Abstract
Diabetic neuropathy (DN), the most common complication of diabetes, frequently leads to foot ulcers and may progress to limb amputations. Despite continuous increase in incidence, there is no clinical therapy to effectively treat DN. Pathogenetically, DN is characterized by reduced vascularity in peripheral nerves and deficiency in angiogenic and neurotrophic factors. We will briefly review the pathogenetic mechanism of DN and address the effects and the mechanisms of cell therapies for DN. To reverse the changes of DN, studies have attempted to deliver neurotrophic or angiogenic factors for treatment in the form of protein or gene therapy; however, the effects turned out to be very modest if not ineffective. Recent studies have demonstrated that bone marrow (BM)-derived cells such as mononuclear cells or endothelial progenitor cells (EPCs) can effectively treat various cardiovascular diseases through their paracrine effects. As BM-derived cells include multiple angiogenic and neurotrophic cytokines, these cells were used for treating experimental DN and found to reverse manifestations of DN. Particularly, EPCs were shown to exert favorable therapeutic effects through enhanced neural neovascularization and neuro-protective effects. These findings clearly indicate that DN is a complex disorder with pathogenetic involvement of both vascular and neural components. Studies have shown that cell therapies targeting both vascular and neural elements are shown to be advantageous in treating DN.
Keywords: Angiogenic factors, cell therapy, diabetes mellitus, diabetic neuropathy, neurotrophic factors, nerves, vasa nervorum
Introduction
Diabetic neuropathy (DN) is a peripheral nervous system disorder and the most common complication of diabetes mellitus. There are 23.6 million children and adults in the United States (around 8% of the population) who have diabetes. DN affects up to 60% of long-standing diabetic patients [1]. Patients afflicted with DN experience decreased quality of life due to chronic pain, loss of sensation in the feet and other areas of the body, and chronic wounds partly caused by impaired pain response [2]. Autonomic nerve dysfunction also contributes to deterioration of the quality of life in diabetic patients [3].
DN can affect sensory, motor and autonomic nerve fibers in any part of the body. The nerves of the lower extremities usually become symptomatic first because they have the longest nerve fibers. There are several distinct syndromes based on the organ systems and types of nerves affected. A patient may have exclusively sensorimotor or autonomic neuropathy or a combination of both. Symptoms develop gradually over time and correlate with the degree of hyperglycemia.
Currently, there are no clinically validated, curative treatments for DN. Optimization of glucose control and foot care may halt disease progression but they cannot reverse nerve damages which often lead to debilitating secondary complications over time. Symptomatic treatment with pain medications is only partially effective and wounds are difficult to treat. Moreover, deficiency of neurotrophic factors has been regarded as one of the likely mechanisms underlying DN [1, 4]. In a clinical trial, a single treatment of injected neurotrophic cytokines was ineffective for treating DN [5]. Since DN lack both angiogenic and neurotrophic factors, cell therapy has recently emerged as an attractive therapeutic strategy in DN.
Pathogenetic Mechanisms Underlying DN
Although DN has been widely explored over the past 20 years [6] and its pathology has been well established, the pathogenesis remains unclear [7]. Pathological findings reported in diabetic patients include axonal atrophy, demyelination, nerve fiber loss, and blunted regeneration of nerves [1, 6]. The pathogenesis of DN is multi-factorial, involving both metabolic and vascular components [8, 9]. On a molecular level, the primary risk factor is hyperglycemia, which is associated with five pathways: the polyol pathway [10]; the advanced glycation end-product pathway [11, 12]; the protein kinase C pathway [13]; the poly ADP-ribose polymerase (PARP) pathway [14, 15]; and the hexosamine pathway [16]. The five pathways contribute to the production of oxidative stress. Accumulation of reactive oxygen species (ROS) increases lipid, DNA, and protein peroxidation, induces cellular apoptosis and, and reduces nerve blood flow [17, 18]. Increased oxidative stress leads to activation of the PARP pathway [19], which regulates the gene expressions involved in inflammatory reactions and neuronal dysfunction. Several studies suggest that oxidative stress and these five pathways are interdependent and central to the pathogenesis of neurovascular dysfunction [20-22].
On a cellular level, hyperglycemia affects sensory, motor, and autonomic neurons by activating the five pathways [1, 23]. Moreover, the induction of microvascular ischemia by reducing blood flow results in nerve dysfunction. ROS and reactive nitrogen species are associated with microvascular complications of diabetes [24-28]. ROS also contributes to impaired vasodilation of epineural blood vessels, resulting in ischemia to the neural tissue [29-31]. Oxidative stress leads to deterioration of Schwann cells, which play a key role as a provider of insulation for neurons, immunologic perineurial blood-nerve-barrier, and effector of nerve regeneration. Such dysfunction via oxidative stress contributes to the phenotype of DN. Thus, antioxidants have become the therapeutic targets in DN studies. However, only a few studies have suggested that antioxidants can prevent or reverse hyperglycemia-induced nerve dysfunction in experimental DN models [32, 33].
Deficiency of Neurotrophic Factors and Vascular Supply as a Cause of DN
In addition to the classical pathogenesis mentioned above, studies have elucidated the major pathophysiologic role of neurotrophic factors and vascular supply in DN. The loss of neurotrophic support and ischemic hypoxia are widely considered to represent the two downstream consequences of the cellular mechanisms described above.
Changes in Growth Factors as a Cause for DN
Many representative growth factors have dual effects of being both neurotrophic and angiogenic [34]. Some examples are vascular endothelial growth factor (VEGF) [35], insulin-like growth factor (IGF) [36-38], nerve growth factor (NGF) [39-41], brain-derived neurotrophic factor (BDNF) [42, 43], and fibroblast growth factor-2 (FGF2) [44, 45]. Recently, the term angioneurin was coined to refer to a growth factor which have both angiogenic and direct neurotrophic effects [46]. The levels of these angioneurins were decreased in diabetic animals and were associated with neural function [47, 48].
VEGF, a major angiogenic factor, is a potent selective mitogenic cytokine for endothelial cells and its expression can be induced by hypoxia through hypoxia-inducible factor-1 [49]. In ischemic tissues, VEGF induces angiogenesis by stimulating the proliferation and migration of endothelial cells [50], leading to the improvement of tissue ischemia. VEGF also enhances Schwann cell migration [51] and proliferation, promotes axonal outgrowth and survival of both the neurons and Schwann cells of superior cervical ganglia and dorsal root ganglia [52]. IGFs induce vessel remodeling [38] and also have neurotrophic effect. IGFs have been shown to promote neurite outgrowth of neuroblastoma cells [53, 54] and accelerate regeneration of sensory [55] and motor nerves [56]. IGF1 is widely expressed in craniofacial sensory ganglia, sciatic nerve, spinal cord, sensory dorsal root ganglia and brain. IGF2 is expressed in the brain, vascular structures of the nervous system, and motor neurons. In neuronal cell bodies, axons and nerve terminals, IGF receptors (IGF1R and IGF2R) are present and IGF-1 expression is reduced in streptozotocin-induced diabetic rats compared to non-diabetic controls. mRNA and protein expression of both IGF1 and IGF2 is decreased in the nerves of streptozotocin-induced diabetic rats and there is also decrease in the mRNA and protein expression level of IGF1R in the superior cervical ganglia of streptozotocin-diabetic rats [57]. Also, Schwann cell mitogenesis and myelination are stimulated by IGF1 [58]. These effects may be important for inter-neuronal signaling and peripheral nervous system function. Sonic hedgehog (SHh) modulates patterning and development of embryonic nervous system. In diabetic animal, SHh mRNA levels are significantly decreased in peripheral nerves. In addition, overexpression of SHh improves blood flow to ischemic nerve and ameliorates nerve function [59]. NGF, a well-known neurotrophic factor, was initially identified as a molecule that promoted survival and differentiation of sensory and sympathetic neurons. Now, NGF has been shown to subserve neuroprotective and repair functions [60]. NGF is synthesized by Schwann cells and target cells of sensory and sympathetic neurons such as epithelial cells, smooth muscle cells, and fibroblasts [61]. NGF homozygous knockout mice do not develop proper sympathetic neurons or small neural crest-derived sensory neurons [62]. In addition to these neurotrophic effects, NGF directly induces angiogenesis [40].
Vascular Deficiency as a Cause for DN
Maintaining adequate blood supply to nerves is crucial in maintaining nerve structure and function. Deficiencies in the blood supply to neural tissues through vasa nervorum, blood vessel within peripheral nerves largely contribute to pathogenetic mechanism of DN [63]. Several mechanisms on vascular structural changes in ischemia on diabetic nerve have been proposed. The most common abnormality in the structure of diabetic vasa nervora is the thickening of basement membrane [64-69], which is highly correlated with neuropathic severity [64, 70, 71]. In addition, decrease in nerve conduction velocity (NCV) in diabetic rats is preceded by impaired vasodilation in epineurial arterioles, which is partly mediated by ROS production [29-31]. In contrast to constricted epineurial arteriole, endoneurial capillaries appear to have a variable patency. Luminal areas of endoneurial capillaries were increased in rodent [72-74] and feline [75] models of DN, whereas those in human showed mixed results of being increased [64, 66, 76], unchanged [65, 69, 77], or decreased [67, 68, 70, 78, 79].
Also, mixed reports on blood vessel number or density in the nerves of diabetic subjects are apparently conflicting. In animal models of diabetes, endoneurial capillary density was reported to be increased [74, 80], unaltered [81], or decreased [47, 48, 82]. In human, the endoneurial capillary density was reported to be higher in early diabetic patients than healthy subjects [77]. Conversely, the endoneurial capillary density in diabetic patients with established neuropathy, showed no significant difference to that of healthy subjects [64, 65, 70]. However, recent studies have showed decreased functional capillary density using lectin perfusion as a method for measuring capillary density [47, 48, 82]. This discrepancy in the number of endoneurial capillaries appears to result from what methods or markers were used to examine capillaries. Studies altogether suggest that the number of capillary increases as compensatory response to ischemia in early diabetic condition and decreases, particularly the functional capillaries, due to impaired neovascularization under prolonged diabetic condition [72, 83, 84].
Despite some controversy on the structural aspects, it appears clear that DN is accompanied by ischemia and hypoxia of microcirculatory nutrient vessels in nerves [22, 63]. Because microcirculation is regulated by humoral, endothelial and neural factors, a vicious pathogenic cycle may develop: microcirculatory dysfunction results in peripheral nerve dysfunction which in turn results in abnormal regulation of the microcirculation leading to further nerve dysfunction. The reduction in endoneurial blood flow has been shown to be ameliorated by treatment with various vasodilatory agents, such as prostaglandin E1 analogues, alpha-adrenergic receptor blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and endothelin receptor antagonists [73, 85-89] in animal models of diabetes.
Further evidence for the impaired vascularization and ischemia in DN are provided by the finding that there is a decrease in factors that promote or maintain blood vessel formation in DN (such as VEGFs, angiopoietins, IGFs, NGF). These observations led to studies of local delivery of the angiogenic factors VEGF-A and VEGF-C into diabetic rats which showed increased vasa nervora density and NCVs [63]. Additionally, VEGFs have direct neurotrophic effects which may underlie improvement of NCVs. For instance, angiogenic medications such as statins have also been shown to improve nerve function in DN [90].
Potential Therapies for DN
Several possible therapies exist for the treatment of DN based on neurovascular pathogenesis. Among them are gene, protein, and cell therapies.
Gene and Protein Transfer
Growth factors are attractive therapeutic option for DN because they can promote neuron survival and functional integrity, as well as repair of damaged nerves. Some growth factors are angiogenic, and their therapeutic effects are mediated by blood vessel growth that supply nutrients and oxygen to nerves [91]. Other growth factors, such as neutrophin 3, are neurotrophic, and their therapeutic effects are via promoting neural regeneration and survival [92]. Growth factors, known as angioneurins (VEGF, FGF2, NGF, BDNF, IGF1), have both angiogenic and neurotrophic properties [46]. The power of these growth factors in the treatment of DN was shown by Schratzberger et al., [51, 63]. They were the first to inject VEGF encoding plasmids into rat and rabbit models of diabetes. The VEGF-treated animals showed normalization in NCV, increase in angiogenesis of vasa nervora, and increase in nerve fiber density. When the plasmid VEGF was applied to human patients, mild, but statistically significant symptomatic improvement was observed in a randomized, double-blinded trial [93]. However, the authors also reported that VEGF therapy was associated with adverse side effects that did not reach statistical significance. As this study has a relatively small sample size, further study is required to conclusively determine the effects of plasmid VEGF therapy. Other growth factors, such as IGF1 and IGF2 have also been studied in animal models of DN and have shown protective effects against development of neuropathy independent of changes in blood glucose [37]. The neurotrophic factor, FGF2 promotes angiogenesis and neurogenesis [94]. Diabetic rats treated with recombinant FGF2 showed improved nerve blood flow, motor NCV and response to mechanical stimuli 30 day post-injection. These results suggest that FGF2 supplementation is a potential therapeutic target of DN [95].
However, most of human clinical trials employing growth factors for DN have not shown beneficial effects [96] except for VEGF [93]. This may reflect the complexities of DN extending to treatment in humans in addition to variables such as period, mode, and delivery dosage of treatment.
Cell Therapy
As mentioned, emerging evidence have indicated that angiogenic factors such as VEGF-A, VEGF-C, SHh, and statin restore microcirculation in the affected nerves accompanied by functional improvement [63, 82, 90]. On the other hand, lack of neurotrophic factors has been regarded as an important pathogenic mechanism of DN [1, 4]. Administration of neurotrophic factors such as NGF [97], IGF1 and IGF2 [36, 37], ciliary neurotrophic factor (CNTF) [98], or glial cell line-derived neurotrophic factor (GDNF) [99] was shown to ameliorate DN in animal models.
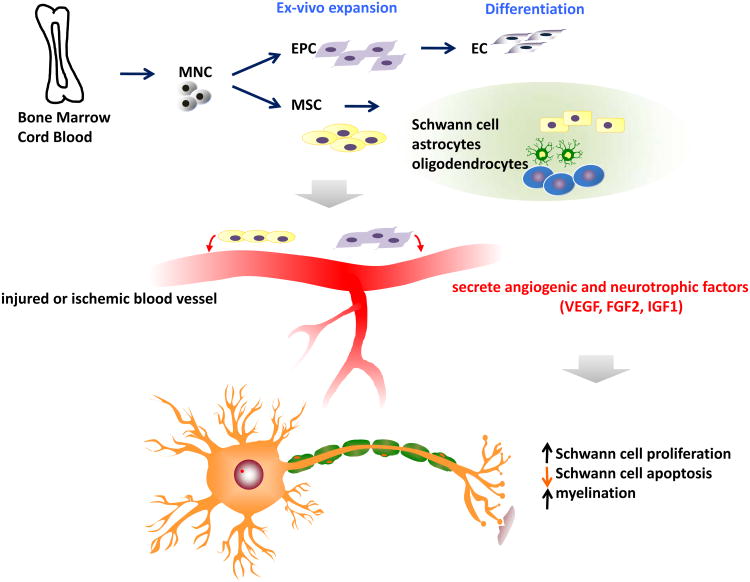
These findings suggest that a therapeutic modality which can target both angiogenic and neurotrophic processes may have more value in treatment of DN. In this sense, cell therapy using stem or progenitor cells has advantages over single gene or protein therapy. Cell therapy can increase multiple angiogenic and neurotrophic factors and potentially supplement specific type of cells required for vascular or neuronal regeneration (Fig. 1). Currently, various bone marrow (BM) cells were shown to have favorable effects for treating DN. An advantage of using circulating or BM-derived cells is that they can be harvested from a patient's own peripheral blood or bone marrow, and re-introduced back to the patient [100, 101].
Fig. 1. Mechanism of action of various bone marrow-derived cells.
Endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) can be cultured from mononuclear cells (MNCs). EPCs can differentiate into ECs and MSCs can give rise to Schwann cells, astrocytes, and oligodendrocytes. When transplanted into diabetic neuropathy animals with injured or ischemic blood vessels, the EPCs and MSCs secret angiogenic and neurotrophic factors including VEGF, FGF2, and IGF1, leading to increase in Schwann cell proliferation and myelination and decrease in Schwann cell apoptosis.
Therapeutic Potential of Bone Marrow Mononuclear Cells
BM-MNCs are derived from BM and isolated using density gradient centrifugation. BM-MNCs are heterogeneous cell population including lymphocytes, hematopoietic stem/progenitor cells, EPCs and MSCs. BM-MNCs have been shown to augment neovascularization by increasing a broad range of angiogenic factors, including FGF2, VEGF, and angiopoietin-1 in the tissue [102, 103]. In animal models, transplantation of BM-MNCs into ischemic limbs [103] and myocardium [102] increased neovascularization and collateral blood vessel formation. These effects of BM-MNCs have also been documented in patients with limb ischemia in randomized controlled trials [104]. BM-MNCs are easily isolated and do not have to be expanded by ex vivo culture. This ease of isolation makes BM-MNCs an attractive source of cells for therapeutic neovascularization.
Recent studies have shown favorable therapeutic effects of BM-MNCs on experimental DN. Hasegawa and colleagues showed that implantation of either peripheral blood (PB)-MNCs or BM-MNCs in a rat model of DN improved motor NCV and blood flow around the sciatic nerve, which is possibly mediated by VEGF secreted from MNCs. This study suggests that BM-MNCs are more effective than PB-MNCs as BM-MNCs include significantly more EPCs than PB-MNCs [81]. Recently Kim et al., reported that intramuscularly transplanted BM-MNCs preferentially localize to the nerves in diabetic rats, especially around vasa nervorum, and increase expression of various angiogenic and neurotrophic factors in the nerves [48]. The vascularity of these nerves improved (Fig. 2) and NCV levels were almost normalized [48]. These studies suggest that the vasa nervorum may play a pathogenic role in both the development and reversal of DN. This study further suggested that angiogenesis is the central mechanism of BM-MNC-induced neovascularization in experimental DN because, from their observation, BM-MNCs do not differentiate into, nor fuse with, endothelial cells in the nerves at a detectable level.
Fig. 2. Localization of transplanted EPCs along the vasa nervorum and transdifferentiation of EPCs into endothelial cells in nerves.
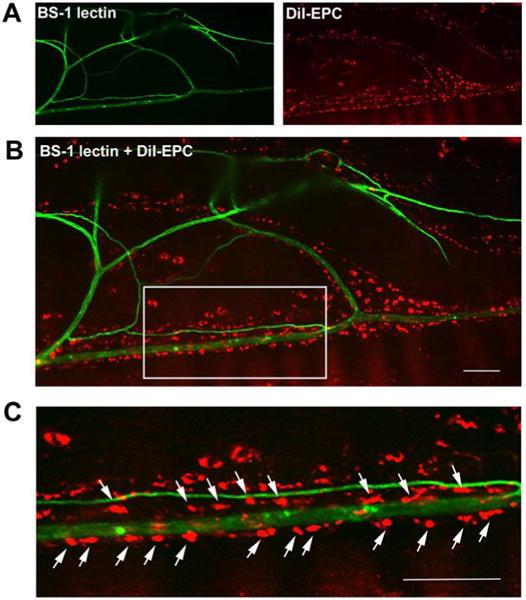
EPCs were isolated from mouse bone marrow and labeled with a red fluorescent dye, CM-DiI. Streptozotocin-induced diabetic mice were injected with the CM-DiI-labeled EPCs into the muscles along the course of the sciatic nerve. Eight weeks after the EPC transplantation, the mice hindlimbs were perfused with BM-1 lectin conjugated with FITC to visualize the blood vessels and the sciatic nerves were harvested. Whole mounted images of a sciatic nerve (A-C) demonstrated that engrafted EPCs (red) preferentially localized along the course of the vasa nervorum (green). Bars, 50 μm in B and C.
Therapeutic Potential of Endothelial Progenitor Cells and Postnatal Vasculogenesis
The development of vascular system consists of two processes: vasculogenesis and angiogenesis. Vasculogenesis refers to the de novo formation of blood vessels from EPCs or angioblasts that differentiate into endothelial cells [105], whereas angiogenesis is growth of pre-existing vasculature by sprouting of new capillaries through proliferation and migration of endothelial cells [106]. Until recently, vasculogenesis was thought to be restricted to embryonic development, while angiogenesis was considered to be responsible for neovascularization in embryos and adults. This view was challenged with the discovery of BM-derived EPCs, which circulate in adult peripheral blood [107], home to ischemic tissue and incorporate into foci of neovascularization [108], leading to de novo blood vessel formation.
The identity of EPCs is complicated by the complexity of the definition and various methods to define the cells. It is now apparent that different subsets of peripheral or BM derived cells, including hematopoietic stem cells, monocytes and circulating endothelial cells, can differentiate into endothelial-like cells. BM-derived EPCs in the adult peripheral blood express a subset of hematopoietic stem cell markers [109, 110]. Specifically, CD34, CD133 and VEGF receptor-2 have been proposed as candidate markers for human EPCs [110-112]. However, there are no known specific markers to identify EPCs without cultivation. Ex vivo expanded human EPCs express various endothelial cells markers such as CD31, CD34, KDR, VE-cadherin, bind lectins, and incorporate Dil-acetylated low-density lipoprotein. The origin of EPCs is further obscured by the two distinctive types of EPCs arising from different culture methods [113]. “Early EPCs”, are mainly derived from mononuclear cells or monocytes and do not proliferate after a few weeks [114-116]. On the other hand, “Late EPCs” form colonies after more than two weeks in culture, have cobblestone morphology, and rapidly proliferate [114, 117]. The distinctive characteristics of these two types of EPCs are reinforced by the different expression of cell surface markers. Early EPCs express pan-leukocyte and monocytic/macrophage markers such as CD45, CD11b and CD14 while late EPCs do not. Early EPCs are also therapeutically effective in vivo while evidence for therapeutic efficacy of late EPCs are limited to date [114, 117].
Endothelial differentiation of EPCs and whether this differentiation plays a main role in the therapeutic benefit of EPCs in recovering damaged tissue function is controversial. Several recent studies have demonstrated differentiation of EPCs into endothelial lineage cells with incorporation into blood vessels [118, 119]. However, other investigators claim that BM-derived cells including EPCs do not undergo endothelial differentiation nor incorporate into vessel walls [120, 121]. These discrepancies may be due to the difference in cell types, the use of different animal models or the rigor criteria to define endothelial differentiation.
One study showed that cord-blood derived EPCs were effective for treating DN [122]. This study claimed that mechanistically, the therapeutic effects are due to the increased differentiation of EPCs into endothelial cells in hindlimb muscles, which then led to an increase in sciatic nerve blood flow. However, this study did not demonstrate the fate of the EPCs in tissues, nor did it address the mechanisms by which transplanted EPCs increase neovas-cularization in muscles or nerve. Given that most recent studies have argued against the endothelial differentiation of EPCs as a major mechanism for neovascularization, endothelial differentiation does not appear to underlie such magnitude of therapeutic effects toward DN [115, 123].
More recently, a study by the author's group reported that local transplantation of BM-derived EPCs improved various manifestations of experimental DN through dual angiogenic and neurotrophic effects on peripheral nerves (Fig. 1) [47]. This study uncovered some important mechanistic insight into the role of EPCs on DN [47]. First, intramuscularly injected EPCs exert therapeutic effects through direct modulation of nerves, not through muscular neovascularization. Second, the therapeutic effects of EPCs are mainly mediated by humoral or paracrine factors released by EPCs, rather than the direct endothelial differentiation of EPCs. Third, the functional capillary (vasa nervora) density in the nerves was significantly increased by the EPC treatment. Fourth, Intramuscularly injected EPCs preferentially homed to sciatic nerves, characteristically localized in close proximity to vasa nervora, and differentiated into endothelial cells albeit infrequently [47]. A large number of engrafted EPCs survived in peripheral nerves for more than 12 weeks and induced prolonged expression of angiogenic and neurotrophic factors. Fifth, EPC transplantation increased proliferation and decreased apoptosis of endothelial and Schwann cells (Fig. 1).
The most notable finding was the direct effect of EPCs on peripheral nerves. The study was the first to demonstrate that EPC transplantation increases capillary density and blood flow in nerves, suggesting that EPCs induce neovascularization in nerves [47]. The differentiation of EPCs into endothelial cells, histologically confirmed as the colocalization of DiI-labeled transplanted cells with BS-1 lectin positive endothelial cells was infrequent, suggesting that angiogenesis could have played a more important role than vasculogenesis. This neural angiogenesis occurred through upregulation of various angiogenic factors in nerves after EPC transplantation. Various paracrine factors including VEGF-A [51, 63], FGF2 [124], BDNF [42], SHh [59, 82], and stromal cell derived factor (SDF)-1α [125, 126] were expressed in the peripheral nerves (Fig. 1). These factors have been shown to have synergistic effects on angiogenesis as well as neuro-protecting effects [63, 95, 127]. In fact, this study was the first to show clear dual angiogenic and neurotrophic effects of EPCs. This upregulation of various classes of biologically important factors may be one of the greatest benefits of stem cell therapy over any single protein or gene therapy, enabling the concerted efforts of multiple neuro-angiogenic cytokines necessary for neurovascular recovery.
Histologically, the author's study also uncovered novel engraftment and retention characteristics of BM-derived cells in tissues [47]. Following a series of reports on the short-term engraftment of any BM cells in a myocardial infarction model [128, 129], the prevailing notion was that adult stem/progenitor cells could not sustain their engraftment more than a few weeks. However, the study by Jeong et al., clearly rebutted this notion that BM-EPCs could survive more than 12 weeks in nerves [47]. The EPCs which were directly injected into the hindlimb muscles disappeared mostly in the muscles within 8 weeks; however the EPCs robustly survived for more than 12 weeks in the sciatic nerves. Interestingly, the study by Naruse et al., showed that capillary density, which had decreased in hindlimb muscles of diabetic rats at 12 weeks of diabetes, was significantly increased after cord-blood EPC treatment [122]. However, this study suggested that blood flow and capillary density in hindlimb were not significantly changed after EPC treatment. This long-lasting cell retention is compatible with the observation that EPCs homed to peripheral nerves far more preferentially than to muscles. This scale of close interaction between any BM cells and steady-state tissues was not previously reported with or without diabetes. The long-term retention of EPCs into the nerves in diabetic mice was not expected when we started this project. Together, our studies with EPCs or BM-MNCs strongly argue that the engraftment characteristics of BM cells depends more on the recipient environment than on the transplanted cells themselves [47, 48]. Another intriguing finding was that the engrafted EPCs were localized in close proximity to the vasa nervora. Such a large magnitude of tropism of BM-derived cells to blood vessels has not been reported in any other tissues. This also clearly supports that despite the controversy of EPCs on blood vessel forming capability in certain models like myocardial infarction [123, 128, 130], it is evident that EPCs can play an important role in vessel homeostasis [123, 131-134]. The distinct properties of BM-derived EPCs such as peripheral neurotropism, sustained engraftment, and vascular localization of EPCs induced robust and prolonged paracrine or humoral effects and reversal of various functional and pathologic features of DN [47, 48, 81, 122].
Perspective
DN is a progressive disease and its manifestations can take many years to develop. Cell therapy may not be a standard treatment option for all stages of DN because different stages of DN are marked by different structural or functional changes. At present, cell therapy may be applied to those patients who suffer from intractable symptoms, acute exacerbation, or combined diseases such as diabetic foot ulcers or critical limb ischemia.
Practically, as the safety of autologous BM-derived cells has been documented by a number of clinical trials [135], it is highly recommended to advance this strategy into a pilot clinical trial for those who are severely affected by DN. Particularly, EPCs will be effective in treating DN when combined with diabetic wounds or peripheral vascular obstruction as the therapeutic effects were already shown in these conditions. However, there are a few remaining concerns in cell therapy strategy. First, the effectiveness of the patient's own cells needs to be evaluated considering the possibility that BM cells derived from diabetic subjects may be impaired in therapeutic potential. Experiments using the autologous cells derived from diabetic subjects are necessary to address these concerns. However, although the efficacy of autologous diabetic cells is less potent, there may be still ways to overcome these defects to a certain extent. One strategy is to enhance their angiogenic and neurotrophic effects by culturing cells and activating necessary pathways with small molecules or growth factors. Second, the long-term effects of cell therapy need to be tested. Given that DN is a disease progressing over a long time, a single injection of cells may not be enough to maintain the nerve function over a long period of time. There are a few approaches to take in this context. One approach is to implant cells repeatedly to maintain their effects. At present, the duration of the beneficial effects of cell therapy in DN is unknown and a critical issue that requires further investigation. In many cases, the first manifestation of DN is a diabetic foot or ulcer which sometimes requires an amputation and a long-term care, which significantly reduces patients' quality of life. Cell therapy in this case can be very critical to rescue further tissue loss.
Cardiovascular autonomic neuropathy (CAN) is associated with increased risk of cardiovascular morbidity and mortality in diabetic patients [136, 137]. Although CAN is one of the most frequently studied complications of diabetes [138] and cell therapy has been reported to be effective in improving ischemic cardiovascular disease [132] and peripheral neuropathy, cell therapy has yet been evaluated in either animal models or human patients with CAN. Future studies are required to determine the effects of cell therapy in CAN.
Future directions of cell therapy for DN will take steps toward enhancing the potency of candidate cells, using both gene and cell therapy, and working with combination of various cell types such as those derived from induced pluripotent stem (iPS) cells Once generated, iPS cells can offer a plentiful and renewal source of cells that can be induced to differentiate into cells of interest [139]. Conclusively, cell therapy may become an innovative alternative therapeutic option for treating advanced DN. However, further research is necessary to overcome some limitations and possible adverse effects of cell therapy.
Acknowledgments
This work was supported in part by NIH grants DP3DK094346, RC1GM092035, AMDCC pilot and feasibility grant, and NIH contract, HHSN268201000043C (Program of Excellence in Nanotechnology Award); NSF EBICS grant, CBET-0939511; and Stem Cell Research Center of the 21st Century Frontier Research Program grant SC4300, funded by the Ministry of Science and Technology, Republic of Korea.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- BM
Bone marrow
- DN
Diabetic neuropathy
- EPC
Endothelial progenitor cell
- FGF
Fibroblast growth factor
- IGF
Insulin-like growth factor
- MNC
Mononuclear cell
- MSC
Mesenchymal stem cell
- NCV
Nerve conduction velocity
- NGF
Nerve growth factor NGF
- PARP
Poly DP-ribose polymerase
- PB
Peripheral blood
- ROS
Reactive oxygen species
- SHh
Sonic hedgehog
- VEGF
Vascular endothelial growth factor
Footnotes
Conflict of Interest: Declared none.
The authors declare no competing financial interests.
References
- 1.Leinninger GM, Vincent AM, Feldman EL. The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst. 2004;9(1):26–53. doi: 10.1111/j.1085-9489.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MP, Dworkin RH, Gammaitoni AR, Olaleye DO, Oleka N, Galer BS. Do pain qualities and spatial characteristics make independent contributions to interference with physical and emotional functioning? J Pain. 2006;7(9):644–653. doi: 10.1016/j.jpain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Verrotti A, Loiacono G, Mohn A, Chiarelli F. New insights in diabetic autonomic neuropathy in children and adolescents. Eur J Endocrinol. 2009;161(6):811–818. doi: 10.1530/EJE-09-0710. [DOI] [PubMed] [Google Scholar]
- 4.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492. doi: 10.1016/S0079-6123(03)46030-5. [DOI] [PubMed] [Google Scholar]
- 5.Apfel SC. Neurotrophic factors and pain. Clin J Pain. 2000;16(2):S7–11. doi: 10.1097/00002508-200006001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell Mol Life Sci. 2003;60(11):2445–2464. doi: 10.1007/s00018-003-3084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons Z, Feldman EL. Update on diabetic neuropathy. Curr Opin Neurol. 2002;15(5):595–603. doi: 10.1097/00019052-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999;12(5):553–563. doi: 10.1097/00019052-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Verrotti A, Giuva PT, Morgese G, Chiarelli F. New trends in the etiopathogenesis of diabetic peripheral neuropathy. J Child Neurol. 2001;16(6):389–394. doi: 10.1177/088307380101600601. [DOI] [PubMed] [Google Scholar]
- 10.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–392. doi: 10.1016/s0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14(10):953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 12.Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16(1):123–125. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 13.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7(1):108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 15.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55(6):1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg HJ, Whiteside CI, Fantus IG. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-beta I and -delta. J Biol Chem. 2002;277(37):33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- 17.Kuhad A, Chopra K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57(4):456–462. doi: 10.1016/j.neuropharm.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19(6):638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54(12):3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron NE, Cotter MA. Effects of protein kinase Cbeta inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev. 2002;18(4):315–323. doi: 10.1002/dmrr.307. [DOI] [PubMed] [Google Scholar]
- 21.Cameron NE, Gibson TM, Nangle MR, Cotter MA. Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann N Y Acad Sci. 2005;1043:784–792. doi: 10.1196/annals.1333.091. [DOI] [PubMed] [Google Scholar]
- 22.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 23.Brown MJ, Asbury AK. Diabetic neuropathy. Ann Neurol. 1984;15(1):2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 26.Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory Neurons and Schwann Cells Respond to Oxidative Stress by Increasing Antioxidant Defense Mechanisms. Antioxid Redox Signal. 2009;11(3):425–438. doi: 10.1089/ars.2008.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 28.Vincent AM, Feldman EL. New insights into the mechanisms of diabetic neuropathy. Rev Endocr Metab Disord. 2004;5(3):227–236. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- 29.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res. 2003;37(1):33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 30.Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res. 2000;1(2):131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50(8):1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 32.Nickander KK, McPhee BR, Low PA, Tritschler H. Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radic Biol Med. 1996;21(5):631–639. doi: 10.1016/0891-5849(96)00172-4. [DOI] [PubMed] [Google Scholar]
- 33.Cameron NE, Cotter MA. Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res Clin Pract. 1999;45(2-3):137–146. doi: 10.1016/s0168-8227(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 34.Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12(21):2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- 35.Simovic D, Isner JM, Ropper AH, Pieczek A, Weinberg DH. Improvement in chronic ischemic neuropathy after intramuscular phVEGF165 gene transfer in patients with critical limb ischemia. Arch Neurol. 2001;58(5):761–768. doi: 10.1001/archneur.58.5.761. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RE, Dorsey DA, Beaudet LN, Plurad SB, Parvin CA, Miller MS. Insulin-like growth factor I reverses experimental diabetic autonomic neuropathy. Am J Pathol. 1999;155(5):1651–1660. doi: 10.1016/S0002-9440(10)65480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang HX, Snyder CK, Pu SF, Ishii DN. Insulin-like growth factors reverse or arrest diabetic neuropathy: effects on hyperalgesia and impaired nerve regeneration in rats. Exp Neurol. 1996;140(2):198–205. doi: 10.1006/exnr.1996.0129. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA. 2004;101(26):9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47(6):1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 40.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47(6):1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 41.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106(17):2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 42.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115(3):653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279(32):33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 44.Unsicker K, Reichert-Preibsch H, Schmidt R, Pettmann B, Labourdette G, Sensenbrenner M. Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc Natl Acad Sci USA. 1987;84(15):5459–5463. doi: 10.1073/pnas.84.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol Cell Neurosci. 2002;20(2):181–197. doi: 10.1006/mcne.2002.1134. [DOI] [PubMed] [Google Scholar]
- 46.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9(3):169–81. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 47.Jeong JO, Kim MO, Kim H, Lee MY, Kim SW, Ii M, Lee JU, Lee J, Choi YJ, Cho HJ, Lee N, Silver M, Wecker A, Kim DW, Yoon YS. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119(5):699–708. doi: 10.1161/CIRCULATIONAHA.108.789297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, Han JW, Lee J, Kim S, Houge MA, Ii M, Yoon YS. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27(7):1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102(18):2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- 50.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 51.Schratzberger P, Schratzberger G, Silver M, Curry C, Kearney M, Magner M, Alroy J, Adelman LS, Weinberg DH, Ropper AH, Isner JM. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6(4):405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 52.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846(2):219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 53.Recio-Pinto E, Ishii DN. Effects of insulin, insulinlike growth factor-II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984;302(2):323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- 54.Recio-Pinto E, Lang FF, Ishii DN. Insulin and insulin-like growth factor II permit nerve growth factor binding and the neurite formation response in cultured human neuroblastoma cells. Proc Natl Acad Sci USA. 1984;81(8):2562–2566. doi: 10.1073/pnas.81.8.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993;607(1-2):117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- 56.Near SL, Whalen LR, Miller JA, Ishii DN. Insulin-like growth factor II stimulates motor nerve regeneration. Proc Natl Acad Sci USA. 1992;89(24):11716–11720. doi: 10.1073/pnas.89.24.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bitar MS, Pilcher CW, Khan I, Waldbillig RJ. Diabetes-induced suppression of IGF-1 and its receptor mRNA levels in rat superior cervical ganglia. Diabetes Res Clin Pract. 1997;38(2):73–80. doi: 10.1016/s0168-8227(97)00077-6. [DOI] [PubMed] [Google Scholar]
- 58.Cheng HL, Russell JW, Feldman EL. IGF-I promotes peripheral nervous system myelination. Ann N Y Acad Sci. 1999;883:124–130. [PubMed] [Google Scholar]
- 59.Calcutt NA, Allendoerfer KL, Mizisin AP, Middlemas A, Freshwater JD, Burgers M, Ranciato R, Delcroix JD, Taylor FR, Shapiro R, Strauch K, Dudek H, Engber TM, Galdes A, Rubin LL, Tomlinson DR. Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J Clin Invest. 2003;111(4):507–514. doi: 10.1172/JCI15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 61.Bandtlow CE, Heumann R, Schwab ME, Thoenen H. Cellular localization of nerve growth factor synthesis by in situ hybridization. EMBO J. 1987;6(4):891–899. doi: 10.1002/j.1460-2075.1987.tb04835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 63.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH, Isner JM. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107(9):1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Britland ST, Young RJ, Sharma AK, Clarke BF. Relationship of endoneurial capillary abnormalities to type and severity of diabetic polyneuropathy. Diabetes. 1990;39(8):909–913. doi: 10.2337/diab.39.8.909. [DOI] [PubMed] [Google Scholar]
- 65.Giannini C, Dyck PJ. Ultrastructural morphometric abnormalities of sural nerve endoneurial microvessels in diabetes mellitus. Ann Neurol. 1994;36(3):408–415. doi: 10.1002/ana.410360312. [DOI] [PubMed] [Google Scholar]
- 66.Malik RA, Newrick PG, Sharma AK, Jennings A, Ah-See AK, Mayhew TM, Jakubowski J, Boulton AJ, Ward JD. Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32(2):92–102. doi: 10.1007/BF00505180. [DOI] [PubMed] [Google Scholar]
- 67.Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48(3):578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 68.Malik RA, Tesfaye S, Thompson SD, Veves A, Sharma AK, Boulton AJ, Ward JD. Endoneurial localisation of microvascular damage in human diabetic neuropathy. Diabetologia. 1993;36(5):454–459. doi: 10.1007/BF00402283. [DOI] [PubMed] [Google Scholar]
- 69.Sima AA, Nathaniel V, Prashar A, Bril V, Greene DA. Endoneurial microvessels in human diabetic neuropathy. Endothelial cell dysjunction and lack of treatment effect by aldose reductase inhibitor. Diabetes. 1991;40(9):1090–1099. doi: 10.2337/diab.40.9.1090. [DOI] [PubMed] [Google Scholar]
- 70.Bradley J, Thomas PK, King RH, Llewelyn JG, Muddle JR, Watkins PJ. Morphometry of endoneurial capillaries in diabetic sensory and autonomic neuropathy. Diabetologia. 1990;33(10):611–618. doi: 10.1007/BF00400205. [DOI] [PubMed] [Google Scholar]
- 71.Khawaja KI, Walker D, Hayat SA, Boulton AJ, Malik RA. Clinico-pathological features of postural hypotension in diabetic autonomic neuropathy. Diabet Med. 2000;17(2):163–166. doi: 10.1046/j.1464-5491.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 72.Sugimoto K, Yagihashi S. Effects of aminoguanidine on structural alterations of microvessels in peripheral nerve of streptozotocin diabetic rats. Microvasc Res. 1997;53(2):105–112. doi: 10.1006/mvre.1996.2002. [DOI] [PubMed] [Google Scholar]
- 73.Yasuda H, Sonobe M, Yamashita M, Terada M, Hatanaka I, Huitian Z, Shigeta Y. Effect of prostaglandin E1 analogue TFC 612 on diabetic neuropathy in streptozocin-induced diabetic rats. Comparison with aldose reductase inhibitor ONO 2235. Diabetes. 1989;38(7):832–838. doi: 10.2337/diab.38.7.832. [DOI] [PubMed] [Google Scholar]
- 74.Uehara K, Sugimoto K, Wada R, Yoshikawa T, Marukawa K, Yasuda Y, Kimura Y, Yagihashi S. Effects of cilostazol on the peripheral nerve function and structure in STZ-induced diabetic rats. J Diabetes Complications. 1997;11(3):194–202. doi: 10.1016/s1056-8727(96)00023-2. [DOI] [PubMed] [Google Scholar]
- 75.Estrella JS, Nelson RN, Sturges BK, Vernau KM, Williams DC, LeCouteur RA, Shelton GD, Mizisin AP. Endoneurial microvascular pathology in feline diabetic neuropathy. Microvasc Res. 2008;75(3):403–410. doi: 10.1016/j.mvr.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuda H, Dyck PJ. Abnormalities of endoneurial microvessels and sural nerve pathology in diabetic neuropathy. Neurology. 1987;37(1):20–28. doi: 10.1212/wnl.37.1.20. [DOI] [PubMed] [Google Scholar]
- 77.Thrainsdottir S, Malik RA, Dahlin LB, Wiksell P, Eriksson KF, Rosen I, Petersson J, Greene DA, Sundkvist G. Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes. 2003;52(10):2615–2622. doi: 10.2337/diabetes.52.10.2615. [DOI] [PubMed] [Google Scholar]
- 78.Dyck PJ, Hansen S, Karnes J, O'Brien P, Yasuda H, Windebank A, Zimmerman B. Capillary number and percentage closed in human diabetic sural nerve. Proc Natl Acad Sci USA. 1985;82(8):2513–2517. doi: 10.1073/pnas.82.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malik RA, Tesfaye S, Thompson SD, Veves A, Hunter A, Sharma AK, Ward JD, Boulton AJ. Transperineurial capillary abnormalities in the sural nerve of patients with diabetic neuropathy. Microvasc Res. 1994;48(2):236–245. doi: 10.1006/mvre.1994.1051. [DOI] [PubMed] [Google Scholar]
- 80.Artico M, Massa R, Cavallotti D, Franchitto S, Cavallotti C. Morphological changes in the sciatic nerve of diabetic rats treated with low molecular weight heparin OP 2123/parnaparin. Anat Histol Embryol. 2002;31(4):193–197. doi: 10.1046/j.1439-0264.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 81.Hasegawa T, Kosaki A, Shimizu K, Matsubara H, Mori Y, Masaki H, Toyoda N, Inoue-Shibata M, Nishikawa M, Iwasaka T. Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp Neurol. 2006;199(2):274–280. doi: 10.1016/j.expneurol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon YS, Curry C, Silver M, Kearney M, Asahara T, Losordo DW. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004;24(11):2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- 83.Zochodne DW, Nguyen C. Increased peripheral nerve microvessels in early experimental diabetic neuropathy: quantitative studies of nerve and dorsal root ganglia. J Neurol Sci. 1999;166(1):40–46. doi: 10.1016/s0022-510x(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 84.Kennedy JM, Zochodne DW. Influence of experimental diabetes on the microcirculation of injured peripheral nerve: functional and morphological aspects. Diabetes. 2002;51(7):2233–2240. doi: 10.2337/diabetes.51.7.2233. [DOI] [PubMed] [Google Scholar]
- 85.Cameron NE, Cotter MA, Ferguson K, Robertson S, Radcliffe MA. Effects of chronic alpha-adrenergic receptor blockade on peripheral nerve conduction, hypoxic resistance, polyols, Na(+)-K(+)-ATPase activity, and vascular supply in STZ-D rats. Diabetes. 1991;40(12):1652–1658. doi: 10.2337/diab.40.12.1652. [DOI] [PubMed] [Google Scholar]
- 86.Cameron NE, Cotter MA, Robertson S. Angiotensin converting enzyme inhibition prevents development of muscle and nerve dysfunction and stimulates angiogenesis in streptozotocin-diabetic rats. Diabetologia. 1992;35(1):12–18. doi: 10.1007/BF00400846. [DOI] [PubMed] [Google Scholar]
- 87.Jarvis MF, Wessale JL, Zhu CZ, Lynch JJ, Dayton BD, Calzadilla SV, Padley RJ, Opgenorth TJ, Kowaluk EA. ABT-627, an endothelin ET(A) receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur J Pharmacol. 2000;388(1):29–35. doi: 10.1016/s0014-2999(99)00865-1. [DOI] [PubMed] [Google Scholar]
- 88.Maxfield EK, Love A, Cotter MA, Cameron NE. Nerve function and regeneration in diabetic rats: effects of ZD-7155, an AT1 receptor antagonist. Am J Physiol. 1995;269(3 Pt 1):E530–E537. doi: 10.1152/ajpendo.1995.269.3.E530. [DOI] [PubMed] [Google Scholar]
- 89.Obrosova IG, Van Huysen C, Fathallah L, Cao X, Stevens MJ, Greene DA. Evaluation of alpha(1)-adrenoceptor antagonist on diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2000;14(11):1548–1558. doi: 10.1096/fj.14.11.1548. [DOI] [PubMed] [Google Scholar]
- 90.Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112(1):93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 91.Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol. 2003;140(4):614–619. doi: 10.1038/sj.bjp.0705458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77(4):503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 93.Ropper AH, Gorson KC, Gooch CL, Weinberg DH, Pieczek A, Ware JH, Kershen J, Rogers A, Simovic D, Schratzberger P, Kirchmair R, Losordo D. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: a randomized, double-blinded trial. Ann Neurol. 2009;65(4):386–393. doi: 10.1002/ana.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mudo G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009;116(8):995–1005. doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 95.Nakae M, Kamiya H, Naruse K, Horio N, Ito Y, Mizubayashi R, Hamada Y, Nakashima E, Akiyama N, Kobayashi Y, Watarai A, Kimura N, Horiguchi M, Tabata Y, Oiso Y, Nakamura J. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes. 2006;55(5):1470–1477. doi: 10.2337/db05-1160. [DOI] [PubMed] [Google Scholar]
- 96.Calcutt NA, Jolivalt CG, Fernyhough P. Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets. 2008;9(1):47–59. doi: 10.2174/138945008783431727. [DOI] [PubMed] [Google Scholar]
- 97.Apfel SC. Neurotrophic factors in the therapy of diabetic neuropathy. Am J Med. 1999;107(2B):34S–42S. doi: 10.1016/s0002-9343(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 98.Mizisin AP, Vu Y, Shuff M, Calcutt NA. Ciliary neurotrophic factor improves nerve conduction and ameliorates regeneration deficits in diabetic rats. Diabetes. 2004;53(7):1807–1812. doi: 10.2337/diabetes.53.7.1807. [DOI] [PubMed] [Google Scholar]
- 99.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tacken PJ, deVries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 101.Prochazka V, Gumulec J, Chmelova J, Klement P, Klement GL, Jonszta T, Czerny D, Krajca J. Autologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic foot. Vnitr Lek. 2009;55(3):173–178. [PubMed] [Google Scholar]
- 102.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 103.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 104.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 105.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 106.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 107.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 108.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 110.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92(2):362–367. [PubMed] [Google Scholar]
- 111.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 112.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 113.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 115.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 116.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 118.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11(3):261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 119.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 120.O'Neill TJT, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97(10):1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 121.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 122.Naruse K, Hamada Y, Nakashima E, Kato K, Mizubayashi R, Kamiya H, Yuzawa Y, Matsuo S, Murohara T, Matsubara T, Oiso Y, Nakamura J. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes. 2005;54(6):1823–1828. doi: 10.2337/diabetes.54.6.1823. [DOI] [PubMed] [Google Scholar]
- 123.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204(13):3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abe K, Saito H. Neurotrophic effect of basic fibroblast growth factor is mediated by the p42/p44 mitogen-activated protein kinase cascade in cultured rat cortical neurons. Brain Res Dev Brain Res. 2000;122(1):81–85. doi: 10.1016/s0165-3806(00)00054-7. [DOI] [PubMed] [Google Scholar]
- 125.Chalasani SH, Baribaud F, Coughlan CM, Sunshine MJ, Lee VM, Doms RW, Littman DR, Raper JA. The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells. J Neurosci. 2003;23(11):4601–4612. doi: 10.1523/JNEUROSCI.23-11-04601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, Vannier J, Soria J, Vasse M, Soria C. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99(6):587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 127.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, Nakagawa K, Hou X, Nagai Y, Hasegawa M, Sugimachi K, Sueishi K. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90(9):966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 128.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 129.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41(5):876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 130.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 132.Kim H, Kim SW, Nam D, Kim S, Yoon YS. Cell therapy with bone marrow cells for myocardial regeneration. Antioxid Redox Signal. 2009;11(8):1897–1911. doi: 10.1089/ars.2009.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Flores-Ramirez R, Uribe-Longoria A, Rangel-Fuentes MM, Gutierrez-Fajardo P, Salazar-Riojas R, Cervantes-Garcia D, Trevino-Ortiz JH, Benavides-Chereti GJ, Espinosa-Oliveros LP, Limon-Rodriguez RH, Monreal-Puente R, Gonzalez-Trevino JL, Rojas-Martinez A. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med. 2010;11(2):72–78. doi: 10.1016/j.carrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 134.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111(9):1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 135.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 136.Habib AA, Brannagan TH., 3rd Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10(2):92–100. doi: 10.1007/s11910-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 137.Schonauer M, Thomas A, Morbach S, Niebauer J, Schonauer U, Thiele H. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res. 2008;5(4):336–344. doi: 10.3132/dvdr.2008.047. [DOI] [PubMed] [Google Scholar]
- 138.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 139.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137(1):13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]