Abstract
Objective:
The present study was designed to investigate the putative antidepressant effects of crocin and crocetin, two major active ingredients of Crocus sativus L. (saffron) using mice in two different regimens of acute and sub-acute administration.
Material and Methods:
In acute treatment, antidepressant-like activities of crocin and crocetin (10, 20 and 40 mg/kg, i.p.) were evaluated using forced swim test (FST). In sub-acute study (21 times with 24-h intervals), antidepressant-like effects of oral administration of drugs were examined using FST and tail suspension test (TST). Locomotor activity and motor coordination were studied using open field and rotarod tests, respectively.
Results:
Acute treatment with crocin (40 mg/kg) and crocetin (20 and 40 mg/kg) produced antidepressant-like effect in FST without affecting the baseline locomotion in mice. Sub-acute oral administration of crocin significantly decreased immobility time only at the highest dose (100 mg/kg). Crocetin (12.5, 25 and 50 mg/kg) was able to decrease immobility time in FST and TST. Locomotor activity and coordination of mice were not affected by crocin or crocetin.
Conclusion:
Since higher doses of crocin was required to show antidepressant effects, more efficacy of crocetin may be concluded. This observation provides further support for metabolism of crocin to crocetin following oral administration.
Key Words: Crocus sativus L., Crocetin, Crocin, Forced swimming test (FST), Tail suspension test (TST)
Introduction
Depressive disorders (depression) are among the most prevalent, recurrent and life threatening psychiatric diseases with high morbidity and mortality (Paykel, 2006 ▶). There are many types of depression that range from mild to extremely severe. Due to the complexity of involved mechanisms in the pathogenesis of this disorder, current drugs (e.g., selective serotonin reuptake inhibitors (SSRIs) and tricyclic anti-depressants (TCA) have not been good enough in the treatment of depression and many patients suffer from different adverse effects (Buckley and McManus, 2002 ▶; Sarko, 2000 ▶). Accordingly, there is a need for development of new drugs with higher efficacies and less adverse effects.
Natural products displaying antidepressant effects are of great interest and may be important sources of new antidepressant drugs (Schulz, 2006 ▶). Saffron, the dried stigmas of Crocus sativus L. (Iridaceae family), a seasoning and coloring agent, has been the world's most expensive spice for decades (Abdullaev, 1993 ▶).
In folk medicine, saffron has been used in the treatment of various kinds of illnesses including cough, asthma, menstruation problems, insomnia, pain relief, colic, chronic uterine hemorrhage, cardiovascular disorders and tumors (Hosseinzadeh and Nassiri-Asl, 2013 ▶; Zargari, 1990 ▶). Main biologically active ingredients of stigmas of saffron including crocetin, a glycosylated carotenoid, and crocin, a carboxylic carotenoid have shown diverse pharmacological functions such as antioxidant and anti-inflammatory (Hosseinzadeh et al., 2009 ▶; Jnaneshwari et al., 2013 ▶; Kazi and Qian, 2009 ▶; Nam et al., 2010 ▶), anti-convulsant (Hosseinzadeh and Talebzadeh, 2005 ▶), anti-tumorigenic (Escribano et al., 1996 ▶; Gutheil et al., 2012 ▶) and hypolipidemic effects (Shirali et al., 2013 ▶). In addition, C. sativus could improve memory and learning ability of rats (Ghadrdoost et al., 2011 ▶; Hosseinzadeh et al., 2012 ▶).
Acute administration of C. sativus stigma extract and crocin have been previously shown to have anti-depressant effects in mice (Hosseinzadeh et al., 2003 ▶). Akhondzadeh et al (2004) ▶, reported that the antidepressant effect of stigmas of C. sativus was as similar as that of imipramine in the treatment of mild to moderate depression. In a recent study, it was suggeested that crocin could be considered as an adjuanctive therapy in the treatment of depressed patients (Talaei et al., 2014 ▶).
Up to now, anti-depressant effect of crocetin has not been evaluated in experimental studies. In this study, we compared the effects of crocin and crocetin following acute and sub-acute administration. In addition, as oral administration of drugs is the prefered rout for patients, the above-mentioned compounds were administered by gavage in the sub-acute study ( for 21 days). The behavioral models for depression evaluation including - forced swim (FST) and tail suspension (TST) tests were performed in mice. Locomotor activity and motor coordination were evaluated by open field and Rotarod tests, respectively. Fluoxetine, a selective serotonin reuptake inhibitor and desipiramine, a tricyclic antidepressant (TCA) that inhibits the reuptake of norepinephrine, were given to mice as positive control groups.
Material and Method
Animals
Male albino mice (weighing 20-30 g, 5 months old), were kept at the animal house of Mashhad University of Medical Sciences, in colony rooms with 12/12 h light/dark cycle at 22 ± 2°C. The animals had free access to food and water. The procedures in this study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by Mashhad University of Medical Sciences.
Materials
Crocin (purity 90%) was extracted from stigmas of saffron in our laboratory as described earlier (Hadizadeh et al., 2010 ▶) and administered in two ways of intraperitoneal (10, 20 and 40 mg/kg) and oral (25, 50 and 100 mg/kg, gavage). Crocetin was received as a gift from Dr Bathaie and administered in two ways of intraperitoneal (10, 20 and 40 mg/kg) and oral (12.5, 25 and 50 mg/kg). Fluoxetine and desipiramine were a gift from Dr Abidi Pharm. Co. (Tehran, Iran) and injected at the dose of 10 mg/kg. Dosage selection was according to the earlier studies (Guo and Lu, 2014 ▶; Higashino et al., 2014 ▶; Hosseinzadeh et al., 2012 ▶). Crocin, fluoxetine and desipiramine were dissolved in normal saline. Crocetin suspension was prepared using few drops of Tween 20 and diluted to desired concentration with saline. Solutions were freshly prepared before administration and were administered at the volume of 1 mL/kg. Treatment procedures were:
Acute treatment
In the acute-dose study, groups were as follows:
Group I (normal saline control group), Groups II-IV (crocin 10, 20 and 40 mg/kg), Groups V-VII (crocetin, 10, 20 and 40 mg/kg), Group VIII (fluoxetine hydrochloride, 10 mg/kg) and Group IX (desipiramine hydrochloride, 10 mg/kg). All drugs were given intraperitoneally, 24, 5 and 1 h before the test.
Sub-acute treatment
In the Sub-acute dose study, groups were as follows:
Group I (normal saline control group), Groups II-IV (crocin 25, 50 and 100 mg/kg), Groups V-VII (crocetin, 12.5, 25 and 50 mg/kg), Group VIII (fluoxetine hydrochloride, 10 mg/kg) and Group IX (desipramine hydrochloride, 10 mg/kg). In this paradigm, drugs were given orally (gavage) once daily for a total of 21 days.
Behavioral tests
In the acute study, animals were evaluated in forced swim test (FST). In the sub-acute study, two tests of TST and FST were used. To minimize tests interference, after 19 days of treatment, mice were tested in the tail suspension test which is a less stressful task and subsequently on day 21, FST which is a more stressful one, was performed (with a rest period of 48 hours) (Crawely, 2000 ▶). All tests took place between 1:30 – 4:30 PM.
Tail suspension test
Animals were suspended on a horizontal beam (height 33 cm) using adhesive tape wrapped around the tip of the tail. The time spent immobile was recorded during 6-min testing (Cryan et al., 2005 ▶).
Forced swim test
One hour after last treatment of acute study or forty-eight hours after tail-suspension testing, mice were gently placed in a Plexiglas cylindrical tank (15 cm in diameter), filled with 35 cm of room-temperature water to ensure that animals could not touch the bottom of the container with their hind paws or their tails. The animals were removed, dried and returned to their home cages after 15 min in water (pre-test). They were again placed in the cylinder 24 h later. The total duration of immobility was measured during a 5-min period. Mice were considered immobile if they made no attempts to escape and remained floating with their heads above the water. A decrease in the duration of immobility is an indicator of antidepressant-like effect (Porsolt et al., 1977 ▶).
Open field test ( OFT)
On the 21th day of chronic study or 45 min after the last treatment of acute study, locomotor activity was measured by the open field test for each animal during a five-minute period, immediately preceding the swim test. Each mouse was placed individually into the center of the open field apparatus. The open field was a wooden box (60 cm x 60 cm x 40 cm), painted white and divided into squares (20 cm x 20 cm) by parallel and perpendicular red strips. The number of squares crossed with all paws (crossing) was counted during a six-minute observation period. The apparatus was cleaned with ethanol 10% between tests in order to remove animal clues (Carola et al., 2002 ▶).
Rota-rod test
In order to determine the effect of compounds on motor coordination, the Rotarod test was performed on day 20 in sub-acute study. Rotarod apparatus consisted of a base platform and a rotating rod (3 cm in diameter). The rod (30 cm in length) was divided into five equal sections by six disks. Thus, up to five mice were tested simultaneously on the apparatus. The integrity of motor coordination was assessed on the basis of endurance time of the animals on the rotating rod. One day before the test, the animals were pre-trained twice on the rod (5 rpm) for at least 5 minutes. On the day of experiment, 30-40 minute after administration of all drugs, coordination was evaluated by increasing rotation cycle from 4 to 40 rpm over 5 minutes (cut-off time). Results were averaged over three trials (Fujimoto et al., 2004 ▶).
Statistics
Data were expressed as mean±SEM. Statistical analysis was performed using One-way ANOVA followed by post hoc test Tukey’s. The SPSS software was used to perform the statistical analysis. The difference was considered statistically significant when p < 0.05.
Results
Acute study
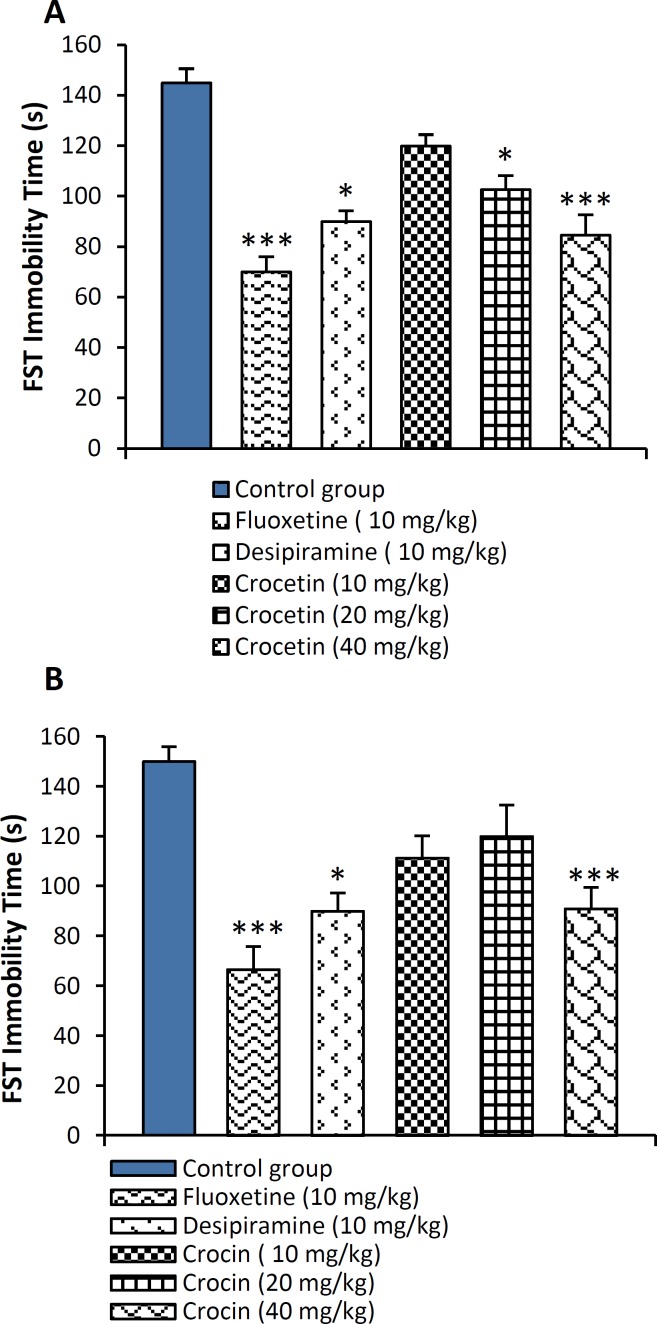
The effects of acute treatment with crocin and crocetin on the total duration of immobility time in mice are shown in Figure 1A and 1B. Crocetin at the dose of 10 mg/kg had no effect on immobility time in mice. Three-time administration of crocetin (20 and 40 mg/kg, p < 0.05 and p < 0.001 respectively) reduced immobility time of animals in forced swimming test (Figure 1). Administration of crocin (40 mg/kg) significantly reduced immobility (p<0.001) (Figure 1B). Duration of immobility was significantly attenuated with intraperitoneal administration of 10 mg/kg of reference drugs, fluoxetine (p<0.001) and desipiramine (p < 0.05).
Figure 1.
Effect of A: crocetin (10, 20 and 40 mg/kg, i.p.) and B: crocin (10, 20 and 40 mg/kg, i.p.) on immobility time as measured by forced swim test (FST) in mice in acute study. The results are presented as mean ± SEM of 6 animals/group and were evaluated by one-way ANOVA followed by the Tukey’s post-hoc test; * p < 0.05, *** p < 0.001 vs. control group. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg).
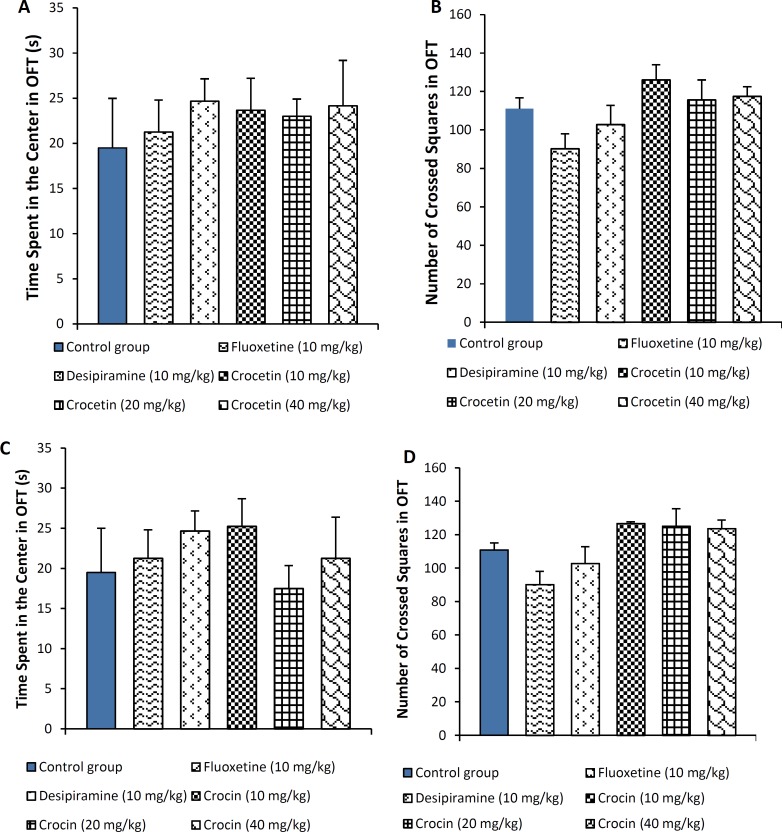
Locomotor activity of animals as measured by the time spent (Figure 2 A and C) as well as number of squares crossed (Figure 2 B and D) in the center of open field apparatus, revealed no significant change among groups.
Figure 2A and C:
Time spent in the center for animals treated with crocetin (10, 20 and 40 mg/kg, i.p.) and crocin (10, 20 and 40 mg/kg, i.p.) in the open field test (OFT). B and D: Number of crossed squares for animals treated with crocetin (10, 20 and 40 mg/kg, i.p.) and crocin (10, 20 and 40 mg/kg, i.p.). The results are presented as mean ± SEM of 6 animals/group and were evaluated by one-way ANOVA. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg
Sub-acute study
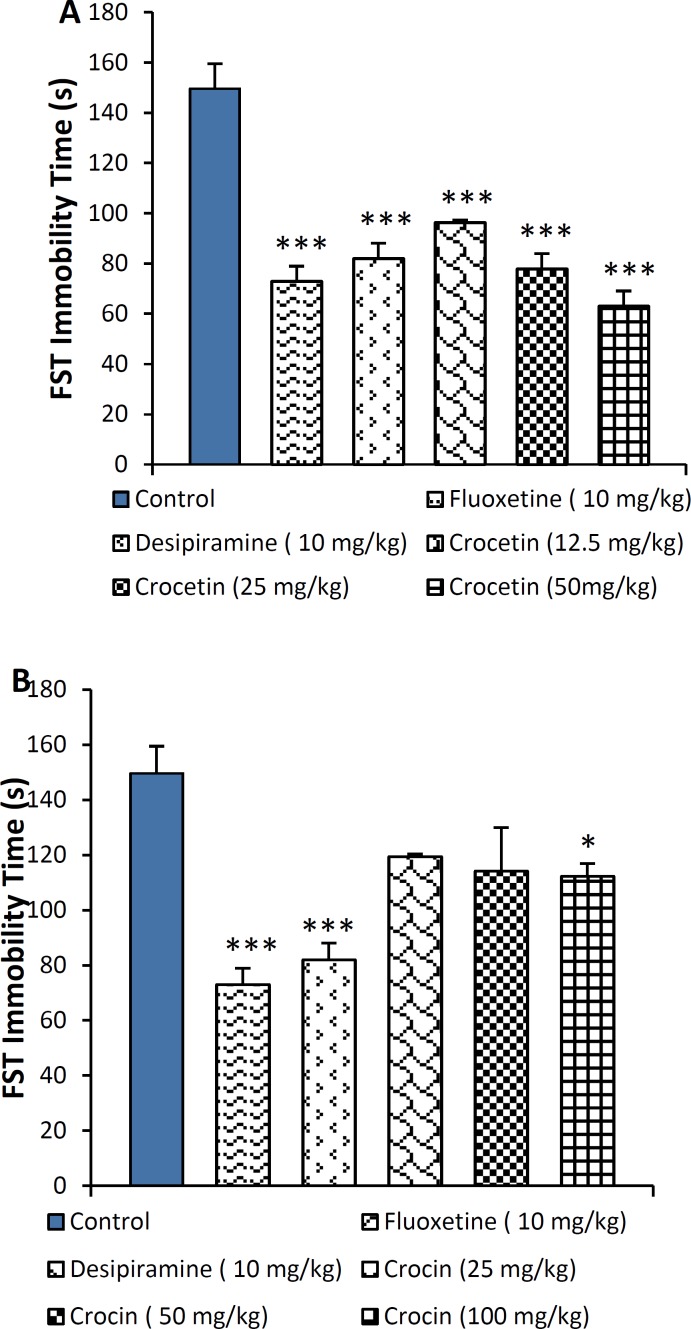
Twenty one-day treatment with crocetin (12.5, 25 and 50 mg/kg) significantly reduced immobility time in FST (p < 0.001) (Figure 3A), while oral administration (gavage) of crocin (25 and 50 mg/kg) had no effect on immobility time in mice. At the highest dose, 100 mg/kg, crocin attenuated immobility time in FST (p < 0.05) as shown in Figure 3B. Similar to the results of FST, crocetin at the doses of 12.5, 25 mg/kg (p < 0.05) as well as 50 mg/kg (p < 0.01) significantly reduced immobility time in TST (Figure 4A). In addition, the highest dose of crocin (100 mg/kg) significantly reduced the time of immobility in TST (p < 0.05) (Figure 4B).
Figure 3.
Effect of A: crocetin (12.5, 25 and 50 mg/kg, i.p.) and B: crocin (25, 50 and 100 mg/kg, i.p.) on immobility time as measured by forced swim test (FST) in mice in sub-acute study. The results are presented as mean±SEM of 6 animals/group and were evaluated by one-way ANOVA followed by Tukey’s post-hoc test; * p < 0.05, *** p < 0.001 vs. control group. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg
Figure 4.
Effect of A: crocetin (12.5, 25 and 50 mg/kg, i.p.) and B: crocin (25, 50 and 100 mg/kg, oral) on immobility time as measured by tail suspension test (TST) in mice in sub-acute study. The results are presented as mean ± SEM of 6 animals/group and were evaluated by one-way ANOVA followed by Tukey’s post-hoc test; * p < 0.05, ** p < 0.01,*** p < 0.001 vs. control group. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg
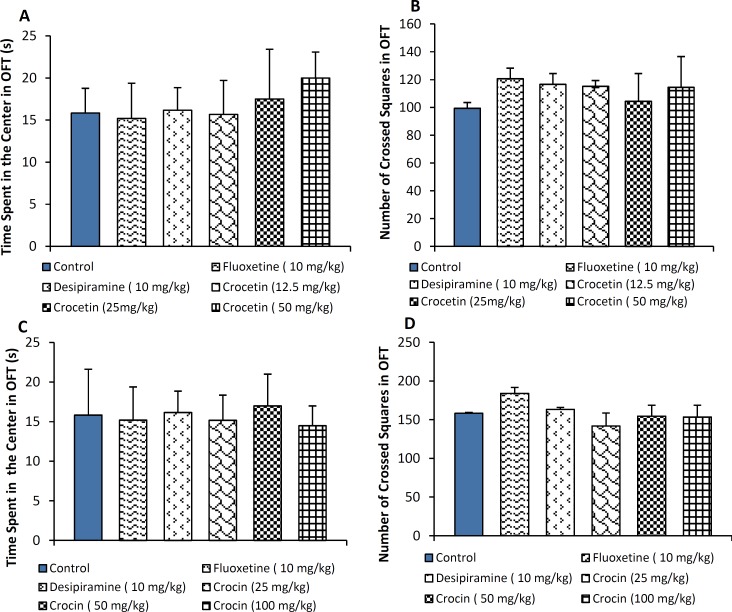
As depicted in Figure 5, the results show that locomotor activity of mice recieving crocin or crocetin for 21 days was not altered compared to normal saline in the open-field test.
Figure 5A and C.
Time (s) spent in the center for animals treated with crocetin (12.5, 25 and 50 mg/kg, i.p.) and crocin (25, 50 and 100 mg/kg, i.p.) in the open field test (OFT). B and D: Number of crossed squares for animals treated with crocetin (10, 20 and 40 mg/kg, i.p.) and crocin (10, 20 and 40 mg/kg, i.p.). The results are presented as mean ± SEM of 6 animals/group and were evaluated by one-way ANOVA. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg)
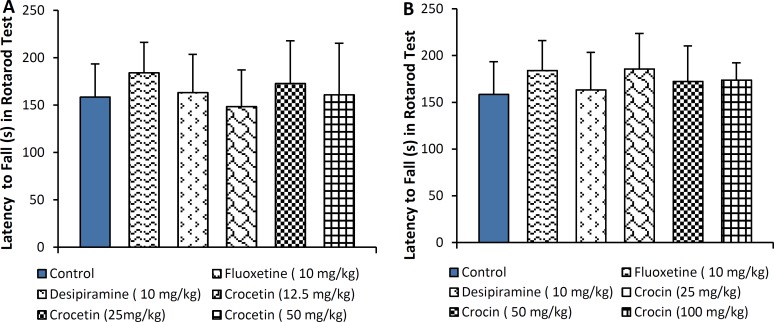
Moreover, 21-day treatment with either crocin or crocetin did not alter motor coordination (Figure 6).
Figure 6.
Effect of A: crocetin (12.5, 25 and 50 mg/kg, i.p.) and B: crocin (25, 50 and 100 mg/kg, i.p.) on the latency (seconds: s) of fall in rotarod test. The results are presented as mean ± SEM of 6 animals/group and were evaluated by one-way ANOVA. Positive control mice were treated with fluoxetine (10 mg/kg) and desipiramine (10 mg/kg).
Discussion
The prevalence of depressive disorders has been rising worldwide. Naturally occurring antidepressants are attractive alternatives for conventional drugs that are used in the treatment of depressive disorders and they have fewer adverse effects (Schulz, 2006 ▶; Szafrański, 2014 ▶).
Immobility time in FST resembles a state of despair and mental depression and was decreased by intraperitoneal crocetin in acute study. It has been reported that neurochemical pathways involved in mediating performance in FST and TST are not exactly the same (Bai et al., 2001 ▶). Employing a battery of tests allows us to explore the effects of the drug on different behavioral tasks to achieve complementary and/or converging information on the antidepressant activities of drugs (Bai et al., 2001 ▶; Cryan et al., 2003 ▶). Reduced immobility time was shown by intraperitoneal crocetin (25, 50 mg/kg) in FST. In addition, oral crocetin (12.5, 25 and 50 mg/kg) displayed antidepressant-like activity in FST and TST. Although intraperitoneal administration of crocin (40 mg/kg) exhibited antidepressant effect, it was not able to decrease immobility time after oral administration except for its highest dose (100 mg/kg). It should be noticed that drugs which induce hyper locomotion may result in a “false” positive effect in FST, and drugs decreasing locomotion may result in a “false” negative result (Borsini and Meli, 1988 ▶). We also studied locomotor activity and coordination performance in open field and Rotarod tests, respectively. Motor coordination of mice pretreated with crocin and crocetin were not impaired as compared with normal saline-pretreated groups of mice. Consequently, it could be suggested that antidepressant effects elicited by drugs are not due to any locomotor effect.
Our results are in line with previous studies demonstrating the antidepressant effects of saffron (Hosseinzadeh et al., 2003 ▶). In a preliminary double-blind randomized control trial study on mild to moderate depressive patients, saffron capsules (30 mg/day, three times daily) was found to be as effective as 100 mg/kg imipramine (Akhondzadeh et al., 2004 ▶). It is now generally accepted that disturbance in the levels of monoamine neurotransmitters (e.g. norepinephrine and serotonin) has an important role in the pathogenesis of depression (Brigitta, 2002 ▶). Selective serotonin reuptake inhibitors increase swimming, while drugs acting on the extracellular levels of norepinephrine or dopamine increase climbing behavior in FST. In our present study, there was no significant difference in the climbing behavior between desipiramine as a selective norepinephrine receptor inhibitor (SNRI) drug and crocetin or crocin. Swimming behavior was also the same in animals treated with fluoxetine, a SSRI drug and crocin or crocetin. Hence, we were not able to estimate the effect of crocin and crocetin on the levels of biogenic amines (serotonin and/or norepinephrine).
Associations between depressive disorders and increased oxidative stress, neuroinflammation, and decreased anti-oxidant defenses have been demonstrated (Black et aL., 2014 ▶; Hurley and Tizabi, 2013 ▶). Antioxidant effects of antidepressants have been shown in the treatment of major depressive disorder (Behr et al. 2012 ▶; Da Silva et al. 2014 ▶). Crocetin and crocin have therapeutic potential in amelioration of many neurodegenerative diseases as evidenced by their antioxidant and anti-inflammatory properties. Crocin and crocetin were able to reduce the production of various neurotoxic molecules such as nitric oxide (NO), reactive oxygen species (ROS), tumor necrosis factor alpha (TNF-α) and interleukin-1β from activated microglia (Nam et al., 2010 ▶). After oral administration, high levels of crocetin were detected in the plasma and brain tissues of rats. Moreover, it prevented ROS-induced oxidative stress in stroke-prone spontaneously hypertensive rats (Yoshino et al., 2011 ▶). Crocetin showed the most potent anti-inflammatory activity among crocin, gentiobiosyl glucosyl crocetin and mono-gentiobiosyl crocetin, which might be a reason for its greater anti-depressant like activity (Hong et al., 2013 ▶).
Different efficacies observed between crocin and crocetin may be due to different absorption characteristics of them. In a study by Asai et al. (2007) ▶, after oral administration, crocetin was rapidly absorbed into the blood circulation and was present in plasma as an intact free form and as glucuronide conjugates (crocetin-monoglucuronide and -diglucuronide). In addition, crocetin and its glucuronide conjugates, but not intact crocins (glycoside forms), were again found in the plasma of mice treated with crocin. These results indicate that orally-administered crocins are hydrolyzed to crocetin before or during intestinal absorption, and absorbed crocetin is partly metabolized to mono- and diglucuronide conjugates (Asai et al., 2007 ▶). Our results are further supported by Xi et al. (2007) ▶ study which reported that orally-administered crocin was not absorbed in plasma after single or repeated doses for 6 days.
Crocin was excreted largely through the intestinal tract following oral administration and low concentrations of crocetin were detected in plasma. In addition, plasma crocetin concentrations did not tend to accumulate with repeated oral doses of crocin. Crocin is not absorbed following gavage administration and is hydrolyzed to crocetin during the absorption (Asai et al., 2005 ▶). In a recent study by Kim et al. (2014) ▶ on the cytotoxicity effects of crocin and crocetin in five human cancer cell lines, approximately 5- to 18-fold higher effect was seen by crocetin. Different mechanisms due to structural characteristics were suggested for their different efficacies. As a result, another hypothesis might be different Mechanisms of action.
Current findings show the effect of crocin and crocetin pretreatment on depressive disorders. It seems that crocetin has more efficacy than crocin. However, further studies on the molecular mechanisms underlying the antidepressant-like effects of crocin and crocetin are required.
Acknowledgments
The authors wish to thank the Vice Chancellor of Research, Mashhad University of Medical Sciences for their financial support. The results described in this paper are part of a Pharm. D. thesis.
Conflict of interest
The authors declare no conflict of interest.
References
- Abdullaev F. Biological effects of saffron. Bio Factors. 1993;4:83–86. [PubMed] [Google Scholar]
- Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. Comparison of Crocus sativus L and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial. BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A, Nakano T, Takahashi M, Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem. 2005;53:7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of behavioral despair. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longevit. 2012;2012:609421. doi: 10.1155/2012/609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psycho Neuroendocrinology. 2014;51c:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Bors W, Saran M, Michel C. Radical intermediates involved in the bleaching of the carotenoid crocin Hydroxyl radicals, superoxide anions and hydrated electrons. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;41:493–501. doi: 10.1080/09553008214550571. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dial Clin Neurosci. 2002;4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NA, McManus PR. Fatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality data. BMJ. 2002;325:1332–1333. doi: 10.1136/bmj.325.7376.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behavior Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley-Liss. Inc; 2000. [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Da Silva AI, Monteiro Galindo LC, Nascimento L, Moura Freitas C, Lagranha CJ, Lopes de Souza S. Fluoxetine treatment of rat neonates significantly reduces oxidative stress in the hippocampus and in behavioral indicators of anxiety later in postnatal life. Can J Physiol Pharmacol. 2014;92:330–337. doi: 10.1139/cjpp-2013-0321. [DOI] [PubMed] [Google Scholar]
- Escribano J, Alonso GL, Coca-Prados M, Fernández JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Guo M, Lu XY. Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry. 2014;4:e486. doi: 10.1038/tp.2014.126. doi: 10.1038/tp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutheil WG, Reed G, Ray A, Anant S, Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Current Pharmaceut Biotech. 2012;13:173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadizadeh F, Mohajeri SA, Seifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak J Biol Sci. 2010;13:691–698. doi: 10.3923/pjbs.2010.691.698. [DOI] [PubMed] [Google Scholar]
- Higashino S, Sasaki Y, Giddings JC, Hyodo K, Sakata SF, Matsuda K, Horikawa Y, Yamamoto J. Crocetin, a carotenoid from Gardenia jasminoides Ellis, protects against hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Phytother Res. 2014;28:1315–1319. doi: 10.1002/ptr.5130. [DOI] [PubMed] [Google Scholar]
- Hong YJ, Yang KS. Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch Pharm Res. 2013;36:933–940. doi: 10.1007/s12272-013-0128-0. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L stigma extracts and their constituents, crocin and safranal, in mice. ISHS Acta Horticulturae. 2003:435–445. [Google Scholar]
- Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the canon of medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L stigma and its bioactive constituents, crocin and safranal. Pharmacogn Mag. 2009;5:419–422. [Google Scholar]
- Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Jnaneshwari S, Hemshekhar M, Santhosh MS, Sunitha K, Thushara R, Thirunavukkarasu C, Kemparaju K, Girish KS. Crocin, a dietary colorant mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J Pharm Pharm. 2013;65:604–614. doi: 10.1111/jphp.12016. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013;23:131–144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–78. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kazi HA, Qian Z. Crocetin reduces TNBS-induced experimental colitis in mice by downregulation of NFkB. Saudi J Gastroenterol. 2009;15:181–187. doi: 10.4103/1319-3767.54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee JM, Kim SC, Park CB, Lee PC. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem Cell Biolog. 2014;92:105–11. doi: 10.1139/bcb-2013-0091. [DOI] [PubMed] [Google Scholar]
- Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I, Hong JW, Lee EH. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Depression: major problem for public health. Epidemiol Psichiatr Soc. 2006;15:4–10. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Sarko J. Antidepressants, old and new A review of their adverse effects and toxicity in overdose. Emergenc Med Clin North Am. 2000;18:637–654. doi: 10.1016/s0733-8627(05)70151-6. [DOI] [PubMed] [Google Scholar]
- Schulz V. Safety of St John's Wort extract compared to synthetic antidepressants. Phytomedicine. 2006;13:199–204. doi: 10.1016/j.phymed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Shirali S, Zahra Bathaie S, Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27:1042–1047. doi: 10.1002/ptr.4836. [DOI] [PubMed] [Google Scholar]
- Szafrański T. Herbal remedies in depression-state of the art. Psychiatr Pol. 2014;48:59–73. [PubMed] [Google Scholar]
- Xi L, Qian Z, Du P, Fu J. Pharmacokinetic properties of crocin (crocetindigentiobiose ester) following oral administration in rats. Phytomedicine. 2007;14:633–636. doi: 10.1016/j.phymed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Talaei A, Hassanpour Moghadam M, SajadiTabassi SA, Mohajeri SA. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord. 2013;174:51–56. doi: 10.1016/j.jad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Yoshino F, Yoshida A, Umigai N, Kubo K, Lee MC. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J Clin Biochem Nutr. 2011;49:182. doi: 10.3164/jcbn.11-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargari A. Medicinal plants. Tehran, University Press; 1990. pp. 574–578. [Google Scholar]