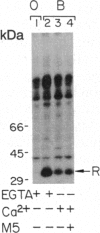
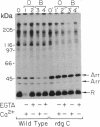
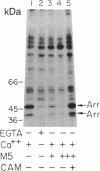
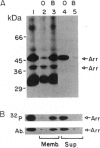
Abstract
Excitation of fly photoreceptor cells is initiated by photoisomerization of rhodopsin to the active form of metarhodopsin. Fly metarhodopsin is thermostable, does not bleach, and does not regenerate spontaneously to rhodopsin. For this reason, the activity of metarhodopsin must be stopped by an effective termination reaction. On the other hand, there is also a need to restore the inactivated photopigment to an excitable state in order to keep a sufficient number of photopigment molecules available for excitation. The following findings reveal how these demands are met. The photopigment undergoes rapid phosphorylation upon photoconversion of rhodopsin to metarhodopsin and an efficient Ca2+ dependent dephosphorylation upon regeneration of metarhodopsin to rhodopsin. Phosphorylation decreases the ability of metarhodopsin to activate the guanine nucleotide-binding protein. Binding of 49-kDa arrestin further quenches the activity of metarhodopsin and protects it from dephosphorylation. Light-dependent binding and release of 49-kDa arrestin from metarhodopsin- and rhodopsin-containing membranes, respectively, directs the dephosphorylation reaction toward rhodopsin. This ensures the return of phosphorylated metarhodopsin to the rhodopsin pool without initiating transduction in the dark. Assays of rhodopsin dephosphorylation in the Drosophila retinal degeneration C (rdgC) mutant, a mutant in a gene previously cloned and predicted to encode a serine/threonine protein phosphatase, reveal that phosphorylated rhodopsin is a major substrate for the rdgC phosphatase. We propose that mutations resulting in either a decrease or an improper regulation of rhodopsin phosphatase activity bring about degeneration of the fly photoreceptor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L. Variations in retinal degenerations. Curr Biol. 1992 Mar;2(3):113–115. doi: 10.1016/0960-9822(92)90238-6. [DOI] [PubMed] [Google Scholar]
- Bennett N., Sitaramayya A. Inactivation of photoexcited rhodopsin in retinal rods: the roles of rhodopsin kinase and 48-kDa protein (arrestin). Biochemistry. 1988 Mar 8;27(5):1710–1715. doi: 10.1021/bi00405a049. [DOI] [PubMed] [Google Scholar]
- Bentrop J., Paulsen R. Light-modulated ADP-ribosylation, protein phosphorylation and protein binding in isolated fly photoreceptor membranes. Eur J Biochem. 1986 Nov 17;161(1):61–67. doi: 10.1111/j.1432-1033.1986.tb10124.x. [DOI] [PubMed] [Google Scholar]
- Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988 Aug 26;54(5):723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Blumenfeld A., Erusalimsky J., Heichal O., Selinger Z., Minke B. Light-activated guanosinetriphosphatase in Musca eye membranes resembles the prolonged depolarizing afterpotential in photoreceptor cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7116–7120. doi: 10.1073/pnas.82.20.7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984 Sep 13;311(5982):160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Devary O., Heichal O., Blumenfeld A., Cassel D., Suss E., Barash S., Rubinstein C. T., Minke B., Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992 Apr;8(4):643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hillman P., Hochstein S., Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev. 1983 Apr;63(2):668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Hyde D. R., Mecklenburg K. L., Pollock J. A., Vihtelic T. S., Benzer S. Twenty Drosophila visual system cDNA clones: one is a homolog of human arrestin. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1008–1012. doi: 10.1073/pnas.87.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Edelman A. M., Blumenthal D. K., Krebs E. G. Rabbit skeletal muscle myosin light chain kinase. The calmodulin binding domain as a potential active site-directed inhibitory domain. J Biol Chem. 1987 Sep 5;262(25):11958–11963. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd, Smith D. P., Whitney M., Malicki D. M., Dolph P. J., Smith G. F., Burkhart W., Zuker C. S. Isolation of a novel visual-system-specific arrestin: an in vivo substrate for light-dependent phosphorylation. Mech Dev. 1990 Dec;33(1):19–25. doi: 10.1016/0925-4773(90)90131-5. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Pak W. L. Light-induced phosphorylation of retina-specific polypeptides of Drosophila in vivo. Science. 1984 Jan 13;223(4632):184–186. doi: 10.1126/science.6419348. [DOI] [PubMed] [Google Scholar]
- Meyertholen E. P., Stein P. J., Williams M. A., Ostroy S. E. Studies of the Drosophila norpA phototransduction mutant. II. Photoreceptor degeneration and rhodopsin maintenance. J Comp Physiol A. 1987 Nov;161(6):793–798. doi: 10.1007/BF00610221. [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E. Characteristics of Drosophila rhodopsin in wild-type and norpA vision transduction mutants. J Gen Physiol. 1978 Nov;72(5):717–732. doi: 10.1085/jgp.72.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Jakes S., Ingebritsen T. S., Hargrave P. A. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989 Sep 25;264(27):15770–15773. [PubMed] [Google Scholar]
- Palczewski K., Rispoli G., Detwiler P. B. The influence of arrestin (48K protein) and rhodopsin kinase on visual transduction. Neuron. 1992 Jan;8(1):117–126. doi: 10.1016/0896-6273(92)90113-r. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Shieh B. H., Zuker C. S. Isolation and structure of an arrestin gene from Drosophila. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1003–1007. doi: 10.1073/pnas.87.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele F. R., Washburn T., Rieger R., O'Tousa J. E. Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell. 1992 May 15;69(4):669–676. doi: 10.1016/0092-8674(92)90230-a. [DOI] [PubMed] [Google Scholar]
- Steele F., O'Tousa J. E. Rhodopsin activation causes retinal degeneration in Drosophila rdgC mutant. Neuron. 1990 Jun;4(6):883–890. doi: 10.1016/0896-6273(90)90141-2. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Takeuchi Y., Komori N., Kobayashi H., Sakai Y., Hotta Y., Matsumoto H. A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science. 1990 Apr 27;248(4954):483–486. doi: 10.1126/science.2158671. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Inoue H., Hotta Y. Absence of phosphatidylinositol phosphodiesterase in the head of a Drosophila visual mutant, norpA (no receptor potential A). J Biochem. 1985 Apr;97(4):1251–1254. doi: 10.1093/oxfordjournals.jbchem.a135171. [DOI] [PubMed] [Google Scholar]