Abstract
Objectives
Enteropathy associated T-cell lymphoma (EATL) is a rare non-Hodgkin lymphoma that may complicate coeliac disease and typically occurs in patients with refractoriness to the gluten-free diet. The majority of these patients harbour a clonal expansion of intraepithelial lymphocytes (IELs) with an aberrant phenotype in the small intestine which are thus considered as the ‘precursor’ lymphoma cells. We describe a 51-year-old female patient with refractory coeliac disease (RCD) who developed an EATL with manifestations in the proximal small intestine and in a mesenteric lymph node that did not evolve from regular type ‘aberrant’ αβ-T-cells but rather from a clonal expansion of γδ-T-cells.
Methods
Duodenal biopsies and lymphoma tissue from a patient with refractory coeliac disease whom developed an EATL were extensively studied by immunophenotypical, T-cell receptor immunogenetic and chromosomal analysis.
Results
Flow cytometric analysis of duodenal IELs revealed an unusual large clonal expansion of CD30 negative γδ-T-cells in a patient with RCD. When the patient clinically deteriorated 18 months later, a substantial part (30%) of this cell population did express CD30. In addition, identical immunogenetic aberrancies had developed in a prehepatic lymph node.
Conclusions
We here report on a case of extraintestinal EATL that originated from a clonal γδ-IEL population rather than from aberrant IEL. This EATL displayed a distinctive pattern of immunophenotypical, T-cell receptor immunogenetic and chromosomal aberrancies as compared to classical EATL, defining this lymphoma as a novel variant of EATL.
Keywords: CELIAC DISEASE, INTESTINAL T CELLS, LYMPHOMA, MOLECULAR IMMUNOLOGY, T-CELL RECEPTOR
Summary box.
What is already known about this subject?
-
▸
Patients refractory to the gluten-free diet may develop an enteropathy-associated T-cell lymphoma (EATL). These patients typically harbour intraepithelial lymphocytes (IELs) with an aberrant phenotype in their duodenal epithelium, that are considered precursor lymphoma cells.
What does this study add?
-
▸
This is the first study to show that an EATL originates from γδ-IELs, rather than from aberrant IELs. Furthermore, it describes a new variant of EATL.
How might this impact on clinical practice?
-
▸
This case underlines the need for a more elaborated EATL classification.
Introduction
Enteropathy-associated T-cell lymphoma (EATL) type I is a rare non-Hodgkin lymphoma that may complicate coeliac disease (CD). It is characterised by large pleomorphic and anaplastic cells that express the αβ-T-cell receptor (TCR), CD30, CD4 and cytotoxic markers but not CD8 and CD56.1 Type II EATL displays a distinctive morphology and immunophenotype and lacks a clear association with CD.2 EATL can be diagnosed simultaneously with the diagnosis of CD or may develop in patients with a known history of CD. Typically, disease in the latter patients is non-responsive to the gluten-free diet (GFD) and is referred to as refractory coeliac disease (RCD).3 Such patients display clonal expansion of a T-cell subset that occurs under physiological circumstances in low frequencies in the small bowel mucosa4 5 which is characterised by the presence of cytoplasmic CD3 (cytCD3) but lacks surface expression of CD3, CD4 and CD8 (referred to as ‘aberrant’ T-cells cytCD3+CD3−CD45+CD7+CD4−CD8− cells).6 In patients who develop EATL following a stage of RCD, a direct clonal relation of the EATL tumour cells to the clonally expanded ‘aberrant’ cells can be demonstrated based on identical TCR rearrangements.7 Thus, such ‘aberrant’ cells can be considered precursor lymphoma cells.
Here, we report on a novel variant of EATL that did not evolve from regular type ‘aberrant’ T-cells but rather from an aberrant clonal duodenal γδ-T-cell population.
Methods
Serum immunoglobulin (Ig)A levels, anti-tissue transglutaminase and antiendomysial IgA and IgG antibodies were detected as previously described.8 For human leucocyte antigen (HLA) genotyping the alleles DQA1*05, DQA*02 and DQB1*02 (encoding the HLA-DQ2 heterodimers) and the alleles DQA1*03 and DQB1*0302 (encoding the HLA-DQ8 heterodimer) were determined by a polymorphism–heteroduplex assay, electrophoresis and gel-staining method on the PhastSystem (Amersham Pharmacia Biotech, Uppsala, Sweden) after PCR amplification.5 For histopathological evaluation biopsy specimens were fixed and preserved in 10% formalin. Histological findings were classified using the Oberhuber's modification of the Marsh criteria.5 Immunohistochemistry was performed on paraffin-embedded tissue sections using standard procedures. Flow-cytometric immunophenotypical analysis was performed on freshly isolated intraepithelial lymphocytes and peripheral blood lymphocytes as previously described.5 T-cell receptor (TCR) δ-chain and γ-chain gene rearrangements were assessed by multiplex PCR and GeneScan analysis, as previously described.5 For oligonucleotide array comparative genomic hybridisation (figure 3). Sample was hybridised to 180 K Agilent microarrays (4×180 k array, Agilent Technologies, Palo Alto, California, USA, custom designed GEO platform GPL8687) accessible through http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL8687. Labelling and hybridisation procedures were performed as previously described.9 The experimental data has been made publicly available through GEO (GSE56425).10 The study was in accordance with the ethical guidelines of the VU University Medical Center and the patient gave written informed consent.
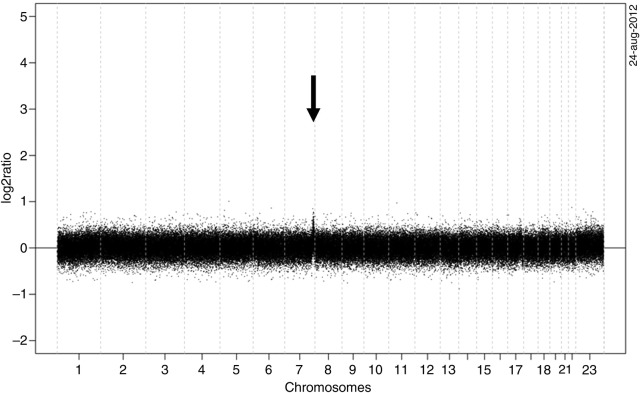
Figure 3.
Chromosomal imbalance in the enteropathy-associated T-cell lymphoma as detected by array comparative genome hybridisation. Only at 7q36 a chromosomal aberration is visible. The X-axis represents the genomic locations of the probes on the array in Mb and the Y-axis represents the log2 ratios of the probes.
Case history (results)
A 51-year-old HLA-DQ 2.5 heterozygous woman was referred to our centre with severe diarrhoea and weight loss. She had been diagnosed with CD 7 years earlier on the basis of antiendomysium antibodies and subtotal villous atrophy (Marsh 3B). She had been on a GFD ever since and dietary adherence was confirmed by negative CD serology and evaluation by a specialised dietitian.
Duodenal histology showed Marsh 3B lesions with focal ulceration. Examination could exclude concomitant autoimmune enteropathy (AIE), immunodeficiency syndrome, Crohn's disease, parasitic infection and malignancy. Flow cytometric analysis of the duodenal IELs revealed a non-significant aberrant IEL population although an unusual large population of 76% γδ-T-cells was present in the epithelial layer (compared to 1–35% in uncomplicated patients with (R)CD).8 These cells did not stain positive for CD30.
The patient was diagnosed with refractory CD without aberrant T-cells (RCD type I) and started on prednisone and 6-tioguanine (6-TG). This resulted in normalisation of the stool frequency, weight gain and a follow-up biopsy 6 months later revealed almost complete mucosal recovery (Marsh 1); the percentage of intra-epithelial γδ-T-cells had decreased to 52%. Eighteen months later her clinical situation deteriorated and duodenal biopsies showed complete villous atrophy (Marsh 3C) and a persisting abnormally high intra-epithelial γδ-T-cells population (78%). Remarkably, fluorescence activated cell sorting analysis now showed CD30 expression on 30% of the intra-epithelial γδ-T-cells while only 10% of αβ-T-cells expressed CD30 within the normal range for activated T-lymphocytes. In the peripheral blood, no significant γδ-T-cell population could be identified. Positron emission tomography (PET)-CT-scan showed diffuse increased fluorodeoxy-glucose-uptake in the jejunum without further signs of lymphadenopathy or hepatosplenomegaly. In addition, a prehepatic intra-abdominal PET-positive mass (diameter 15×9 mm) was identified, which was not present on the CT scan of the abdomen that was performed 18 months earlier during the RCD workup.
Histological analysis
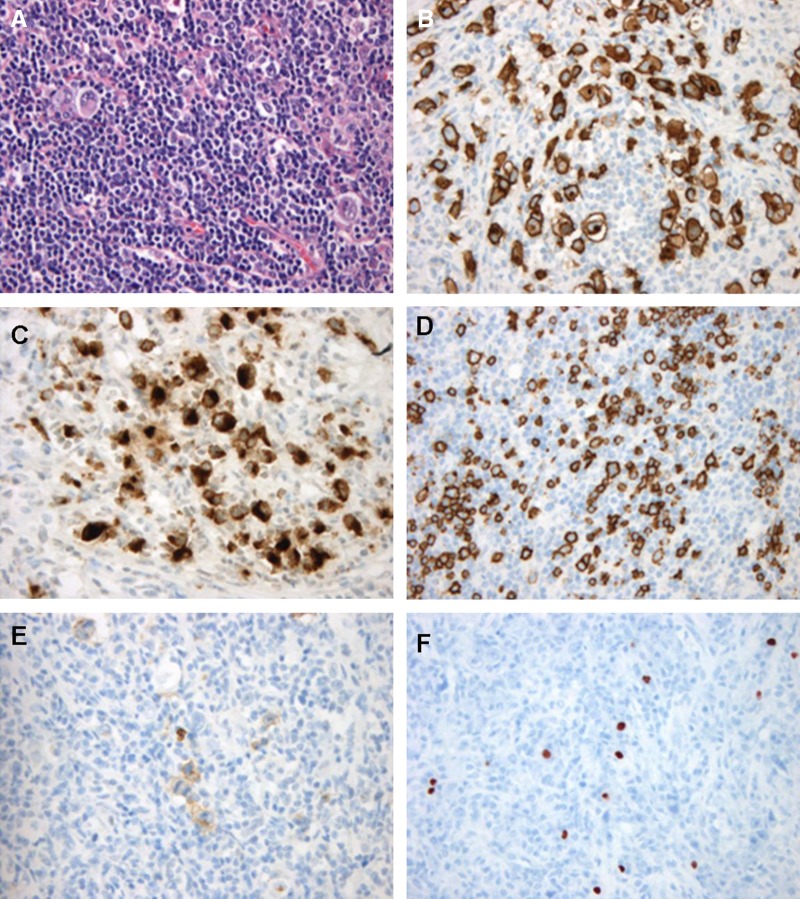
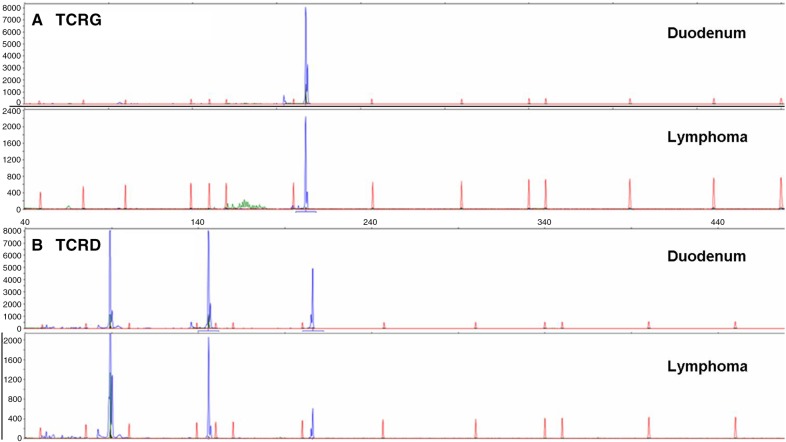
An excisional biopsy was taken from the lymph node. The lymph node showed a complete effaced architecture with a diffuse, sheet-like infiltrate of large lymphoid blasts with polymorphic nuclei, multiple large ovoid nucleoli and clear cytoplasm. Immunohistochemistry showed a very unusual immunophenotype (figure 1). The tumour cells were uniformly positive for CD7, granzymeB, CD30 as well as CD20. TIA1 and CD15 were positive in a smaller percentage of the tumour cells. There was extensive T-cell marker loss as CD3, CD5, CD4 and CD8 were negative. Additional B-cell markers (CD79a and PAX5) were also negative. Epstein-Barr virus-encoded RNA (EBER) occasionally stained large cells, while the reactive small cell infiltrate showed more EBER-positive cells. Gene-rearrangement studies showed a polyclonal immunoglobulin heavy chain rearrangement pattern but a monoclonal T-cell receptor γ (TCRG) and δ (TCRD) rearrangements, confirming a T-cell origin of the proliferation. The monoclonal TCRG and TCRD rearrangement pattern in the tumour was identical to that observed in the duodenum (figure 2), indicating that the monoclonal γδ-T-cell population was already present in the duodenum at time of the initial RCDI diagnosis.
Figure 1.
Histology and immunophenotype of a novel variant of enteropathy-associated T-cell lymphoma. (A) H&E (B) CD30 (C) GranzymeB (D) CD20 (E) CD15 (f) Epstein-Barr virus-encoded RNA.
Figure 2.
Rearrangement analysis of the T-cell receptor γ (TCRG) (A) and T-cell receptor δ (TCRD) (B) genes on DNA derived from a duodenal sample 2 years prior to tumour development (top) and the extraintestinal enteropathy-associated T-cell lymphoma (bottom).
Genetic studies
Array comparative genomic hybridisation (aCGH) revealed only one chromosomal gain on the chromosome region 7q36 (figure 3). Fluorescent in situ hybridisation for MYC did not show a MYC break.
Treatment
Initially, the patient was treated with a 5-day course of Cladribine 0.1 mg/kg/day. Malabsorption-related symptoms did not improve and villous atrophy persisted. Therefore, the patient was subsequently treated with three courses of rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin en prednisone (R-CHOP). This resulted in clinical improvement as defaecation frequency normalised and a 4 kg weight gain was reached after 3 months. A follow-up PET-scan revealed a diminished lymphoma lesion. The duodenal mucosa showed complete recovery (Marsh 0). Moreover, the γδ-T-cell population decreased to 29% of total IELs, a percentage that is considered normal in patients with (R)CD, with absence of CD30 expression.
Currently, 2 years after R-CHOP treatment, the patient is clinically well and repeated follow-up endoscopy showed normal mucosa without histological abnormalities. The γδ-IEL population varies between 25 and 30% of total IELs.
Discussion
This case involves a patient with CD who developed a T-cell lymphoma with an unusual phenotype and genotype. As far as we are aware, this is the first report of an extraintestinal lymphoma originating from a duodenal γδ-IEL population. Peripheral γδ-T-cell lymphomas frequently concern hepatosplenic T-cell lymphomas but our patient had dissimilar clinical, phenotypical and chromosomal characteristics. Intestinal γδ-T-cell lymphomas have been reported but consist of monomorphic cells with a CD103+CD3+CD8+CD56+ phenotype that can be categorised as type 2 EATL and are not associated with CD.11
The observed genetic aberrations in this patient involved gain of the 7q region, which shows frequent alteration in EATL as well as in many other T-cell and B-cell lymphomas.12 13 Other common chromosomal aberrations observed in EATL type 1 and 2, including gains in 1q, 5q and 9q, were absent in this patient (table 1). Moreover, aberration of the MYC oncogene, as often present in type 2 EATL, was absent. In addition, the recently described EATL that arose in an patient with AIE who also displayed aberrations on chromosome 7 involved a distinctly more complex pattern with alterations on 8p22-23.2 and 10q23 which, again, were absent in our patient.14 The 7q region consists of a highly dense gene region that includes multiple genes with regulatory functions and it is tempting to speculate that these play a role in the onset of this tumour.
Table 1.
Overview of the characteristics of the at presently reported various types of EATL
| Characteristics | EATL type 1 | EATL type 2 | variant type EATL | AIE-associated T-cell lymphoma |
|---|---|---|---|---|
| Association with enteropathy | + | − | + | + |
| HLA-DQ2/-DQ8 | + | − | + | + |
| Putative precursor cell | CD3s−IEL | Clonal aberrant T-cell | Clonal γδ-T-cells | Clonal CD8 αβ-T-cells |
| Morphology | Polymorphic | Monomorphic | Polymorphic | Monomorphic |
| Phenotype | ||||
| CD3 | + | + | − | + |
| CD4 | Mostly− | − | − | − |
| CD5 | Mostly− | Mostly+(70%) | − | + |
| CD8 | Mostly−(20%+) | + | − | + |
| CD7 | + | + | + | − |
| CD15 | − | − | + | nd |
| CD20 | − | − | − | − |
| CD30 | Mostly+(80%) | − | + | +/− |
| CD56 | − | + | − | − |
| Granzyme | + | + | + | + |
| TIA1 | + | + | + | + |
| Chromosomal aberrantions | ||||
| Gains | 1q, 5q, 7q, 9q, | MYC, 7q, 8q, 9q | 7q | 7q |
| Loss | 8p, 13q,16q | 8p, 10q | ||
HLA, human leucocyte antigen.
The current classification for EATL is unsatisfactory as it is becoming clearer that EATL type 2 is not associated with enteropathy and seems a separate entity rather than a variant of type 1 EATL.2 In addition, as in the case described here, the AIE-associated EATL as well as other intestinal lymphomas with a broader spectrum of this disease15 clearly show a need for a more elaborate division (table 1). The precursor cells from which these various EATLs arise form an important area of research to better understand these lymphomas.
In conclusion, we here report a novel variant of EATL, with an unusual phenotype and genotype in a patient with type I RCD, that evolved from a clonal γδ-IEL population.
This case provides additional support for the much needed development of a novel classification for EATL.
Footnotes
Contributors: RLJvW analysed data and wrote the manuscript. DdJ performed histopathological analysis and wrote the manuscript. AWL and DAMH performed and analysed TCR clonality studies. BY and HFvE performed and analysed comparative genomic hybridisation studies. HJB analysed flow cytometric studies. CJJM and GB evaluated clinical studies, supervised the study and wrote the manuscript.
Funding: This work was supported by the Coeliac Disease Consortium, the Netherlands.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. . WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edn. Lyon, France: IARC Press, 2008. [Google Scholar]
- 2.Chan JK, Chan AC, Cheuk W, et al. . Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent gammadelta T-cell receptor expression. Am J Surg Pathol 2011;35:1557–69. doi:10.1097/PAS.0b013e318222dfcd [DOI] [PubMed] [Google Scholar]
- 3.Di SA, Biagi F, Gobbi PG, et al. . How I treat enteropathy-associated T-cell lymphoma. Blood 2012;119:2458–68. doi:10.1182/blood-2011-10-385559 [DOI] [PubMed] [Google Scholar]
- 4.Schmitz F, Tjon JM, Lai Y, et al. . Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 2013;62:509–19. doi:10.1136/gutjnl-2012-302265 [DOI] [PubMed] [Google Scholar]
- 5.Tack GJ, van Wanrooij RL, Langerak AW, et al. . Origin and immunophenotype of aberrant IEL in RCDII patients. Mol Immunol 2012;50:262–70. doi:10.1016/j.molimm.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 6.Cellier C, Patey N, Mauvieux L, et al. . Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998;114:471–81. doi:10.1016/S0016-5085(98)70530-X [DOI] [PubMed] [Google Scholar]
- 7.Cellier C, Delabesse E, Helmer C, et al. . Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000;356:203–8. doi:10.1016/S0140-6736(00)02481-8 [DOI] [PubMed] [Google Scholar]
- 8.Verbeek WH, Goerres MS, von Blomberg BM, et al. . Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin Immunol 2008;126: 48–56. doi:10.1016/j.clim.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Buffart TE, Israeli D, Tijssen M, et al. . Across array comparative genomic hybridization: a strategy to reduce reference channel hybridizations. Genes Chromosomes Cancer 2008;47:994–1004. doi:10.1002/gcc.20605 [DOI] [PubMed] [Google Scholar]
- 10.Barrett T, Troup DB, Wilhite SE, et al. . NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res 2009;37(Database issue):D885–90. doi:10.1093/nar/gkn764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson AL, Swerdlow SH, Przybylski GK, et al. . Intestinal gammadelta T-cell lymphomas are most frequently of type II enteropathy-associated T-cell type. Hum Pathol 2013;44: 1131–45. doi:10.1016/j.humpath.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brito-Babapulle V, Matutes E, Parreira L, et al. . Abnormalities of chromosome 7q and Tac expression in T cell leukemias. Blood 1986;67:516–21. [PubMed] [Google Scholar]
- 13.Deleeuw RJ, Zettl A, Klinker E, et al. . Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology 2007;132:1902–11. doi:10.1053/j.gastro.2007.03.036 [DOI] [PubMed] [Google Scholar]
- 14.Malamut G, Verkarre V, Callens C, et al. . Enteropathy-associated T-cell lymphoma complicating an autoimmune enteropathy. Gastroenterology 2012;142:726–9. doi:10.1053/j.gastro.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 15.Attygalle AD, Cabecadas J, Gaulard P, et al. . Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward—report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology 2014;64:171–99. doi:10.1111/his.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]