Abstract
Purpose: To determine the efficacy and safety of topical bevacizumab treatment in patients with ocular surface squamous neoplasia (OSSN).
Methods: Six eyes of 6 patients with primary OSSN confirmed by impression cytology received topical 5 mg/mL bevacizumab 4 times daily for a period of 8 weeks. Patients were evaluated in 2-week intervals. Digital photography images were obtained at each visit and changes in the size of the lesions were analyzed by image analysis software.
Results: The mean age of the patients was 66±13 (±SD) years. Four tumors were nasal in origin and 2 tumors were temporal. The mean reduction observed in the lesion area was 43%±24.2% (range, 20%–71%) in the first month and 68%±29.7% (range, 42%–100%) in the second month when compared with the baseline area. Four patients required tumor excision at the end of the treatment period. Surgical treatment was not necessary in 2 patients due to complete disappearance of the tumor, which was confirmed by impression cytology. The visual acuity was stable in all patients and no systemic or visual side effects were observed during the study period.
Conclusions: Topical bevacizumab is effective as a neoadjuvant therapy combined with surgical excision for the treatment of OSSN. Topical bevacizumab may be used before surgery to decrease the size of the excision. Excision may be unnecessary in responsive patients.
Introduction
Ocular surface squamous neoplasia (OSSN) is a dysplasia of the conjunctival, limbal, or corneal epithelium.1 It is the most common nonpigmented tumor of the conjunctiva.2 OSSN is described as a relatively low-grade malignancy because invasive disease is uncommon and tends to be preceded by dysplasia and carcinoma in situ.1 However, a lack of appropriate and effective treatment for OSSN can result in malignant changes, followed by local invasion and, rarely, metastasis. OSSN includes conjunctival and/or corneal intraepithelial neoplasia, intraepithelial carcinoma of the conjunctiva, and conjunctival squamous cell carcinoma.3
Treatment modalities include combinations of excision, cryotherapy, and topical chemotherapy, including 5-flourouracil (5-FU), mitomycin C (MMC), and interferon alpha-2b (IFN-α2b).2 Surgical excision with adequate margins and adjunctive cryotherapy is a well-established treatment for OSSN, although this is an invasive option with numerous disadvantages. Chemotherapeutic agents can be used as primary or adjunctive therapy.4 A review of individual studies reveals similar efficacy between MMC, 5-FU, and IFN-α2b.5 The cumulative and delayed ocular surface toxicity of MMC, resulting in ocular irritation, conjunctival hyperemia, and punctate keratopathy, and the commonly observed side effects of 5-FU, such as lid toxicity, superficial keratitis, and corneal epithelial defects, make these agents less preferred in the treatment of OSSN.6,7 Interferon-α2b has a better toxicity profile with occasional flu-like symptoms and rare ocular surface irritation. However, the high cost remains a major disadvantage.8
Antivascular endothelial growth factors (anti-VEGFs) have been increasingly used for a range of cancers, including other epithelial cancers of the head and neck, with the goal of depriving the tumor of its vascular tissue. Bevacizumab (Avastin®; Genentech, Inc.), a full-length humanized monoclonal anti-VEGF antibody that was initially approved by the FDA for the treatment of patients with colorectal cancer, found off-label clinical implications in ophthalmology for the successful treatment of age-related choroidal neovascularization, proliferative and nonproliferative diabetic retinopathy, neovascular glaucoma, and anterior segment neovascular disorders.9–11
Subconjunctival anti-VEGF agents have been used in a few studies, for primary or recurrent OSSN, mostly with favorable clinical results and without any ocular or systemic side effects.12–15 However, the efficacy of topical anti-VEGF treatment for OSSN was not evaluated previously. The aim of this study was to determine the efficacy and safety of topical bevacizumab treatment in patients with OSSN.
Methods
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Baskent University. Patients were given detailed information regarding the off-label use of bevacizumab before treatment and informed consent was obtained from each patient.
Six eyes of 6 patients with primary OSSN confirmed by impression cytology received topical 5 mg/mL bevacizumab 4 times daily for a period of 8 weeks. Patients with a history of medical or surgical treatments were excluded from the study. Regional metastasis was ruled out in all patients by palpation of the cervical and periocular areas and by magnetic resonance imaging. Excisional biopsy of the remaining tumor and cryotherapy of the conjunctival borders were performed at the end of the topical treatment period, if necessary.
Bevacizumab eye drops were aseptically prepared in the hospital pharmacy by diluting the commercially available intravenous bevacizumab (Avastin) solution (25 mg/mL) with sterile saline to a concentration of 5 mg/mL, as previously described.16,17
Patients were evaluated in 2-week intervals. A complete ophthalmological examination was performed and digital photography images were obtained at baseline and at each follow-up visit. Changes in the size and extent of the lesions were analyzed by image analysis software (ImageJ 1.44p Wayne Rasband; National Institutes of Health) using the standardized digital slit-lamp photographs (32× magnification). The lesion area was calculated in terms of pixels. The percentage decrease in the area was calculated by the following formula: (baseline surface–post-treatment surface)/baseline surface ×100.
Data were tabulated and analyzed using customized database software (SPSS, Inc.). Correlations between data were analyzed using the Wilcoxon signed-rank test. A P value less than 0.05 was considered statistically significant.
Results
The mean age of the patients (4 men and 2 women) was 66±13 (±SD) years. Four tumors were nasal in origin and 2 tumors were temporal. The mean reduction observed in the lesion area was 43%±24.2% (range, 20%–71%; P=0.026) in the first month and 68%±29.7% (range, 42%–100%; P=0.026) in the second month when compared with the baseline area. Reduction in the tumor size was observed both in the conjunctival and corneal portions of the tumors (Fig. 1). Four patients (66.7%) required tumor excision at the end of the treatment period, and histological examination revealed conjunctival intraepithelial neoplasia in all of these patients. Surgical treatment was not necessary in 2 patients (33.3%) due to complete disappearance of the tumor, which was confirmed by impression cytology (Fig. 2). The visual acuity was stable in all patients and no systemic or ocular side effects were observed during the study period. Recurrence was not observed in any patient for a follow-up period of 6 months.
FIG. 1.
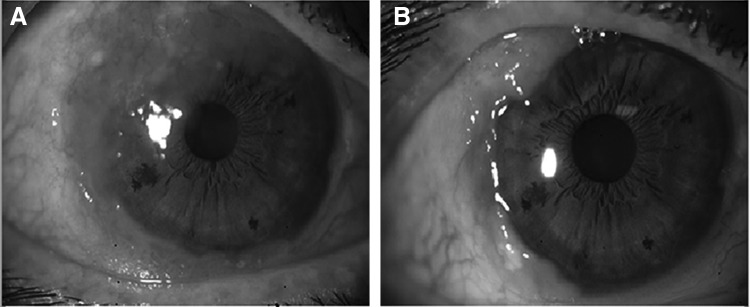
Patient 1 before (A) and after (B) topical bevacizumab treatment. Reduction in the tumor size was observed both in the conjunctival and corneal portions of the tumor.
FIG. 2.
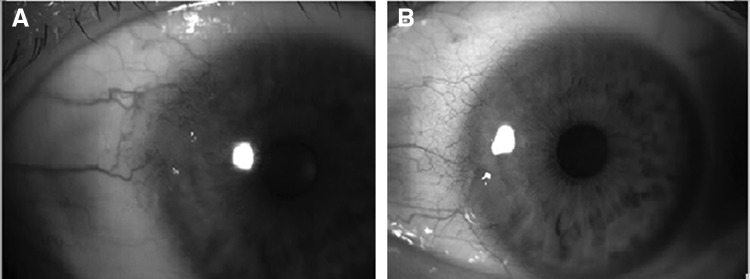
Patient 2 before (A) and after (B) topical bevacizumab treatment. Surgical treatment was not necessary due to complete disappearance of the tumor, which was confirmed by impression cytology.
Discussion
Bevacizumab, which is a full-length immunoglobulin, has a 12-nm-long Y-shaped configuration with a molecular weight of 149 kDa. Its three arms are ∼3.5 nm in diameter. Topical administration of full-length immunoglobulins is typically considered ineffective because such molecules are too large to penetrate the intact cornea. However, the clinical effectiveness of topical bevacizumab in the treatment of corneal NV, which has been shown before,16–19 indirectly implies that topical bevacizumab can go through the epithelial barrier in patients with ocular inflammation and corneal NV, which may affect the integrity of the epithelial barrier. In the light of these previous studies, we aimed to evaluate the efficacy of topical bevacizumab treatment in patients with OSSN.
Subconjunctival anti-VEGF agents have been used for the treatment of OSSN previously. Teng et al. evaluated the efficacy of subconjunctival ranibizumab for the treatment of refractory squamous cell carcinoma of the conjunctiva with corneal extension in 4 patients.13 Their results revealed that ranibizumab can decrease the size and vascularity of the tumors; however, complete disappearance of the tumor did not occur in any of the cases. Finger and Chin evaluated the safety, tolerability, and efficacy of subconjunctival ranibizumab in 5 patients with recurrent squamous cell carcinoma of the conjunctiva and cornea with 16–24 injections and reported a clinically defined complete response in three cases.14 They stated that anti-VEGF chemotherapy may offer a new strategy, complement excision and cryotherapy, or provide an alternative to radiation and/or exenteration.
The first case report evaluating the intralesional use of bevacizumab as an adjunctive therapy in a patient with OSSN stated that no significant change was observed in the tumor grossly and histologically.15 In another recent study,12 efficacy and safety of subconjunctival bevacizumab were evaluated in 10 eyes with primary OSSN. Two subconjunctival injections were given with a 2-week interval. The mean tumor area was reduced by 25%±5.65%, 2 weeks after the first injection, and by 42%±33%, 2 weeks after the second injection. Reduction in the tumor size occurred mainly in the conjunctival portion of the tumors and the corneal portions remained approximately stable. In 2 cases with exclusive conjunctival involvement, complete resolution of the tumor was observed and confirmed with impression cytology. In our study, the mean reduction observed in the lesion area was higher compared with the results of this study [43%±24.2% (range, 20%–71%; P=0.026) in the first month and 68%±29.7% (range, 42%–100%; P=0.026) in the second month]. Moreover, reduction in the tumor size was observed both in the conjunctival and corneal portions of the tumors.
To our knowledge, this is the first study evaluating topical bevacizumab for the treatment of OSSN. Treatment of OSSN using topical therapy has the advantage of treating the whole ocular surface, including subclinical atypical cells, and of avoiding injection-related complications, including pain and subconjunctival hemorrhage. Most recently, Kim et al. showed that topically administered bevacizumab was more long-standing than a subconjunctivally injected drug in inhibiting corneal neovascularization.18 Moreover, the antiangiogenic effect of topically administered and subconjunctivally injected bevacizumab did not differ at 1 week after treatment in 2 previous comparative studies.19,20 The superior or equal efficacy of topically applied bevacizumab with subconjunctival bevacizumab might be due to the altered permeability of the diseased cornea to bevacizumab or the the limbal penetration of antibodies, as occurs with other peptides or F(abs).21
Topical interferon α-2b (IFNα-2b) is currently the mainstay of medical treatment of OSSN because it is relatively nontoxic to the ocular surface when compared with MMC and 5-FU.5 It can be used in the form of subconjunctival injections or topical administration, as well as a combination of these.5,22–24 In a recent study evaluating the efficacy of topical IFNα-2b for the treatment of OSSN, the mean tumor surface area reduction was 58% in the first 3 months of therapy.24 This is similar to the 68% reduction in the second month with topical bevacizumab, observed in the current study. However, some side effects were observed relating to the use of topical IFNα-2b, including conjunctival hyperemia, follicular hypertrophy, giant papillary conjunctivitis, irritation, corneal epithelial defect, and flu-like symptoms, which resolved within 1 month of discontinuation of topical therapy.24 Although the duration of therapy was shorter in our study, we did not observe any ocular or systemic adverse effects with the use of topical bevacizumab for 2 months. The major disadvantage of topical IFNα-2b is the high cost when compared with other chemotherapeutic options for OSSN.
It is known that VEGF is an important neurotrophic growth factor. Hence, anti-VEGF therapies might have several side effects on the ocular surface. However, in an experimental study analyzing the safety profile of VEGF-A neutralization at the ocular surface, no significant side effects were observed on normal corneal epithelial wound healing, normal corneal integrity, or normal nerve fiber density.25 Topical bevacizumab is a generally well-tolerated treatment. No side effects were observed in the literature with 5 mg/mL topical bevacizumab treatment up to 12 months used for different corneal neovascular diseases.26 However, the possible dose-dependent inhibitory effect on corneal wound healing should be kept in mind and care should be taken in patients with epithelial defects and neurotrophic keratopathy. In our study, we did not observe any ocular or systemic adverse effects with a dose of 5 mg/mL administered 4 times daily for 8 weeks.
As a result, topical bevacizumab is effective as a neoadjuvant therapy combined with surgical excision for the treatment of OSSN. It may be used before surgery to decrease the size of the excision. Excision may be unnecessary in responsive patients. In the present study, the treatment was effective as a neoadjuvant therapy in 66.7% of the cases that required surgical excision at the end of the topical treatment period and it was effective as a primary and sole therapy in 33.3% of our cases. Topical treatment also seems superior to subconjunctival administration, especially for the treatment of the corneal portion of the tumor. This might be due to the larger treatment area achieved by topical administration. The combination of topical and subconjunctival bevacizumab should be considered for future research in a larger group of patients.
Since recurrence of OSSN can occur even years after treatment, follow-up is of high importance in continuing to evaluate the efficacy and side effects of various therapies. In our study, recurrence was not observed in any patient during a follow-up period of 6 months. In 4 of the 6 cases, this could be attributed to surgical resection in addition to or in lieu of treatment with topical bevacizumab as well. Combinations of various chemotherapeutic agents as well as surgical therapy may be needed in the long-term treatment of OSSN. Topical bevacizumab therapy was combined with surgical excision in 66.7% of our cases as well. Further studies with a longer follow-up duration, different bevacizumab concentrations, and a larger number of patients, including histology experiments to explain the mechanism of action of the treatment drug, are needed to determine the safety and efficacy of topical bevacizumab treatment for OSSN.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lee G.A., and Hirst L.W. Ocular surface squamous neoplasia. Surv. Ophthalmol. 39:429–450, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Shields C.L., and Shields J.A. Tumors of the conjunctiva and cornea. Surv. Ophthalmol. 49:3–24, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Erie J.C., Campbell R.J., and Liesegang T.J. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 93:176–183, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Sepulveda R., Pe'er J., Mİdena E., et al. . Topical chemotherapy for ocular surface squamous neoplasia: current status. Br. J. Ophthalmol. 94:532–535, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Nanji A.A., Sayyad F.E., and Karp C.L. Topical chemotherapy for ocular surface squamous neoplasia. Curr. Opin. Ophthalmol. 24:336–342, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Rudkin A.K., and Muecke J.S. Adjuvant 5-fluorouracil in the treatment of localised ocular surface squamous neoplasia. Br. J. Ophthalmol. 95:947–950, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Shields C.L., Demirci H., Marr B.P., et al. . Chemoreduction with topical mitomycin C before resection of extensive squamous cell carcinoma of the conjunctiva. Arch. Ophthalmol. 123:109–113, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mittal R., Rath S., and Vemuganti G.K. Ocular surface squamous neoplasia—review of etio-pathogenesis and an update on clinico-pathological diagnosis. Saudi. J. Ophthalmol. 27:177–186, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashshur Z.F., Bazarbachi A., Schakal A., et al. . Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am. J. Ophthalmol. 142:1–9, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Avery R.L. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 26:352–354, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Hosseini H., Nowroozzadeh M.H., Salouti R., et al. . Anti-VEGF therapy with bevacizumab for anterior segment eye disease. Cornea. 31:322–334, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Faramarzi A., and Feizi S. Subconjunctival bevacizumab injection for ocular surface squamous neoplasia. Cornea. 32:998–1001, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Teng C.C., Chin K.J., and Finger P.T. Subconjunctival ranibizumab for squamous cell carcinoma of the conjunctiva with corneal extension. Br. J. Ophthalmol. 93:837–838, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Finger P.T., and Chin K.J. Refractory squamous cell carcinoma of the conjunctiva treated with subconjunctival ranibizumab (Lucentis): a two-year study. Ophthal. Plast. Reconstr. Surg. 28:85–89, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Paul S., and Stone D.U. Intralesional bevacizumab use for invasive ocular surface squamous neoplasia. J. Ocul. Pharmacol. Ther. 28:647–649, 2012 [DOI] [PubMed] [Google Scholar]
- 16.DeStefano J.J., and Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch. Ophthalmol. 125:834–836, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Asena L., Akova Y.A., Cetinkaya A., et al. . The effect of topical bevacizumab as an adjunctive therapy for corneal neovascularization. Acta. Ophthalmol. 91:e246–e248, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Kim D., Kim E.S., et al. . Topically administered bevacizumab had longer standing anti-angiogenic effect than subconjunctivally injected bevacizumab in rat corneal neovacularization. Int. J. Ophthalmol. 6:588–591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed A., Berati H., Nalan A., et al. . Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin. Experiment. Ophthalmol. 37:730–736, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hashemian M.N., Moghimi S., Kiumehr S., et al. . Prevention and treatment of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with corticosteroid in experimental rats. Ophthalmic. Res. 42:90–95, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Brereton H.M., Taylor S.D., Farrall A., et al. . Influence of format on in vitro penetration of antibody fragments through porcine cornea. Br. J. Ophthalmol. 89:1205–1209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp C.L., Galor A., Chhabra S., Barnes S.D., and Alfonso E.C. Subconjunctival/perilesional recombinant interferon α2b for ocular surface squamous neoplasia: a 10-year review. Ophthalmology. 117:2241–2246, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Schechter B.A., Koreishi A.F., Karp C.L., and Feuer W. Long-term follow-up of conjunctival and corneal intraepithelial neoplasia treated with topical interferon alfa-2b. Ophthalmology. 115:1291–1296, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Shah S.U., Kaliki S., Kim H.J., et al. . Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American Joint Committee on Cancer classification. Arch. Ophthalmol. 130:159–164, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Bock F., Onderka J., Rummelt C., et al. . Safety profile of topical VEGF neutralization at the cornea. Invest. Ophthalmol. Vis. Sci. 50:2095–2102, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Koenig Y., Bock F., Horn F., et al. . Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes. Arch. Clin. Exp. Ophthalmol. 247:1375–1382, 2009 [DOI] [PubMed] [Google Scholar]


