gastric pit cells along the rostro-caudal axis. Cell Tissue Res 328, 365–374 (2007) [DOI] [PubMed] [Google Scholar]
- 10.Kouznetsova I, Peitz U, Vieth M, Meyer F, Vestergaard EM, Malfertheiner P, Roessner A, Lippert H, Hoffmann W: A gradient of TFF3 (trefoil factor family 3) peptide synthesis within the normal human gastric mucosa. Cell Tissue Res 316, 155–165 (2004) [DOI] [PubMed] [Google Scholar]
- 11.Paulsen F, Varoga D, Paulsen A, Tsokos M: Trefoil factor family (TFF) peptides of normal human Vater’s ampulla. Cell Tissue Res 321, 67–74 (2005) [DOI] [PubMed] [Google Scholar]
- 12.Wiede A, Jagla W, Welte T, Köhnlein T, Busk H, Hoffmann W: Localization of TFF3, a new mucus-associated peptide of the human respiratory tract. Am J Respir Crit Care Med 159, 1330–1335 (1999) [DOI] [PubMed] [Google Scholar]
- 13.Wiede A, Hinz M, Canzler E, Franke K, Quednow C, Hoffmann W: Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res 303, 109–115 (2001) [DOI] [PubMed] [Google Scholar]
- 14.Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U: Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem 55, 505–513 (2007) [DOI] [PubMed] [Google Scholar]
- 15.Rinnert M, Hinz M, Buhtz P, Reiher F, Lessel W, Hoffmann W: Synthesis and localization of trefoil factor family (TFF) peptides in the human urinary tract and TFF2 excretion into the urine. Cell Tissue Res 339, 639–647 (2010) [DOI] [PubMed] [Google Scholar]
- 16.Langer G, Jagla W, Behrens-Baumann W, Walter S, Hoffmann W: Secretory peptides TFF1 and TFF3 synthesized in human conjunctival goblet cells. Invest Ophthalmol Vis Sci 40, 2220–2224 (1999) [PubMed] [Google Scholar]
- 17.Paulsen FP, Hinz M, Schaudig U, Thale AB, Hoffmann W: TFF peptides in the human efferent tear ducts. Invest Ophthalmol Vis Sci 43, 3359–3364 (2002) [PubMed] [Google Scholar]
- 18.Jackerott M, Lee YC, Møllgård K, Kofod H, Jensen J, Rohleder S, Neubauer N, Gaarn LW, Lykke J, Dodge R, Dalgaard LT, Søstrup B, Jensen DB, Thim L, Nexø E, Thams P, Bisgaard HC, Nielsen JH: Trefoil factors are expressed in human and rat endocrine pancreas: differential regulation by growth hormone. Endocrinology 147, 5752–5759 (2006) [DOI] [PubMed] [Google Scholar]
- 19.Jagla W, Wiede A, Dietzmann K, Rutkowski K, Hoffmann W: Co-localization of TFF3 peptide and oxytocin in the human hypothalamus. FASEB J 14, 1126–1131 (2000) [DOI] [PubMed] [Google Scholar]
- 20.Fu T, Stellmacher A, Znalesniak EB, Dieterich DC, Kalbacher H, Hoffmann W: TFF3 is expressed in neurons and microglial cells. Cell Physiol Biochem 34, 1912–1919 (2014) [DOI] [PubMed] [Google Scholar]
- 21.Bernstein HG, Dobrowolny H, Trübner K, Steiner J, Bogerts B, Hoffmann W: Differential regional and cellular distribution of TFF3 peptide in the human brain. Amino Acids 47, 1053–1063 (2015) [DOI] [PubMed] [Google Scholar]
- 22.Cook GA, Familari M, Thim L, Giraud AS: The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett 456, 155–159 (1999) [DOI] [PubMed] [Google Scholar]
- 23.Teng X, Xu LF, Zhou P, Sun HW, Sun M: Effects of trefoil peptide 3 on expression of TNF-α, TLR4, and NF-κB in trinitrobenzene sulphonic acid induced colitis mice. Inflammation 32, 120–129 (2009) [DOI] [PubMed] [Google Scholar]
- 24.Poulsen SS, Kissow H, Hare K, Hartmann B, Thim L: Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat. Regul Pept 126, 163–171 (2005) [DOI] [PubMed] [Google Scholar]
- 25.Marchbank T, Cox HM, Goodlad RA, Giraud AS, Moss SF, Poulsom R, Wright NA, Jankowski J, Playford RJ: Effect of ectopic expression of rat trefoil factor family 3 (intestinal trefoil factor) in the jejunum of transgenic mice. J Biol Chem 276, 24088–24096 (2001) [DOI] [PubMed] [Google Scholar]
- 26.Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L: Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 127, 502–513 (2004) [DOI] [PubMed] [Google Scholar]
- 27.Mashimo H, Wu DC, Podolsky DK, Fishman MC: Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274, 262–265 (1996) [DOI] [PubMed] [Google Scholar]
- 28.Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK: Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126, 796–808 (2004) [DOI] [PubMed] [Google Scholar]
- 29.Chwieralski CE, Schnurra I, Thim L, Hoffmann W: Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am J Respir Cell Mol Biol 31, 528–537 (2004) [DOI] [PubMed] [Google Scholar]
- 30.Taupin DR, Kinoshita K, Podolsky DK: Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A 97, 799–804 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rösler S, Haase T, Claassen H, Schulze U, Schicht M, Riemann D, Brandt J, Wohlrab D, Müller-Hilke B, Goldring MB, Sel S, Varoga D, Garreis F, Paulsen FP: Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum 62, 815–825 (2010) [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann W: Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J Med Chem 52, 6505–6510 (2009) [DOI] [PubMed] [Google Scholar]
- 33.Madsen J, Sorensen GL, Nielsen O, Tornøe I, Thim L, Fenger C, Mollenhauer J, Holmskov U: A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PloS One 8, e64441 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves EP, Ali T, Leonard P, Hearty S, O’Kennedy R, May FE, Westley BR, Josenhans C, Rust M, Suerbaum S, Smith A, Drumm B, Clyne M: Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 135, 2043–2054 (2008) [DOI] [PubMed] [Google Scholar]
- 35.Stürmer R, Müller S, Hanisch FG, Hoffmann W: Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell Physiol Biochem 33, 895–904 (2014) [DOI] [PubMed] [Google Scholar]
- 36.Hanisch FG, Bonar D, Schloerer N, Schroten H: Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J Biol Chem 289, 27363–27375 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright NA: Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos Trans R Soc Lond B Biol Sci 353, 925–933 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP: Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193, 1027–1034 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podolsky DK, Gerken G, Eyking A, Cario E: Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137, 209–220 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riaño A, Ortiz-Masià D, Velázquez M, Calatayud S, Esplugues JV, Barrachina MD: Nitric oxide induces HIF-1α stabilization and expression of intestinal trefoil factor in the damaged rat jejunum and modulates ulcer healing. J Gastroenterol 46, 565–576 (2011) [DOI] [PubMed] [Google Scholar]
- 41.Johansson ME, Thomsson KA, Hansson GC: Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 8, 3549–3557 (2009) [DOI] [PubMed] [Google Scholar]
- 42.YuH He Y, Zhang X, Peng Z, Yang Y, Zhu R, Bai J, Tian Y, Li X, Chen W, Fang D, Wang R: The rat IgGFcgammaBP and Muc2 C-terminal domains and TFF3 in two intestinal mucus layers bind together by covalent interaction. PloS One 6, e20334 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK: Trefoil peptide protection of intestinal epithelial barrier function: Cooperative interaction with mucin glycoprotein. Gastroenterology 109, 516–523 (1995) [DOI] [PubMed] [Google Scholar]
- 44.Johansson ME, Sjövall H, Hansson GC: The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10, 352–361 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann W: TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int J Oncol 47, 806–816 (2015) [DOI] [PubMed] [Google Scholar]
- 46.Johansson ME, Hansson GC: Keeping bacteria at a distance. Science 334, 182–183 (2011) [DOI] [PubMed] [Google Scholar]
- 47.Chen GY, Stappenbeck TS: Mucus, it is not just a static barrier. Sci Signal 7, pe11 (2014) [DOI] [PubMed] [Google Scholar]
- 48.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, Elinav E, Finlay BB, Flavell RA: NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, Guan JL, Saitoh T, Akira S, Seglen PO, Dinauer MC, Virgin HW, Stappenbeck TS: Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J 32, 3130–3144 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A: Mucus enhances gut home-ostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belkaid Y, Grainger J: Mucus coat, a dress code for tolerance. Science 342, 432–433 (2013) [DOI] [PubMed] [Google Scholar]
- 52.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD: Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29, 306–317 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heimesaat MM, Dunay IR, Schulze S, Fischer A, Grundmann U, Alutis M, Kühl AA, Tamas A, Toth G, Dunay MP, Göbel UB, Reglodi D, Bereswill S: Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PloS One 9, e108389 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasper L, Courret N, Darche S, Luangsay S, Mennechet F, Minns L, Rachinel N, Ronet C, Buzoni-Gatel D: Toxoplasma gondii and mucosal immunity. Int J Parasitol 34, 401–409 (2004) [DOI] [PubMed] [Google Scholar]
- 55.Dunay IR, Sibley LD: Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr Opin Immunol 22, 461–466 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM: CD8α+ dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 35, 249–259 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biswas A, Bruder D, Wolf SA, Jeron A, Mack M, Heimesaat MM, Dunay IR: Ly6Chigh monocytes control cerebral toxoplasmosis. J Immunol 194, 3223–3235 (2015) [DOI] [PubMed] [Google Scholar]
- 58.Dunay IR, Fuchs A, Sibley LD: Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun 78, 1564–1570 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McBerry C, Egan CE, Rani R, Yang Y, Wu D, Boespflug N, Boon L, Butcher B, Mirpuri J, Hogan SP, Denkers EY, Aliberti J, Herbert DR: Trefoil factor 2 negatively regulates type 1 immunity against Toxoplasma gondii. J Immunol 189, 3078–3084 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR: Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 209, 607–622 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lubka M, Müller M, Baus-Loncar M, Hinz M, Blaschke K, Hoffmann W, Pfister M, Löwenheim H, Pusch CM, Knipper M, Blin N: Lack of TFF3 peptide results in hearing impairment and accelerated presbyacusis. Cell Physiol Biochem 21, 437–444 (2008) [DOI] [PubMed] [Google Scholar]
- 62.Blaschke K: Vergleichende neurobiologische Untersuchungen von TFF3-defizienten Mäusen und Wildtyptieren (MD Thesis). Medical Faculty, Otto-von-Guericke-Universität Magdeburg (2010) [Google Scholar]
- 63.Fu T: In vivo and in vitro studies on the expression and function of TFF peptides in the gastrointestinal tract and the central nervous system. Fakultät für Naturwissen schaften, Otto-von-Guericke-Universität Magdeburg (2014) [Google Scholar]
- 64.Fu T, Kalbacher H, Hoffmann W: TFF1 is differentially expressed in stationary and migratory rat gastric epithelial cells (RGM-1) after in vitro wounding: influence of TFF1 RNA interference on cell migration. Cell Physiol Biochem 32, 997–1010 (2013) [DOI] [PubMed] [Google Scholar]
- 65.Kouznetsova I, Chwieralski CE, Bälder R, Hinz M, Braun A, Krug N, Hoffmann W: Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am J Respir Cell Mol Biol 36, 286–295 (2007) [DOI] [PubMed] [Google Scholar]
- 66.Heimesaat MM, Dunay IR, Alutis M, Fischer A, Möhle L, Göbel UB, Kühl AA, Bereswill S. Nucleotide-oligomerization-domain-2 affects commensal gut microbiota composition and intracerebral immunopathology in acute Toxoplasma gondii induced murine ileitis. PloS One 9, e105120 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baus-Loncar M, Kayademir T, Takaishi S, Wang T: Trefoil factor family 2 deficiency and immune response. Cell Mol Life Sci 62, 2947–2955 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurt-Jones EA, Cao L, Sandor F, Rogers AB, Whary MT, Nambiar PR, Cerny A, Bowen G, Yan J, Takaishi S, Chi AL, Reed G, Houghton J, Fox JG, Wang TC: Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect Immun 75, 471–480 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]


