Abstract
Purpose
Data on the role of angiogenesis inhibitors (AIs) rechallenge in the treatment of advanced non-small-cell lung cancer (NSCLC) patients who previously received bevacizumab remain limited. We aim to investigate the efficacy of AIs in the treatment of advanced NSCLC in this setting.
Methods
Studies from PubMed, Web of Science, and abstracts presented at American Society of Clinical Oncology meeting up to December 1, 2014 were searched to identify relevant studies. Eligible studies included prospective randomized controlled trials evaluating AIs in advanced NSCLC, with survival data on patients who previously received bevacizumab. The end points were overall survival and progression-free survival. Statistical analyses were conducted by using either random effects or fixed effect models according to the heterogeneity of included studies.
Results
A total of 452 patients with advanced NSCLC who previously received bevacizumab were identified for analysis. The meta-analysis results demonstrated that AI rechallenge significantly improved progression-free survival (hazard ratio: 0.72, 95% confidence interval: 0.58–0.89, P=0.002) when compared to non-AI containing regimens. Additionally, a nonsignificant improvement in overall survival was also observed in advanced NSCLC in this setting (hazard ratio: 0.82, 95% confidence interval: 0.65–1.03, P=0.087). Similar results were also observed in subgroup analysis according to treatment regimens.
Conclusion
The findings of this study suggest that NSCLC patients who relapsed after a first-line bevacizumab-containing chemotherapy obtain improved clinical benefits from AI rechallenge. Prospective clinical trials investigating the role of AI rechallenge in this setting are recommended.
Keywords: non-small-cell lung cancer, rechallenge, second-line, angiogenesis inhibitors, randomized controlled trials, meta-analysis
Introduction
Lung cancer is the leading cause of cancer-related mortality with an estimated 1.4 million deaths each year.1 Non-small-cell lung cancer (NSCLC) accounts for 85% of the cases. Most of NSCLC patients have advanced disease at diagnosis. For these patients, platinum-based doublet therapy is the standard of care.2 After the first-line failure, conventional monotherapy with docetaxel or pemetrexed, and more recently with erlotinib, gains a low response rate, patients have a relatively short progression-free survival (PFS) and overall survival (OS).3–5 Clearly, novel therapeutic approaches to improve outcomes for patients with NSCLC are badly needed.6
Angiogenesis, the process of vasculature formation, is critical for tumor progression, invasion, and metastasis in solid tumors.7 Pathways that promote angiogenesis have been targeted as a therapeutic approach in multiple types of cancer, including NSCLC.8–11 Of these, the vascular endothelial growth factor (VEGF) pathway has been the most well studied. Bevacizumab is the only approved antiangiogenic agent for NSCLC patients, when added to first-line carboplatin/paclitaxel chemotherapy.12–14 More recently, the platelet-derived growth factor (PDGF) and fibroblast growth factor pathways have been identified as regulators of angiogenesis and potential mediators of resistance to VEGF inhibition. Thus, new antiangiogenic treatment strategies are being evaluated that target multiple receptors within a family (VEGF receptor [VEGFR]-1, VEGFR-2)15–17 or multiple angiogenic pathways (targets VEGFR and PDGF receptor pathways).15,18,19
Several previously preclinical and observational studies demonstrate that sustained VEGF inhibition with bevacizumab can be beneficial in some patients with solid tumors.20 Preclinical data suggest that sustained VEGF inhibition achieves and maintains tumor regression.21–23 Rapid tumor revascularization has also been observed to occur after stopping the anti-VEGF therapy, indicating that vascular regrowth may be a normal physiological response to the stoppage of anti-VEGF therapy.24,25 Sustained VEGF inhibition has thus been shown to achieve and maintain tumor regression. Insight into the effect of treatment with bevacizumab beyond disease progression has been provided by the randomized, prospective bevacizumab treatment trials known as the ML18147 study for metastatic colorectal cancer (mCRC).26 In this prospective study, continued antiangiogenic treatment with bevacizumab plus chemotherapy beyond first progressive disease correlated with prolonged survival versus no continuation of bevacizumab (median OS: 11.1 versus 9.6 months). Thus, retreatment with angiogenesis inhibitors (AIs) could be proposed, hypothetically, for patients with NSCLC. However, in the NSCLC setting, no Phase III trials have been published in a second-line setting to prove the value of AIs rechallenge. In this study, we assess the effect on OS of AIs rechallenge in advanced NSCLC patients, who had previously been given bevacizumab plus standard first-line chemotherapy.
Materials and methods
Selection of studies
We searched the PubMed (data from January 2000 to October 2014), Embase (data from January 2000 to October 2014), and the Cochrane Library electronic databases. The search criteria included only randomized controlled trials (RCTs) which were published in English language, and the key words “aflibercept”, “VEGFR-TKIs”, “sorafenib”, “Nexavar”, “sunitinib”, “Sutent”, “SU1248”, “vandetanib”, “Caprelsa”, “ZD6474”, “axitinib”, “pazopanib”, “Votrient”, “GW786034”, “regorafenib”, “apatinib”, “ramucirumab”, “nintedanib”, “BIBF1120”, “thalidomide”, “lenalidomide”, “angiogenesis inhibitors”, “randomized”, “non-small-cell lung cancer” were used for the search. We also searched abstracts and virtual meeting presentations from the American Society of Clinical Oncology (http://www.asco.org/ASCO) conferences that took place between January 2004 and June 2014. Each publication was reviewed, and in cases of duplicate publication, only the most complete, recent, and updated report of the clinical trial was included for analysis. The authors confirm they did not require approvals and/or patient consent for these case studies.
Data extraction and clinical end point
Data extraction was conducted independently by two investigators according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement,27 and any discrepancy between the reviewers was resolved by consensus. For each study, the following information was extracted: first author’s name, year of publication, number of patients who received bevacizumab already, treatment arms, primary end points, and median follow-up. Phase I trials and single-group Phase II trials were omitted from analysis because of the lack of controls. Trials that met the following criteria were included in our analysis: 1) prospective randomized controlled trails comparing therapies with or without AIs (aflibercept, sorafenib, sunitinib, vandetanib, pazopanib, axitinib, regorafenib, apatinib, cediranib, ramucirumab, nintedanib, thalidomide, lenalidomide); 2) trials with patients who were pathologically confirmed to have NSCLC; and 3) studies having sufficient survival data of patients who received bevacizumab already. If multiple publications of the same trial were retrieved or if there was a case mix between publications, only the most recent publication (and the most informative) was included. The quality of reports of clinical trials was assessed and calculated using the 5-item Jadad scale, including randomization, double-blinding, and withdrawals as previously described.28
Data analysis
The analysis was undertaken on an intention-to-treat basis: patients were analyzed according to treatment allocated, irrespective of whether they received that treatment. Statistical analysis of the overall hazard ratio (HR) for OS and PFS was calculated using Version 2 of the Comprehensive Meta-Analysis program (Biostat, Englewood, NJ, USA). A statistical test with a P-value less than 0.05 was considered significant. HR > 1 reflects more deaths or progression in AIs-containing regimens group and vice versa. Between-study heterogeneity was estimated using the χ2-based Q statistic.29 The I2 statistic was also calculated to evaluate the extent of variability attributable to statistical heterogeneity between trials. Heterogeneity was considered statistically significant when Pheterogeneity <0.05 or I2>50%. If heterogeneity existed, data were analyzed using a random effects model. In the absence of heterogeneity, a fixed effects model was used. To investigate the sources of heterogeneity, we also conducted predefined subgroup analysis according to treatment regimens. The presence of publication bias was evaluated by using the Begg and Egger tests.30 All P-values were two sided. All confidence intervals (CIs) had a two-sided pro bability coverage of 95%.
Results
Search results
A total of 270 potentially relevant studies were retrieved electronically, 264 of which were excluded for the reasons shown in Figure 1. Six published RCTs with subgroup analysis assessing the efficacy of AIs rechallenge in NSCLC patients were included in the meta-analysis.16,31–35 The baseline characteristics of each trial are presented in Table 1. A total of 452 patients were included in the study. According to the inclusion criteria of each trial, patients were required to have adequate renal, hepatic, and hematologic function. The quality of each included study was roughly assessed according to Jadad scale. All of the included trials were double-blind, placebo-controlled randomized trials, and thus had Jadad score of 5.
Figure 1.
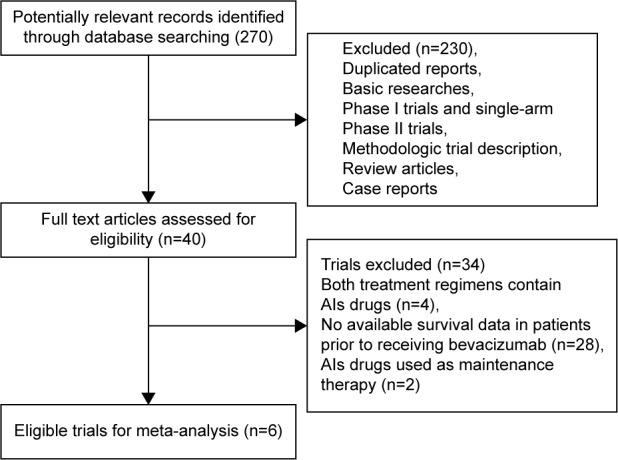
Studies eligible for inclusion in the meta-analysis.
Abbreviation: AIs, angiogenesis inhibitors.
Table 1.
Baseline characteristic of the six trials included for analysis
| Study | Total patients | Number of patients who received bevacizumab already | Treatment regimens | Primary end point | Median follow-up (month) | Jadad score |
|---|---|---|---|---|---|---|
| Garon et al31 | 1,253 | 180 | Ramucirumab 10 mg/kg + docetaxel | OS | 9.5 | 5 |
| Placebo + docetaxel | ||||||
| Reck et al16 | 562 | 38 | Nintedanib 200 mg qd po + docetaxel | PFS | 7.1 | 5 |
| Placebo + docetaxel | ||||||
| Scagliotti et al32 | 960 | 96 | Sunitinib 17.5 mg qd po + erlotinib | OS | 21.3 | 5 |
| Placebo + erlotinib qd po | ||||||
| Natale et al33 | 1,240 | 50 | Vandetanib 300 mg qd po + erlotinib | PFS | NR | 5 |
| Placebo + erlotinib | ||||||
| de Boer et al34 | 534 | 44 | Vandetanib 100 mg qd po + pemetrexed | PFS | NR | 5 |
| Placebo + pemetrexed | ||||||
| Herbst et al35 | 1,391 | 44 | Vandetanib 100 mg qd po + docetaxel | PFS | 12.8 | 5 |
| Placebo + docetaxel |
Abbreviations: OS, overall survival; PFS, progression-free survival; qd, four times a day; po, oral administration; NR, not reported.
Overall survival
All of the six trials reported OS data of AIs rechallenge in NSCLC patients who previously received bevacizumab. The pooled results demonstrated that AIs rechallenge had a tendency to improve OS in comparison with non-AIs-containing regimens (HR: 0.82, 95% CI: 0.65–1.03, P=0.087, Figure 2), using a fixed-effects model (I2=0%, P=0.735). We then performed subgroup analysis according to treatment regimens and found that both AIs rechallenge plus chemotherapy (HR: 0.84, 95% CI: 0.63–1.12, P=0.24) or erlotinib (HR: 0.78, 95% CI: 0.53–1.14, P=0.20) had a tendency to improve OS when compared to non-AIs-containing regimens.
Figure 2.
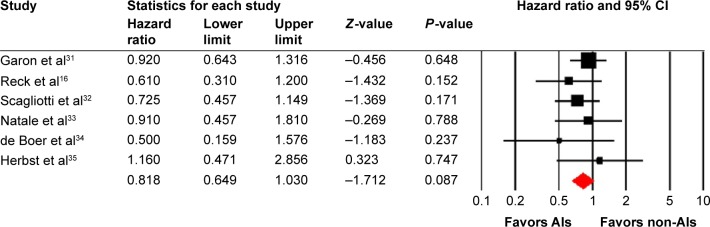
Fixed-effects model of hazard ratio (95% CI) of OS associated with therapies with or without AIs.
Abbreviations: CI, confidence interval; OS, overall survival; AIs, angiogenesis inhibitors.
Progression-free survival
Six trials reported PFS data. The pooled HR for PFS demonstrated that AIs rechallenge significantly improved PFS, with a HR of 0.72 (95% CI: 0.58–0.89, P=0.002, Figure 3), compared with non-AIs containing therapy. There was no significant heterogeneity between trials (I2=0%, P=0.49), and the pooled HR for PFS was performed by using fixed-effects model. We then did subgroup analysis according to treatment regimens and found that both AIs rechallenge plus chemotherapy (HR: 0.73, 95% CI: 0.57–0.95, P=0.018) or erlotinib (HR: 0.70, 95% CI: 0.48–1.00, P=0.05) regimens significantly improved PFS when compared with non-AIs-containing regimens.
Figure 3.
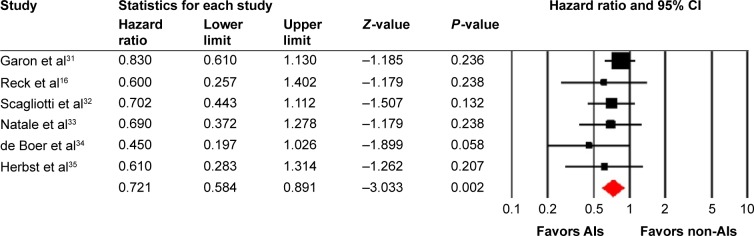
Fixed-effects model of hazard ratio (95% CI) of PFS associated with therapies with or without AIs.
Abbreviations: CI, confidence interval; AIs, angiogenesis inhibitors; PFS, progression-free survival.
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of literatures. The Begg’s funnel plots did not reveal any evidence of obvious asymmetry (P=0.70 for OS and P=0.06 for PFS, respectively). Then, Egger’s test was used to provide statistical evidence of funnel plot symmetry. The results still did not suggest any evidence of publication bias for OS (P=0.53). However, there was publication bias for PFS using Egger’s test (P=0.009). The difference in the results obtained from the two methods might be due to a greater statistical power of the regression methods.36
Discussion
The mechanism of resistance to chemotherapy is typically the result of changes in tumor cell biology and is often agent-specific.37 By contrast, the mechanism of resistance for bevacizumab is not well understood, which might result from the development of alternative angiogenesis pathways.38 Consequently, failure of chemotherapy does not necessarily mean failure of antiangiogenic treatment, implying that the disease may still be partially or significantly dependent on VEGF-mediated endothelial cell mitogenesis and survival. The angiogenic signal continues throughout the lifespan of the tumor. One hypothesis is that persistent VEGF suppression, along with secondary and tertiary cytotoxic regimens, may result in continued clinical benefit. This hypothesis is supported by the experience of patients with mCRC enrolled in the bevacizumab ML18147 study.26 In addition, the use of angiogenesis inhibitor aflibercept in patients with mCRC, previously treated with oxaliplatin-based regimen, also significantly improved OS when compared to chemotherapy alone (13.5 versus 12.06 months, P=0.0032).39 Based on these considerations, we conducted the current meta-analysis to evaluate whether AIs rechallenge in combination with standard treatment versus standard of care alone in second-line treatment could obtain clinical benefits in NSCLC patients who have progressed after first-line treatment with bevacizumab-containing chemotherapy.
To the best of our knowledge, this study is the first meta-analysis with a focus on investigating the value of AIs rechallenge in pretreated patients with advanced NSCLC, who have been previously exposed to bevacizumab. This study included six RCTs, incorporating 452 patients who previously received bevacizumab. The pooled results confirm that AIs rechallenge significantly improve PFS compared to non-AIs-containing regimens. Additionally, there is a tendency of improved OS in AIs rechallenge groups. Similar results are observed in subgroup analysis according to treatment regimens. Therefore, this study suggests that, in patients with advanced NSCLC who previously received bevacizumab, AIs rechallenge in combination with standard treatment could be a preferable treatment option over standard second-line therapy alone, although this recommendation cannot be conclusive because the overall comparisons are not based on randomization. Furthermore, the toxicity outcome is not assessed.
Our analysis has some obvious limitations. First, a large number of trials do not report the bevacizumab treatment status. This is largely because these trials are performed to investigate the efficacy of AIs in NSCLC, but not specifically for patients who previously received bevacizumab. Although all of these trials carry out the randomization process adequately, an imbalance of patient characteristics between the two treatment groups of the previously treated bevacizumab subgroup could exist. Therefore, these data should be interpreted cautiously, because the extracted data used for this analysis could not be considered randomized. Second, the published articles provide the crossover rate only for the entire group of enrolled patients without previous bevacizumab treatment subgroup data. Therefore, we could not examine the effect of treatment crossover on the outcomes. Third, the toxicity profile is another important factor for choosing treatment options. However, it is not possible to perform an analysis to deal with such a concern because reports of adverse events from each subgroup are not available. Fourth, although we included all RCTs investigating the efficacy of AIs rechallenge in NSCLC, these is no specific trial to investigate the efficacy of bevacizumab rechallenge in NSCLC patients, who were previously treated with bevacizumab. Thus, the efficacy of bevacizumab rechallenge in the setting remains to be determined. Finally, in the meta-analysis of published studies, publication bias is important because trials with positive results are more likely to be published, while trials with null results tend to be unpublished. Our research detects no publication bias for OS, but not for PFS. With these limitations in mind, a definite conclusion about the usefulness and benefit of AIs rechallenge in the second-line setting cannot be stated. A robust confirmation through a solid Phase III trial is needed before any recommendation can be performed.
Conclusion and future prospective
In conclusion, our study demonstrates that AIs rechallenge is an effective treatment option for patients who were previously exposed to bevacizumab. Further, well-conducted, randomized-controlled biomarker-enriched trials are fundamental to better evaluate this treatment strategy with attention to details such as the toxicities and other accompanying drugs.
Footnotes
Author contributions
QLG and LDZ designed the research; WL, HYZ, and NH conducted the research; LWG and QLG analyzed data; QLG wrote the draft; all authors read, reviewed, and approved the final paper. QLG had primary responsibility for final content. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the paper. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this paper. The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Group NM-AC Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 4.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18(12):2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 5.Carney DN. Lung cancer – time to move on from chemotherapy. N Engl J Med. 2002;346(2):126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 6.Qi WX, Wang Q, Jiang YL, et al. Overall survival benefits for combining targeted therapy as second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of published data. PLoS One. 2013;8(2):e55637. doi: 10.1371/journal.pone.0055637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallis AG, Syrigos KN. Targeting tumor neovasculature in non-small-cell lung cancer. Crit Rev Oncol Hematol. 2013;86(2):130–142. doi: 10.1016/j.critrevonc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Lammers PE, Horn L. Targeting angiogenesis in advanced non-small cell lung cancer. J Natl Compr Canc Netw. 2013;11(10):1235–1247. doi: 10.6004/jnccn.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reckamp KL. Antiangiogenic agents as second-line therapy for advanced non-small cell lung cancer. Cancer Lett. 2012;321(2):101–109. doi: 10.1016/j.canlet.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Qi WX, Tang LN, He AN, Shen Z, Yao Y. The role of vandetanib in the second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of four randomized controlled trials. Lung. 2011;189(6):437–443. doi: 10.1007/s00408-011-9332-1. [DOI] [PubMed] [Google Scholar]
- 12.Lauro S, Onesti CE, Righini R, Marchetti P. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res. 2014;34(4):1537–1545. [PubMed] [Google Scholar]
- 13.Soria JC, Mauguen A, Reck M, et al. Meta-analysis of bevacizumab in advanced Ncg Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(1):20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Belani CP, Yamamoto N, Bondarenko IM, et al. Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer. 2014;14:290. doi: 10.1186/1471-2407-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Kaiser R, Mellemgaard A, et al. for the L-LSG Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 17.Clarke JM, Hurwitz HI. Targeted inhibition of VEGF receptor 2: an update on ramucirumab. Expert Opin Biol Ther. 2013;13(8):1187–1196. doi: 10.1517/14712598.2013.810717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur J Cancer. 2014;50(4):706–712. doi: 10.1016/j.ejca.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Felip E, Besse B, et al. An open-label, multicenter, randomized, phase ii study of pazopanib in combination with pemetrexed in first-line treatment of patients with advanced-stage non-small-cell lung cancer. J Thorac Oncol. 2013;8(12):1529–1537. doi: 10.1097/JTO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Rivera F, Berry S, et al. First BEAT Investigators Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 21.Melnyk O, Zimmerman M, Kim KJ, Shuman M. Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol. 1999;161(3):960–963. [PubMed] [Google Scholar]
- 22.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153(4):1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105(8):R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vosseler S, Mirancea N, Bohlen P, Mueller MM, Fusenig NE. Angiogenesis inhibition by vascular endothelial growth factor receptor-2 blockade reduces stromal matrix metalloproteinase expression, normalizes stromal tissue, and reverts epithelial tumor phenotype in surface heterotransplants. Cancer Res. 2005;65(4):1294–1305. doi: 10.1158/0008-5472.CAN-03-3986. [DOI] [PubMed] [Google Scholar]
- 26.Bennouna J, Sastre J, Arnold D, et al. Investigators MLS Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 29.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbroucke JP. Bias in meta-analysis detected by a simple, graphical test. Experts’ views are still needed. BMJ. 1998;316(7129):469–470. author reply 470–461. [PMC free article] [PubMed] [Google Scholar]
- 31.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 32.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol. 2012;30(17):2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 33.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 34.de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 37.Mellor HR, Callaghan R. Resistance to chemotherapy in cancer: a complex and integrated cellular response. Pharmacology. 2008;81(4):275–300. doi: 10.1159/000115967. [DOI] [PubMed] [Google Scholar]
- 38.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]


