Abstract
Background
Alveolar echinococcosis (AE) is a neglected zoonosis presenting with focal liver lesions (FLL) with a wide range of imaging patterns resembling benign as well as malignant FLLs. Complementary serology and histopathology may be misleading.
Objective
The objective of our study is to highlight pitfalls leading to wrong diagnoses and harmful interventions in patients with AE.
Design
This retrospective sentinel case series analyses diagnostic and treatment data of patients with confirmed AE.
Results
80 patients treated between 1999 and 2014 were included in the study. In 26/80 patients treatment decisions were based on a wrong diagnosis. AE was mistaken for cystic echinococcosis (CE) in 12/26 patients followed by cholangiocellular carcinoma (CCA) in 5/26 patients; 61/80 patients had predominantly infiltrative liver lesions and 19/80 patients had a predominantly pseudocystic radiological presentation. Serology correctly differentiated between Echinococcus multilocularis and Echinococcus granulosus in 53/80 patients. Histopathology reports attributed the right Echinococcus species in 25/58 patients but failed to differentiate E. multilocularis from E. granulosus in 25/58 patients. Although contraindicated in AE 8/25 patients treated surgically had instillation of a protoscolicidal agent intraoperatively. One of the eight patients developed toxic cholangitis and liver failure and died 1 year after liver transplantation.
Conclusions
Misclassification of AE leads to a critical delay in growth inhibiting benzimidazole treatment, surgical overtreatment and bares the risk of liver failure if protoscolicidal agents are instilled in AE pseudocysts.
Keywords: HEPATOBILIARY PATHOLOGY, HEPATOBILIARY RADIOLOGY, INFECTIOUS DISEASE
Summary box.
What is already known about this subject?
-
▸
Alveolar echinococcosis (AE) plays an increasing role in clinical practice.
-
▸
A substantial proportion of AE focal liver lesions (FLLs) do not exhibit the imaging pattern regarded as characteristic for AE.
-
▸
Serology cannot reliably discriminate between AE and cystic echinococcosis (CE).
What are the new findings?
-
▸
There is a risk of associating cystic FLLs wrongly with CE instead of AE.
-
▸
Histopathology may fail to differentiate between AE and CE.
-
▸
Mistaking AE for CE leads to non-curative surgical interventions with the risk of toxic cholangitis if protoscolicidal solutions are applied.
How might it impact on clinical practice in the foreseeable future?
-
▸
Awareness of AE as an important differential diagnosis in the workup of FLLs. Deleterious consequences for patients if AE is not treated timely or treated with substances with biliary toxicity due to misclassification of pseudocystic AE as CE.
Introduction
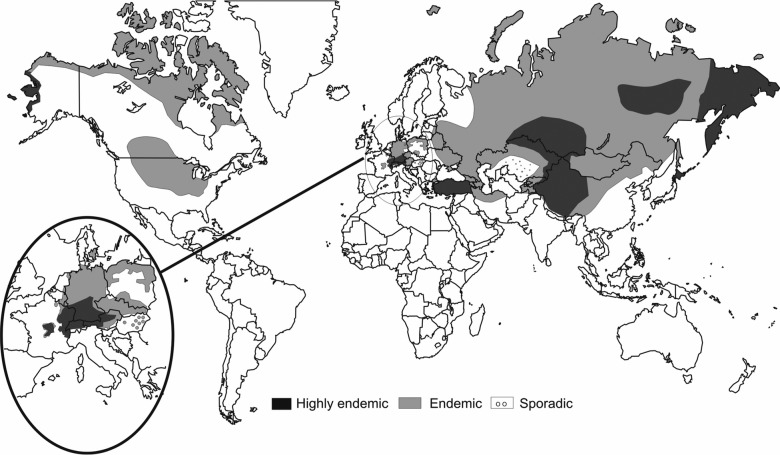
Alveolar echinococcosis (AE) is a neglected zoonosis presenting with focal liver lesions (FLL).1 In endemic regions, awareness of this disease is high due to the known transmission risk (figure 1).2 AE should, however, always be included in the differential diagnosis of FLLs due to the high mobility of patients. The clinical problem is thus to identify AE in ‘a sea’ of differential diagnoses.
Figure 1.
Global distribution of alveolar echinococcosis.2
The ‘typical’ AE FLL is described as a mixed pattern of infiltration, necrosis (‘pseudocysts’) and calcification with multivesicular, honeycomb-like areas which brings solid and cystic differential diagnoses into play.3 Hepatocellular carcinoma in liver cirrhosis, hepatocellular adenoma, haemangioma and focal nodular hyperplasia are solid FLLs that can be diagnosed by imaging alone, whereas intrahepatic cholangiocarcinoma (ICCA) and alveolar echinococcosis cannot. Similarly, the cystic FLLs, simple hepatic cysts, polycystic liver disease and cystic echinococcosis, have specific radiological features, whereas biliary cystadenoma, biliary cystadenocarcinoma and alveolar echinococcosis represent cystic FLLs which cannot be diagnosed by imaging alone.1 3–5
Consequently, serology complements imaging in the diagnostic process of AE. Antibody testing follows a 2-step approach with sensitive Echinococcus species and E. multilocularis ELISAs followed by a more specific western blot.4 6 Cross-reactivity of first-line tests is common with cystic echinococcosis (CE) and has been described with malignancies.7 If serology is inconclusive, liver biopsy is warranted with a high likelihood that histopathology and PCR confirm the diagnosis. The latter, however, is not routinely available and pathologists may not be familiar with the former.4 8–10
The aim of this study is to identify pitfalls leading to erroneous diagnoses and harmful interventions in AE through analysis of diagnostic and therapeutic pathways of patients with confirmed AE treated in our centre.
Methods
Design
Retrospective case series with the aim of identifying clinically relevant sentinel events in the diagnostic and therapeutic pathway of patients with confirmed final diagnosis AE according to the WHO-IWGE working group criteria.11 The study was approved by the Ethical Board of the University of Heidelberg (S039/2013).
Study population and clinical setting
Since 1999, 83 patients with AE and 240 patients with CE have been treated and followed up at the Section of Clinical Tropical Medicine at Heidelberg University Hospital. The clinic for echinococcosis is run interdisciplinary in cooperation with the Department of Diagnostic and Interventional Radiology, the Department of Surgery and the Department of Gastroenterology. Our unit is a national clinical reference centre for echinococcosis. All patients, who presented to our clinic between 1999 and 2014 and were finally confirmed as those with AE, and in whom cross-sectional imaging and serology were available, were included in the analysis (n=80). Patients were referred from tertiary care centres in 32/80 patients, secondary care centres in 12/80 centres, community hospitals in 18/80 patients and from primary care in 18/80 patients. The majority of patients were of German origin (64/80) followed by patients from countries of the Former Soviet Union, Turkey and other countries. Diagnosis of AE was established according to the expert consensus guidelines3 9 by imaging and serology and, where necessary, histopathology. If histopathology reports failed to differentiate AE from CE in primary reporting, they were re-evaluated by a pathologist experienced in the diagnosis of AE and PCR was performed from paraffin blocks.
Patient age ranged from 17 to 86 years with a mean age of 53.8 years, and gender distribution was comparable with 44 female and 36 male patients. Most liver lesions were discovered incidentally (42/80) or presented with upper abdominal symptoms (22/80). In total 7/80 patients presented with obstructive jaundice, whereas 6 patients had other symptoms and 3 unknown symptoms.
Data extraction from patient notes
Diagnosis
Diagnosis before referral to the echinococcosis clinic on which treatment decisions were initially based was extracted from patient notes.
Imaging
CT and MRI of patients were reanalysed by a board-certified radiologist addressing the predominant radiological pattern and patients were allocated to one of two categories of discernible morphological patterns: (1) infiltrative liver lesions and (2) pseudocystic liver lesions. Infiltrative liver lesions were defined as FLLs with ill-defined margins and a significant solid matrix component. Pseudocystic liver lesions were defined as FLLs with predominantly cystic components. All lesions were assessed for multivesicular morphology, calcification (CT only) and contrast enhancement.
Serology
Serology was interpreted as positive for AE when one of the screening ELISAs (Echinococcus species (ELISA), E. multilocularis (ELISA)) and AE-specific bands were demonstrated in the Immunoblot at first presentation. Correspondingly serology was interpreted as CE when CE-specific bands were demonstrated in the Immunoblot. Unspecified echinococcosis (E) was defined as Echinococcus-specific bands in the Immunoblot without a species specific pattern or a positive screening ELISA. ELISA kits (‘Bordier Affinity Products SA’, 1023 Crissier, Switzerland), with antigens from E. granulosus hydatid fluid and recombinant and affinity purified antigens (Em2-Em18) from E. multilocularis and Immunoblot (ECH-WB96G, Biorepair, Sinsheim, Germany), were used.
Histopathology
Histopathology was either available from liver biopsy or from operated patients (liver resection). Conclusions of histopathology reports were divided into four categories: (1) AE—alveolar echinococcosis, if the report contained terms like alveolar echinococcosis, E. multilocularis and ‘Echinococcus alveolaris’, (2) CE—cystic echinococcosis, if the report contained terms like cystic echinococcosis or E. granulosus, (3)E—echinococcosis if the report did not discriminate AE and CE and terms like echinococcosis and “echinococcal cyst” were used, (4) U—non-specific result, if necrosis or any other non-specific inflammatory infiltrate was described.
Treatment data
The surgical reports of all patients with AE were analysed. Surgical treatment was judged as appropriate if R0 liver resection was aimed at. If surgical techniques for CE were described and terms like endocystectomy, partial cyst resection with or without the use of a protoscolicidal solution was described, surgical treatment was judged as inappropriate.
Medical reports were screened for percutaneous treatment with instillation of protoscolicidal solution (Puncture, Aspiration, Instillation and Reaspiration-PAIR) as used for CE—this intervention was judged as inappropriate.
Results
Diagnosis
In 26/80 patients, therapeutic decisions were based on a wrong diagnosis. Of these, 12/26 patients were mistakenly diagnosed for CE, followed by ICCA in 5/26 patients, other malignancy in 5/26 patients, haemangioma in 3/26 patients and simple cyst in 1/26 patients (see figures 2–4).
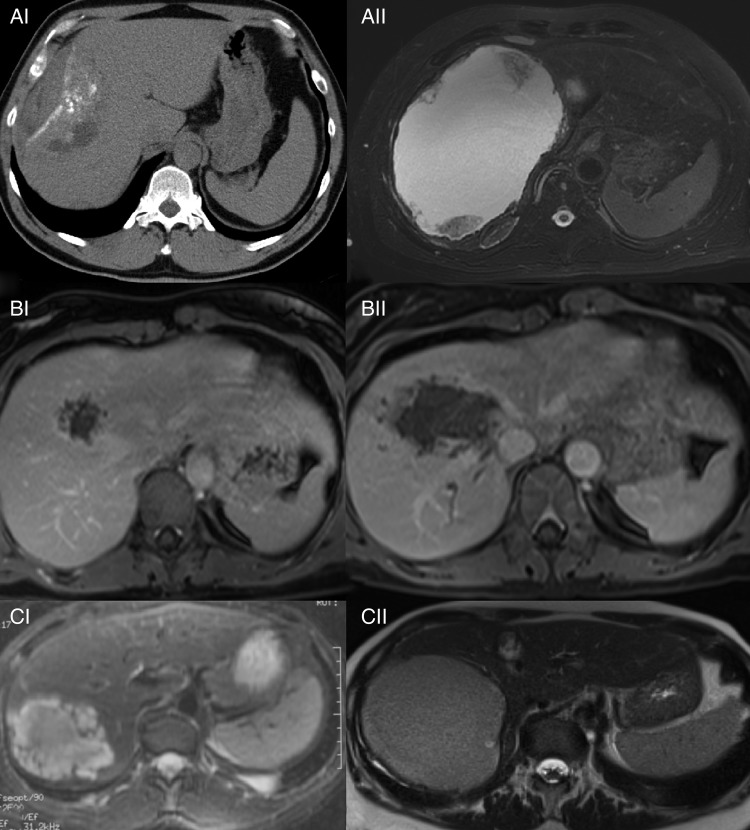
Figure 2.
Evolution of AE lesions over time in 3 patients initially diagnosed as haemangioma. Patient A: (AI) irregular defined lesion with mixed texture and calcification misdiagnosed as haemangioma (2008), CT without contrast enhancement, (AII) after an interval of 4 years, a pseudocystic mass containing fluid and debris developed and alveolar echinococcosis was diagnosed (2012), T2-weighted MRI. Patient B: (BI) irregularly defined microcystic lesion with faint peripheral contrast enhancement misdiagnosed as haemangioma (2008), contrast enhanced T1-weighted MRI, (BII) significant increase in size of liver lesion with perivascular infiltration misdiagnosed as cystic echinococcosis (2012), contrast-enhanced T1-weighted MRI. Patient C: (CI) lobulated, multivesicular fluid-containing lesion with solid components misdiagnosed as haemangioma (2005), T2-weighted MRI, (CII) liquefaction of lesion with increase in size and decay of former solid components misdiagnosed as cystic echinococcosis (2014), T2-weighted MRI.
Figure 3.
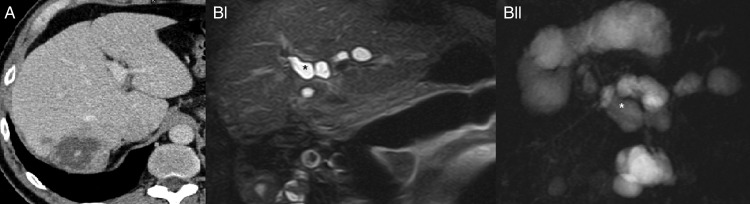
Consequences of erroneous instillation of protoscolicidal agent in an AE liver lesion. (A) Ill-defined lesion with cystic and solid components and calcification before surgery, CT with contrast enhancement. (BI and BII) after instillation of protoscolicidal solution, both images show saccular dilation (asterisk*) of bile ducts indicative of bile duct necrosis, coronary T2-weighted MRI, MR cholangiopancreatography.
Figure 4.
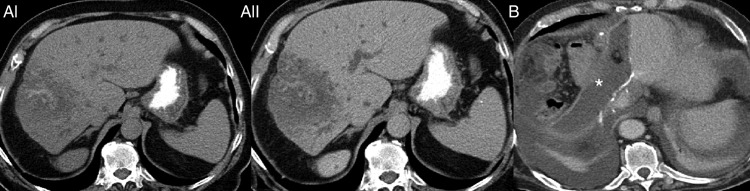
78-year-old patient with post op confirmed AE operated as cholangiocarcinoma. (AI) diffuse infiltrative lesion of the right liver lobe with calcification, CT without contrast enhancement. (AII) faint contrast enhancement in the periphery of the lesion, CT with contrast enhancement. (B) After an extended right hemihepatectomy with biliodigestive anastomosis, a large bilioma and consecutively abscess (asterisk *) with fistula to the abdominal wall developed and was drained for several months.
Eight of 19 (42%) patients in the pseudocyst group were mistakenly diagnosed for CE compared to 4/61 (6%) patients in the infiltrative group.
Imaging
Of 80 eligible patients, 61 showed predominantly infiltrative lesions and 19 pseudocystic or cystic morphology. Calcification was present in 62 of 69 patients where CT was available. The majority of patients (46/80) showed no contrast enhancement. In 34 patients, faint contrast enhancement of liver lesions was demonstrable. Multivesicular morphology of lesions was found in 55/80 patients.
Serology
Serology correctly confirmed AE in the majority of our patients (53/80; 65%). In the remaining patients, it either failed to differentiate AE from CE (27%) or wrongly pointed towards CE (8%).
Histopathology
Histopathology was available in 58 patients. The first histopathological examination by external or internal pathologists was assessed for histopathological diagnosis. The correct diagnosis of AE was made in 25/58 patients, whereas in 25/58 patients histopathology reports failed to assign the species (E. multilocularis or E. granulosus) and in 2/58 patients reports attributed findings to the wrong species (E. granulosus instead of E. multilocularis) or reported only non-specific findings in 6/58 patients.
Treatment data
Twenty five patients were treated surgically. Fifteen patients had R0 resection, of which in two patients a protoscolicidal solution was used. Ten patients had an R2 resection of which a protoscolicidal solution was used in six patients. In total, 8/25 patients had instillation of a protoscolicidal solution during surgery, a method used in CE. One patient developed subacute liver failure due to toxic cholangitis with liver transplantation 7 months after instillation of a protoscolicidal solution into a pseudocystic AE liver lesion (figure 3). The patient died 1 year after transplantation due to sepsis. One patient had instillation of a protoscolicidal solution by percutaneous puncture (PAIR), a treatment used for CE.
Discussion
The widespread use of imaging not only brings to light the awaited diagnoses of hypothesis-driven work-up but also confronts physicians with unsuspected findings. AE is a pertinent example. To get as close to the real life, day-to-day clinical challenge of identifying AE among patients with FLLs, we analysed the diagnostic and treatment pathways of patients with a finally confirmed diagnosis of AE.
It is striking that roughly one-third of treatment decisions taken before referral to our centre were based on wrong diagnoses. Most frequently, AE was mistaken for CE.
The spectrum of misinterpretations differs between lesions with infiltrative and pseudocystic imaging patterns. Infiltrative AE was more commonly confused with hepatic malignancy, and pseudocystic AE was frequently mistaken for CE. In part, this may be due to a lack of awareness of AE being a possible differential diagnosis of other FLLs with a wide range of imaging features from solid to cystic.
Whereas malignancies and benign lesions are in most cases serologically distinguishable from AE, CE is not. Once positive serology had set the track towards echinococcosis, serology correctly confirmed AE in 2/3 of our patients. In the remaining patients, it either failed to differentiate AE from CE or rarely pointed wrongly towards CE.7 Also, histopathology failed to assign the species (E. multilocularis or E. granulosus) in more than half of our patients or attributed findings to the wrong species (E. granulosus instead of E. multilocularis).
Consequences of misdiagnosis:
Misclassification of AE as a benign lesion results in progression to a stage in which curative R0 resection may no longer be possible or in complications preventable by timely initiation of benzimidazole treatment (figure 2).
Misclassification of AE as a malignancy, most frequently ICCA in our series, may cause surgical overtreatment of AE. To achieve a cure, AE and ICCA FLLs are ideally surgically removed, although the threshold for surgery differs. In some patients with AE, one would rather abstain from surgery if curative resection is not safely achievable (figure 4). Suppressive drug treatment with benzimidazoles is a good alternative with excellent 10 year survival rates.12
Misclassification of AE as CE is alarmingly common in our patient series. This is most striking in patients with AE presenting with pseudocysts, that is, large necrotic cavities where almost half of the patients were wrongly diagnosed as having CE. In contrast, it concerned only very few of our patients with finally confirmed AE in the infiltrative group. Apart from this, we could not find any pattern of serological or histopathological results which was particularly misleading. Misclassifying ‘cystic’ AE as CE results in a disastrous treatment outcome when protoscolicidal agents such as 95% alcohol or 20% saline are applied. In our series, eight patients had instillation of protoscolicidal solutions during surgery or PAIR. Of those, seven had been misclassified as CE and in one patient a protoscolicidal solution was applied despite the correct diagnosis of AE. One patient has been referred to our hospital for liver transplantation a few months after surgical treatment for suspected CE and instillation of formalin.
The main limitation of our study is its design which makes the definition of type and direction of biases difficult if not impossible. In rare diseases such as AE, however, retrospective case series are a legitimate tool to recognise sentinel events which are potentially harmful for patients.11 In our analysis we have identified several pivotal elements in the diagnostic process and treatment of AE. Familiarity with these pitfalls will make treatment of patients with AE safer. Our analysis underpins that once AE is suspected, the diagnostic process and treatment decisions should be accompanied by a specialised centre.
Footnotes
Contributors: MS and TJ designed the study, analysed the data and wrote the first draft of the manuscript. CM extracted and analysed the patient data. TFW analysed the radiological imaging data. All authors contributed to the final version of the manuscript, and read and approved the final document.
Competing interests: None declared.
Ethics approval: Ethics committee of the Medical Faculty Heidelberg.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328–47. doi:10.1038/ajg.2014.213 [DOI] [PubMed] [Google Scholar]
- 2.Torgerson PR, Keller K, Magnotta M, et al. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis 2010;4:e722 doi:10.1371/journal.pntd.0000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresson-Hadni S, Delabrousse E, Blagosklonov O, et al. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int 2006;55(Suppl):S267–72. doi:10.1016/j.parint.2005.11.053 [DOI] [PubMed] [Google Scholar]
- 4.Eckert J, Gemmell MA, Pawlowski ZS, eds. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: WHO/OIE, 2001. [Google Scholar]
- 5.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657–69. doi:10.1136/gutjnl-2011-301748 [DOI] [PubMed] [Google Scholar]
- 6.Poretti D, Felleisen E, Grimm F, et al. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg 1999;60:193–8. [DOI] [PubMed] [Google Scholar]
- 7.Reiter-Owona I, Gruner B, Frosch M, et al. Serological confirmatory testing of alveolar and cystic echinococcosis in clinical practice: results of a comparative study with commercialized and in-house assays. Clin Lab 2009;55:41–8. [PubMed] [Google Scholar]
- 8.Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis 2010;23:505–12. doi:10.1097/QCO.0b013e32833d7516 [DOI] [PubMed] [Google Scholar]
- 9.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica 2010;114:1–16. doi:10.1016/j.actatropica.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Stojkovic M, Gottstein B, Junghanss T. Echinococcosis. In: Farrar J, Hotez PJ, Junghanss T, et al. eds Manson's tropical diseases. 23rd edn Elsevier Saunders, 2014:795–819. [Google Scholar]
- 11.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol 2011;151:7–10.e1. doi:10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torgerson PR, Schweiger A, Deplazes P, et al. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol 2008;49:72–7. doi:10.1016/j.jhep.2008.03.023 [DOI] [PubMed] [Google Scholar]