Abstract
Goals
To test the hypothesis that the use of a low-residue breakfast (LRB) the day prior to colonoscopy was not inferior to consuming clear fluids alone (CFD) in patients undergoing outpatient colonoscopy with a polyethylene glycol (PEG) bowel preparation.
Background
Optimal colon cleansing is essential for complete visualisation of the mucosa during colonoscopy. Few studies have examined the effect of diet on the quality of bowel cleansing or tolerance in patients using a PEG bowel preparation for colonoscopy.
Methods
Randomised, single-blinded non-inferiority trial. Adult patients scheduled for outpatient colonoscopy with PEG solution were randomised to an LRB followed by clear fluids or CFD using either a traditional or split-dose PEG solution for bowel preparation. The primary outcome was colon cleansing based on the Ottawa Bowel Preparation Score (OBPS).
Results
On an intention-to-treat (ITT) basis, a total of 109 and 105 patients were included in the CFD and LRB arms, respectively, with 116 and 98 patients, respectively, for the per-protocol (PP) analysis. Although there was no difference in the mean total OBPS between the CFD or LRB arms in either the ITT or PP analysis, the threshold for non-inferiority was not met. Patient acceptance of the regimens was higher in the LRB arm than in the CFD arm in the ITT and PP analyses.
Conclusions
This study failed to show the non-inferiority of an LRB in patients receiving bowel preparation with a PEG-based solution. A CFD should be prescribed when using a PEG bowel preparation.
Trial registration number
This trial is registered at ClinicalTrials.gov (NCT01454388).
Keywords: COLONOSCOPY, DIET, CLINICAL DECISION MAKING
Summary box.
What do we already know?
-
▸
Optimal colon cleansing is essential for achieving a thorough colonoscopy.
-
▸
Many patients are instructed to adhere to a clear fluid diet prior to colonoscopy, despite a lack of evidence supporting this practice.
-
▸
Intolerability of the bowel preparation is a major reason for patients to forgo undergoing a colonoscopy.
What does this study demonstrate?
-
▸
A low-residue breakfast the day before colonoscopy was better tolerated compared with a clear fluid only diet.
-
▸
Non-inferiority was not met for the low-residue diet group for colon cleansing.
How is this clinically relevant?
-
▸
A low-residue diet before colonoscopy may be inferior to a clear fluid diet for colon cleansing, but is better tolerated.
-
▸
Clinicians can use this information to individualise their recommendations to patients based on their anticipated compliance with the bowel preparation instructions.
Introduction
Since the introduction of colonoscopy over four decades ago,1 optimising the bowel preparation for the examination has been an active field of research. To date, research has focused largely on which cleansing agents to use and the timing of their administration, with few studies evaluating the effect of the type of diet consumed during the preparation. While bowel cleanliness is foremost in most clinicians’ minds, patient tolerance of the bowel preparation is critical to ensure acceptance and compliance, as the need to undergo a bowel cleansing regimen is one of the main reasons why people avoid colonoscopy.2 In an effort to maximise the efficacy of the preparation, many physicians prescribe a clear fluid only diet3–9 the day prior to colonoscopy, despite the lack of evidence supporting this practice.
Few studies have looked at the specific effect of diet on bowel cleanliness or patient tolerance using a polyethylene glycol (PEG) bowel preparation. Investigators from Korea randomised patients to prepackaged low-residue meals or clear fluids and found no difference in the quality of bowel cleansing but did see an improvement in patient tolerance in those randomised to the low-residue meal.10 These results may be difficult to translate to real life, as the stringent use of prepackaged meals may not be feasible on a large scale. Additionally, patients in the study had their colonoscopies in the afternoon and ingested a full 4 L of PEG on the morning of the procedure. Thus, the results may not be applicable to the bowel cleansing schedules that are commonly used in North America (full doses the evening prior to colonoscopy or a split-dose11 12). Several other studies have attempted to evaluate the effect of diet on bowel preparation;13 14 however, the type of bowel preparation prescribed and timing of the procedures varied across randomisation groups. Further, the split-dose bowel preparation, which has been more commonly recommended for colonoscopies,11 12 has not been adequately evaluated in combination with alternations in dietary regimens.
Our group and others have previously studied the effect of a low-residue diet in patients undergoing outpatient colonoscopy with ingestion of low-volume bowel preparations.13 15 The consumption of the low-residue diet has been consistently better tolerated than a clear fluid diet (CFD) without affecting the quality of the bowel preparation. It is unclear, however, whether these results are generalisable to patients ingesting a large volume PEG preparation. This is of particular importance, as a PEG-based bowel preparation is often used in the elderly or those with medical conditions that make large volume shifts inadvisable.
The aim of this non-inferiority trial was to test the hypothesis that the use of a low-residue breakfast (LRB) the day prior to colonoscopy was not inferior to consuming a CFD alone in patients undergoing outpatient colonoscopy with a PEG bowel preparation. The secondary outcome was the effect of the diet on patient tolerability of the bowel preparation.
Methods
Study design
This is a prospective randomised, endoscopist-blinded, non-inferiority trial that compared two dietary regimens: LRB followed by clear fluids the day prior to colonoscopy versus a CFD over the entire day prior to colonoscopy. All colonoscopies were performed at Hotel Dieu Hospital in Kingston, Ontario, which is a teaching hospital affiliated with Queen's University. We are a tertiary referral centre performing an average of 3500 colonoscopies per year. This trial is registered at ClinicalTrials.gov (NCT01454388) and was approved by the Queen's University research ethics board.
Participants
Patients were recruited from outpatient gastrointestinal (GI) clinics, which represent a subset of all patients with colonoscopy seen in our tertiary care centre. Patients ≥18 years of age scheduled for outpatient colonoscopy for whom the gastroenterologist ordering the colonoscopy had chosen to prescribe a polyethylene glycol electrolyte lavage solution (PEG-ELS)-based bowel cleansing preparation (as opposed to a low-volume solution) were eligible for inclusion and recruited by a research assistant. Exclusion criteria included pregnancy, prior bowel resection, recent (within 6 months) acute coronary syndrome, significant constipation (<3 spontaneous bowel movements per week) and active inflammatory bowel disease.
Intervention
All study participants received clear verbal and written instructions on bowel preparation, specific to their randomisation arm, from the research assistant at the time of their initial clinic appointment after randomisation had been completed. On the day prior to colonoscopy, those in the LRB were instructed to ingest a low-residue breakfast no later than 10:00 followed by a clear fluid diet thereafter. An information sheet containing acceptable low-residue options was given to the patients in the LRB group (Appendix A). No specific prepackaged dietary products were used or recommended. The CFD group received the endoscopy unit's usual instructions of ingesting clear fluids only over the entire day prior to colonoscopy. Patients in both groups were allowed to continue ingesting clear fluids until 2 h prior to their scheduled colonoscopy appointment.
Bowel cleansing regimen
All patients were instructed to ingest 4 L in total of the PEG-ELS solution. The PEG solution was not provided centrally, and therefore participants were able to select a flavour of their own choice. Patients whose colonoscopy was scheduled before 11:00 were asked to drink the entire solution over 1–3 h, starting at 19:00 the evening prior to the procedure (traditional dosing). Those scheduled on or after 11:00 were asked to drink 2 L over 1 h the evening before the procedure, starting at 19:00, and the remaining 2 L over 1 h, starting 4 h before the scheduled procedure (split-dosing).
Reasons for prescribing both traditional and split-dose regimens were twofold. The first was related to the practice of the endoscopy unit at the time the study was submitted for ethics review (September 2009) where data publication was evolving regarding the superior efficacy of a split-dose regimen. At this time, we had begun to use a split-dose schedule for all cases booked after 11:00. The second related to patient preference, as some individuals living at a far distance from the endoscopy unit would not accept a split-dose schedule.
Study setting
Patients were recruited from outpatient gastroenterology clinics at Hotel Dieu Hospital, Kingston, Ontario and all colonoscopies were performed at the endoscopy unit of the same centre. Colonoscopies were performed by one of eight Canadian board certified Gastroenterologists participating in the study between 08:00 and 16:00 Monday to Friday. All patients were sedated using interval doses of intravenous fentanyl and midazolam to achieve minimal to moderate conscious sedation.
We are a teaching facility, with four GI fellows annually, who were involved in performing a subset of the study procedures under the constant and direct supervision of the attending physician. The attending physician was responsible for the assessment and documentation of the OBPS and Aronchick scoring assessments.
Outcomes
The primary outcome was bowel cleansing, as measured by the Ottawa Bowel Preparation Scale (OBPS), which is a validated and reliable scale.16 Secondary outcomes included patient hunger prior to the time of colonoscopy, as measured by a validated visual analogue scale (VAS),17 patient acceptance of the preparation regimen, assessed by a questionnaire and bowel cleansing, as measured using the Aronchick bowel cleansing scale.18
The efficacy of colon cleansing was assessed in the endoscopy suite on completion of the colonoscopy by the gastroenterologist performing the procedure. Nine gastroenterologists participated in the study and were blinded to the randomisation group. At the end of the procedure, each colonoscopist completed preprinted OBSP and Aronchick scoring sheets for evaluation of colon cleansing. The endoscopists had undergone training in the use of the OBPS previously.19 The scale ranges from 0 and 14, with 14 being the poorest preparation. The endoscopist assessed the OBPS score before cleaning the colon with water through the colonoscope. The Aronchick scale was used as a secondary outcome due to concern of the validity of the OBPS score in the setting of foot pedal washers.
On the day of the procedure, before entering the endoscopy suite, patients were asked to complete a questionnaire to assess their tolerance of the assigned dietary regimen. The questionnaire included a validated 50 mm VAS to gauge the patient's intensity of hunger at that point in time.17 A 5-point ordinal scale was also used to inquire about the ease/difficulty in following the assigned dietary regimen.
Sample size
Using a non-inferiority design with a margin of 1 in the OBPS, and assuming an SD of 2.6 (based on prior research at our centre), 107 patients would be required in each arm to achieve 80% power and a two-sided α of 0.05. To account for an anticipated 15% dropout rate, 123 patients were recruited to each group.
Randomisation and blinding
Randomisation was achieved through permuted random size blocks of four and eight, prepared by an independent research office. After consent was obtained, the research assistant would open the opaque envelope and reveal the diet group to which the subject was randomised. All involved healthcare providers (physicians and nursing staff) were blinded to the study participants’ group assignment and patients were reminded not to reveal their randomisation group to the staff on entry into the endoscopy unit. If an endoscopist became unblinded to the study assignment, a second endoscopist would be asked to come into the endoscopy suite, view the colonoscopy and grade the cleanliness based on the OBPS and Aronchick scores.
Statistical methods
Baseline demographics were compared using the Student t test for normally distributed data and χ2 test for categorical data. The primary outcome of total OBPS and secondary outcome of the hunger rating were compared between arms using the Student t test after confirming a normal distribution. The secondary outcome of the Aronchick Score was compared using the Wilcoxon Rank Sum test and the patient acceptance was analysed using χ2 analysis. To limit bias that could be introduced with the analysis of only complete case data, multiple imputation techniques20 were used to generate values for the total OBPS scores on the individuals with missing data. Variables included to perform the imputation were sex, age, randomisation arm, traditional versus split-dosing and history of a previous colonoscopy. We performed a sensitivity analysis by analysing the data with and without the imputation of OBPS scores. The results did not differ, and therefore the results using the imputed OBPS scores are reported. Results were analysed in intention-to-treat (ITT) and per-protocol (PP) analyses. All analyses were performed using STATA V.12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, Texas, USA: StataCorp LP).
Results
Study recruitment and participant flow are shown in figure 1. From January 2010 to March 2012, 124 patients were randomised to each group, with 15 patients (CFD group) and 19 patients (LRB group) withdrawing prior to the colonoscopy. Non-participation was largely due to withdrawal of consent, with one subject in each arm having an undisclosed hemicolectomy (exclusion criteria). This resulted in 109 and 105 patients in the CFD and LRB groups, respectively, for the ITT analysis. Full OBPS bowel cleansing data were unavailable for 12% (n=13) in the CFD group (technically incomplete (n=9), incomplete data form (n=2) and poor preparation (n=2) and for 10% (n=11) of patients in the LRB group (technically incomplete procedures (n=7), incomplete data forms (n=3) or preparation too poor to complete the procedure (n=1). As described in the methods section, all of these individuals were included in the ITT and PP analyses with the generation of OBPS scores using multiple imputation. A number of patients in the LRB arm (n=7) by default remained on clear fluids only over the day prior to colonoscopy because they normally did not consume breakfast and therefore did not change their habits before colonoscopy preparation. This resulted in 116 and 98 patients in the CFD and LRB groups, respectively, for the PP analysis.
Figure 1.
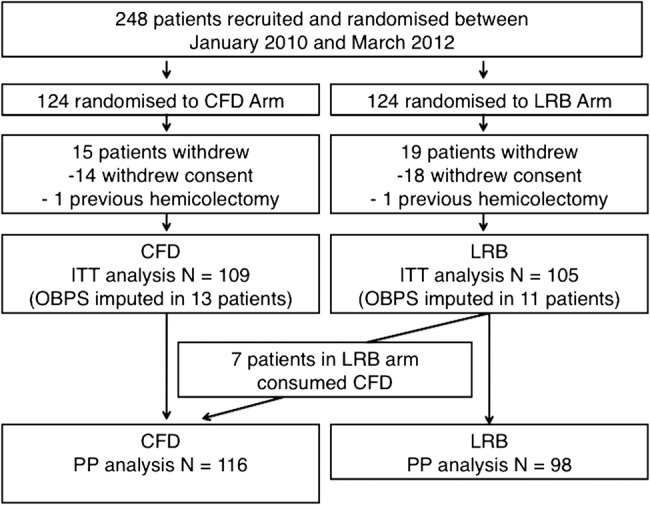
Flow of patients in the study.
Baseline data are shown in table 1. The median age was 65 years (IQR 54–74) in the CF arm and 62 years (IQR 52–73) in the LRB arm. There were no differences seen between groups with respect to age, sex or indication for colonoscopy. There was also no difference between the CFD and LRB arms in terms of caecal intubation rates (91.7% vs 93.3%, respectively, p=0.505), polyp detection rates (46.8% vs 49.5% p=0.689) or adenoma detection rates (36.7% vs 34.3% p=0.452; table 1). Finally, polyp detection rates did not differ between endoscopists (p=0.428).
Table 1.
Baseline patient demographic information
| Clear fluid diet (n=109) | Low-residue breakfast (n=105) | p Value | |
|---|---|---|---|
| Male sex, % (n) | 45 (49) | 41 (43) | 0.554 |
| Age, median (IQR) | 65 (54–74) | 62 (52–73) | 0.366 |
| Indication for colonoscopy, % (n) | |||
| GI symptoms | 66.9 (71) | 60.0 (63) | 0.777 |
| CRC screening | 19.8 (21) | 18.1 (19) | |
| CRC surveillance | 16.0 (17) | 21.9 (23) | |
| Split-dose preparation, % (n) | 55.1 (60) | 58.6 (61) | 0.595 |
| Completed bowel prep, % (n) | 97.3 (106) | 97.2 (102) | 0.548 |
| Caecal intubation rate, % (n) | 91.7 (100) | 93.3 (98) | 0.505 |
| Polyp detection rate, % (n) | 46.8 (51) | 49.5 (52) | 0.689 |
| Polyp number, median (IQR) | 2 (1–3) | 2 (1–3) | 0.475 |
| Polyp type, % (n) | |||
| Adenoma | 41.1 (21) | 31.4 (16) | 0.518 |
| Advanced adenoma | 37.3 (19) | 39.2 (20) | |
| Non-adenoma | 21.6 (11) | 29.4 (15) | |
| Adenoma detection rate, % (n) | 36.7 (40) | 34.3 (36) | 0.452 |
| Constipating medications, % (n) | 30.3 (33) | 36.2 (38) | 0.358 |
| Previous colonoscopy, % (n) | 50.5 (55) | 46.2 (49) | 0.531 |
| Previous bowel prep, % (n) | |||
| Colyte | 23.2 (25) | 24 (25) | 0.532 |
| PicoSalax | 11.1 (12) | 5.8 (6) | |
| NaPhos | 2.8 (3) | 4.8 (5) | |
| Citromag | 0 | 1 (1) | |
| Unknown | 13.9 (15) | 10.6 (11) | |
| NA | 49.1 (54) | 53.9 (56) | |
CRC, colorectal cancer; GI, gastrointestinal; Hx, history of; NA: not applicable; NaPhos, sodium phosphate.
Bowel preparation
On an ITT basis, there was no difference in total OBPS between the LRB and CFD arms (mean total OBPS 4.97 vs 4.97 respectively, p=0.99, table 2). Additionally, no significant difference in total OBPS was seen when analysed in a PP analysis (mean total OBPS in the LRB arm, 5.32 vs 4.68 in the CFD arm, p=0.12). Although the threshold for non-inferiority was met on the ITT analysis as the 95% CI for the difference did not cross the non-inferiority margin of −1 (95% CI for the difference −0.83 to 0.83), this was not true for the PP analysis (95% CI for the difference −1.47 to 0.18), and therefore non-inferiority of the LRB diet was not met. As a secondary outcome, we compared the quality of bowel preparation between the two arms using the Aronchick Score. There was again no difference seen in the quality of the preparation between the groups in either the ITT (p=0.95, figure 2) or PP analysis (p=0.64).
Table 2.
Ottawa Bowel Preparation Scores
| Clear fluid diet | Low-residue breakfast | p Value | |
|---|---|---|---|
| Intention-to-treat analysis | n=109 | n=105 | |
| Total OBPS, mean±SD | 4.97±3.28 | 4.97±2.83 | |
| (95% CI) | (4.35 to 5.59) | 4.42 to 5.52) | 0.999 |
| Total OBPS, mean difference | −0.01 | ||
| (95% CI) | (−0.83 to 0.83) | ||
| Right OBPS, mean±SD | 1.72±1.18 | 1.80±1.05 | 0.597 |
| (95% CI) | (1.48 to 1.95) | (1.59 to 2.02) | |
| Fluid score, mean±SD | 0.47±0.66 | 0.59±0.67 | |
| (95% CI) | (0.34 to 0.61) | (0.46 to 0.72) | 0.220 |
| Per-protocol analysis | n=116 | n=98 | |
| Total OBPS, mean±SD | 4.68±3.14 | 5.32±2.94 | 0.124 |
| (95% CI) | (4.10 to 5.25) | (4.73 to 5.92) | |
| Total OBPS, mean difference | −0.65 | ||
| (95% CI) | (−1.47 to 0.18) | ||
| Right OBPS, mean±SD | 1.62±1.16 | 1.93±1.04 | 0.050 |
| (95% CI) | (1.39 to 1.84) | (1.71 to 2.15) | |
| Fluid Score, mean±SD | 0.45±0.64 | 0.63±0.69 | 0.069 |
| (95% CI) | (0.33 to 0.58) | (0.48 to 0.77) | |
| Stratified by bowel preparation timing | |||
| Traditional dosing (n=93) | |||
| Total OBPS, mean±SD | 5.77±3.32 | 5.41±2.83 | 0.585 |
| (95% CI) | (4.81 to 6.72) | (4.54 to 6.28) | |
| Split-dosing (n=121) | |||
| Total OBPS, mean±SD | 4.32±3.13 | 4.56±2.72 | 0.651 |
| (95% CI) | (3.51 to 5.12) | (3.86 to 5.26) | |
Figure 2.
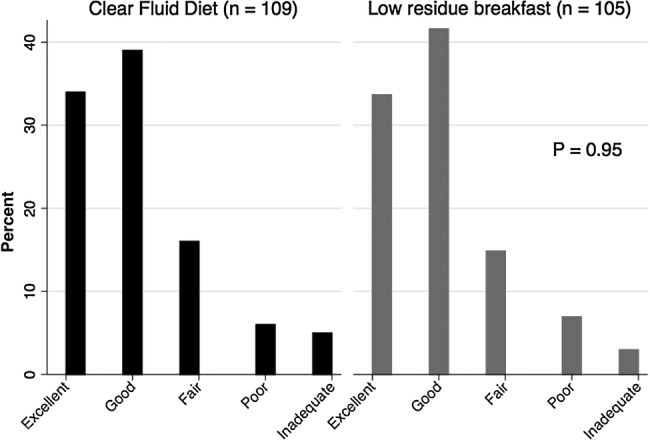
Intention-to-treat Aronchick bowel preparation scale results.
In an a priori subgroup analysis, split-dosing resulted in better OBPS scores overall (mean total OBPS in the split group was lower than in traditional dosing 4.44 vs 5.60, respectively, p=0.006). When looking at each bowel preparation timing group separately, the type of diet consumed had no affect on the total OBPS (table 2).
Patient tolerance
Patients’ hunger rating just prior to colonoscopy using the VAS was not significantly different between groups in the ITT (LRB mean 20.8±15.4 mm vs CFD 20.4±15.3 mm, p=0.86) or PP analysis (LRB mean 21.4±15.3 mm vs CFD mean 19.8±15.3 mm, p=0.45). However, patient acceptance of the regimens, as measured through a questionnaire, showed that a higher proportion of patients in the LRB arm rated the preparation as very easy or easy compared to those assigned to the CFD arm in the ITT (67.3% vs 51.4% respectively, p=0.04, figure 3) and PP analysis (68.8% vs 51.3% respectively, p=0.03).
Figure 3.
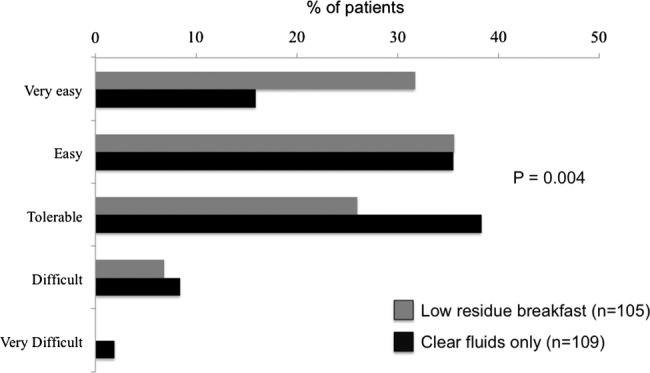
Tolerance of the bowel preparation based on randomisation to low-residue breakfast or clear fluid diet CFD.
Discussion
In this work, we examined the effect of an LRB on both the quality of bowel cleansing and patient tolerance of a PEG-based regimen during outpatient colonoscopy. Although we have previously shown that an LRB did not affect colon cleansing when using a low-volume bowel preparation,13 we were unable to claim non-inferiority in patients who were prescribed a PEG-based solution. The likely explanation for the difference in results relates to differences in the patient populations recruited into the different studies. In both cases, the decision on which type of bowel preparation to prescribe was left to the discretion of the attending gastroenterologist. In the current era, PEG-based preparations are generally reserved for elderly patients or those individuals with comorbid illness in whom the risk of volume shifts that may occur with a low-volume preparation is high. These individuals have also been shown to be at a higher risk of poor bowel preparation, independent of the type of diet consumed,21 and therefore the addition of diet liberalisation with an LRB may not be advisable. When comparing this study cohort with that of our previous study, those prescribed PEG versus a low-volume preparation were older (median age 65 years vs 57 years, respectively) and were more likely to be receiving a colonoscopy for non-colorectal cancer screening purposes (70% vs 20%, respectively).13 This suggests that the type of patients in our practice who are prescribed a PEG bowel preparation differ from those receiving a low-volume preparation and provides further insight as to why this current study failed to show non-superiority with an LRB. While non-inferiority was not met, it is worth noting that there was no difference between groups in polyp detection and adenoma detection rates.
As one of our secondary outcomes, we were able to show that patients better tolerated the LRB than the CFD. Therefore, even though the threshold for non-inferiority was not met in the PP analysis, and thus non-inferiority at a statistical level cannot be claimed, our results still provide important information to clinicians selecting bowel cleansing regimens if tolerance is anticipated to be a barrier to colonoscopy acceptance. We opted for an a priori margin of 1 point on the OBPS to claim non-inferiority. This stringent margin was chosen, as we did not want to sacrifice any degree of bowel cleansing with the use of the LRB. However, our CI around the per-protocol point estimate was −1.47, suggesting that the LRB could be, at most, worse by ∼1.5 points on the OBPS.
Splitting the doses of bowel purgatives to include one dose the morning of the procedure has been shown to increase the efficacy of cleansing,4 22–26 and several investigators have attempted to look at the effect on bowel preparation of a split-dose preparation along with varying dietary regimens in randomised trials. A recent trial comparing the use of a traditional (evening prior) PEG bowel preparation plus a clear fluid diet versus a split-dose regimen without dietary restrictions showed that the split-dose arm without dietary restriction was superior for colon cleansing.14 However, given that the type of diet was not constant in both arms, the specific effect of a non-fluid diet on bowel preparation or tolerability using a traditional or split-dose PEG preparation was not addressed. A second study looking at varying diets and oral sulfate solution preparation did not find any difference in the quality of bowel preparation in patients who had a low-residue diet over the entire day prior to colonoscopy compared with those taking clear fluids for the full day.13 However, all the patients took the preparation according to a split-dose schedule, raising the possibility that this timing may obviate the need for dietary restrictions. Our subgroup analysis comparing those individuals who received a split-dose preparation versus the traditional dosing is consistent with previous literature suggesting that split-dose timing results in a superior bowel cleanse when compared with traditional dosing. However, unlike these previous studies, we found that the LRB did not appear to affect the bowel preparation whether the timing was traditional or a split-dose. These results must be interpreted in the context of a subgroup analysis, where we did not have adequate statistical power to claim non-inferiority.
A surprising result was that while a higher proportion of patients in the LRB group ranked the preparation as easy or very easy to tolerate compared with patients in the CFD only group, there was no difference in the overall hunger scores. One possible explanation for this may involve the timing of the administration of the visual analogue scale for hunger. Both the overall tolerance questionnaire and the VAS for hunger were given to patients as they arrived in the endoscopy suite on the day of their procedure. As the question of overall tolerance encompassed the whole regimen and the VAS question addressed hunger at a specific moment of time, any improvement in the hunger rating due to the LRB may have been most evident if the VAS had have been completed on the day prior to colonoscopy. Future studies looking to address the question if hunger is related to diet may be best asked before the patients present for the procedure.
There are several limitations to our study that readers will need to consider. First, we had missing data for the total OBPS in 10% of the study population, mostly due to incomplete data forms. Analysis of complete case data only can result in bias. To overcome this, we used multiple imputation to derive OBPS scores for those with missing data and used these values in our ITT and PP analyses. This statistical technique has been suggested to be the best way to handle missing data, as it has been shown to produce results where statistically valid inferences that properly reflect the uncertainty due to missing data can be made.20 Second, our population did not include those individuals with a history of a previous poor bowel preparation and was not enriched with individuals with other predictors of difficult preparations such as chronic narcotic use and diabetes. These patients were excluded, as historically we find they have difficulty achieving an adequate preparation and risk skewing the results. Therefore, whether these individuals would have the same results using an LRB cannot be determined. Third, it is problematic that split-dosing was not utilised for all participants. At the time of this study, this practice was just emerging as the preferred method of cleansing. We attempted to account for this through a subgroup analysis based on conventional versus split-dosing. This showed that regardless of the timing of the bowel preparation, the LRB did not affect the quality of the preparation (table 2) and a split-dose preparation had lower OBPS scores than the conventional dosing. Fourth, the recruitment for this study took longer than anticipated as the majority of clinicians were preferentially using a low-volume bowel preparation and patients who were triaged direct to colonoscopy (∼30% of all procedures in our centre) were not eligible for inclusion to the study, as they were not reviewed in clinic prior to their procedure. Finally, as this study looked at outpatient procedures, we are not able to determine whether the use of an LRB is feasible for inpatients undergoing colonoscopy.
In summary, our study suggests that the use of an LRB the day prior to colonoscopy with a PEG-based solution may be inferior to a CFD with respect to colon cleansing, but is better tolerated by patients. Our results indicate that CFD should be used when using a PEG bowel preparation; however, patient tolerability is an important factor and clinicians may choose to use this information to individualise their approach to diet recommendations depending on the anticipated acceptance of the preparation.
Supplementary Material
Acknowledgments
The authors would like to thank Darlene Brady and Jackie McKay for their invaluable assistance in conducting this trial as well as the Hotel Dieu Hospital for its support of our clinical research programme.
Footnotes
Contributors: JAF took part in study design, study protocol, statistical analysis and writing the first draft of manuscript. JG participated in data collection and critical review of the manuscript. AM took part in study design, study protocol and critical review of the manuscript. SV participated in study design, critical review of the manuscript and funding support. LH took part in study design, study protocol, critical review of the manuscript and funding support.
Funding: This study was funded in full by the Queen's University Department of Medicine.
Competing interests: LH has served as a consultant for Pentax, a speaker for Boston Scientific and has received research funding from Ferring Canada. SV has received research funding from Ferring Canada.
Ethics approval: Queen's University Research Ethics Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Wolff WI. Colonoscopy: history and development. Am J Gastroenterol 1989;84:1017–25. [PubMed] [Google Scholar]
- 2.Harewood GC, Wiersema MJ, Melton LJ III. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol 2002;97:3186–94. doi:10.1111/j.1572-0241.2002.07129.x [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Baki H, Hashash JG, Elhajj II, et al. A randomized, controlled, double-blind trial of the adjunct use of tegaserod in whole-dose or split-dose polyethylene glycol electrolyte solution for colonoscopy preparation. Gastrointest Endosc 2008;68:294–300. doi:10.1016/j.gie.2008.01.044 [DOI] [PubMed] [Google Scholar]
- 4.Flemming JA, Vanner SJ, Hookey LC. Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc 2012;75:537–44. doi:10.1016/j.gie.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 5.Gupta T, Mandot A, Desai D, et al. Comparison of two schedules (previous evening versus same morning) of bowel preparation for colonoscopy. Endoscopy 2007;39:706–9. doi:10.1055/s-2007-966375 [DOI] [PubMed] [Google Scholar]
- 6.Hookey LC, Vanner S. A review of current issues underlying colon cleansing before colonoscopy. Can J Gastroenterol 2007;21:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz PO, Rex DK, Epstein M, et al. A dual-action, low-volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II study. Am J Gastroenterol 2013;108:401–9. doi:10.1038/ajg.2012.441 [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Katz PO, Bertiger G, et al. Split-dose administration of a dual-action, low-volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest Endosc 2013;78:132–41. doi:10.1016/j.gie.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc 2009;69:700–6. doi:10.1016/j.gie.2008.09.047 [DOI] [PubMed] [Google Scholar]
- 10.Park DI, Park SH, Lee SK, et al. Efficacy of prepackaged, low residual test meals with 4L polyethylene glycol versus a clear liquid diet with 4L polyethylene glycol bowel preparation: a randomized trial. J Gastroenterol Hepatol 2009;24:988–91. doi:10.1111/j.1440-1746.2009.05860.x [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–50. doi:10.1038/ajg.2009.104 [DOI] [PubMed] [Google Scholar]
- 12.Rex DK. Does the use of sedation, or the level of sedation, affect detection during colonoscopy? Am J Gastroenterol 2012;107:1849–51. doi:10.1038/ajg.2012.354 [DOI] [PubMed] [Google Scholar]
- 13.Sipe BW, Fischer M, Baluyut AR, et al. A low-residue diet improved patient satisfaction with split-dose oral sulfate solution without impairing colonic preparation. Gastrointest Endosc 2013;77:932–6. doi:10.1016/j.gie.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 14.El Sayed AM, Kanafani ZA, Mourad FH, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc 2003;58:36–40. doi:10.1067/mge.2003.318 [DOI] [PubMed] [Google Scholar]
- 15.Melicharkova A, Flemming J, Vanner S, et al. A low-residue breakfast improves patient tolerance without impacting quality of low-volume colon cleansing prior to colonoscopy: a randomized trial. Am J Gastroenterol 2013;108:1551–5. doi:10.1038/ajg.2013.21 [DOI] [PubMed] [Google Scholar]
- 16.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc 2004;59:482–6. doi:10.1016/S0016-5107(03)02875-X [DOI] [PubMed] [Google Scholar]
- 17.Cardello AV, Schutz HG, Lesher LL, et al. Development and testing of a labeled magnitude scale of perceived satiety. Appetite 2005;44:1–13. doi:10.1016/j.appet.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 18.Aronchick CA, Lipshutz WH, Wright SH, et al. Validation of an instrument to assess colon cleansing [abstract] Am J Gastroenterol 1999;94:2667. [Google Scholar]
- 19.McKnight LC, Day AG, Hookey LC. A formal assessment of the need for teaching prior to the use of the Ottawa Bowel Preparation Scale. Am J Gastroenterol 2006;101:s508. [Google Scholar]
- 20.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley & Sons, 1987. [Google Scholar]
- 21.Romero RV, Mahadeva S. Factors influencing quality of bowel preparation for colonoscopy. World J Gastrointest Endosc 2013;5:39–46. doi:10.4253/wjge.v5.i2.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex DK. Split dosing for bowel preparation. Gastroenterol Hepatol (NY) 2012;8:535–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Di PJ, Rex D. Advances in bowel preparations: new formulation and clinical results. Gastroenterol Nurs 2011;34(Suppl 2):S2–8. doi:10.1097/SGA.0b013e31823080ef [DOI] [PubMed] [Google Scholar]
- 24.Lasisi F, Rex DK. Improving protection against proximal colon cancer by colonoscopy. Expert Rev Gastroenterol Hepatol 2011;5:745–54. doi:10.1586/egh.11.78 [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Di Palma JA, Rodriguez R, et al. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc 2010;72:328–36. doi:10.1016/j.gie.2010.03.1054 [DOI] [PubMed] [Google Scholar]
- 26.Unger RZ, Amstutz SP, Seo DH, et al. Willingness to undergo split-dose bowel preparation for colonoscopy and compliance with split-dose instructions. Dig Dis Sci 2010;55:2030–4. doi:10.1007/s10620-009-1092-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


