Abstract
Background
Pegylated interferon and ribavirin (PEG-IFN+RBV) may be more cost-effective than direct-acting antivirals in resource-limited settings. Current literature suggests sustained virological response (SVR) in hepatitis C virus genotype 4 (HCV-4) is similar to genotype 1 (HCV-1), but worse than 2 and 3 (HCV-2/3). However, few studies have compared treatment response between these groups and these have been limited by small sample sizes with heterogeneous designs. We performed a meta-analysis of SVR predictors in HCV-4 versus HCV-1, 2, and 3 patients treated with PEG-IFN+RBV.
Methods
In November 2013, we searched for ‘genotype 4’ in MEDLINE/EMBASE databases and scientific conferences. We included original articles with ≥25 treatment-naïve HCV-4 and comparisons to HCV-1, 2, and/or 3 patients treated with PEG-IFN+RBV. Random effects modelling was used with heterogeneity defined by Cochrane Q-test (p value<0.10) and I2 statistic (>50%).
Results
Five studies with 20 014 patients (899 HCV-4; 12 033 HCV-1; and 7082 HCV-2/3 patients) were included. SVR was 53% (CI 43% to 62%) for HCV-4, 44% (CI 40% to 47%) for HCV-1; and 73% (CI 58% to 84%) for HCV-2/3. SVR with EVR (early virological response) was 75% (CI 61% to 86%) in HCV-4; 64% (CI 46% to 79%) in HCV-1; and 85% (CI 71% to 93%) in HCV-2/3. SVR without EVR was 10% (CI 6% to 17%) for HCV-4; 13% (CI 12% to 15%) for HCV-1; and 23% (CI 16% to 33%) for HCV-2/3.
Conclusions
SVR rates are similar in HCV-4 (∼50%) and HCV-1 (∼40%). Lack of EVR is a good stopping rule for HCV-4 and HCV-1 since only 10% subsequently achieve SVR. In HCV-4 patients with EVR, three-quarters can expect to achieve SVR with PEG-IFN+RBV.
Keywords: HEPATITIS C, GENOTYPE, HCV
Summary box.
What is already known about this subject?
-
▸
There are six major HCV genotypes (HCV-1 to HCV-6), which are geographically distributed and demonstrate variable response to antiviral treatment.
-
▸
While HCV-1, 2, and 3 have been well-represented in large registration trials, data on HCV-4 has been limited.
-
▸
Treatment guidelines recommend the same length of treatment with PEG-IFN+RBV in HCV-4 and HCV-1; however, there is conflicting published data regarding the rate of SVR in HCV-4 compared to HCV-1.
What are the new findings?
-
▸
In our meta-analysis of five studies with a total of 20 014 patients treated with PEG-IFN+RBV, we observed pooled SVR rates of 53% for HCV-4, 44% for HCV-1, and 73% for HCV-2/3.
-
▸
SVR was higher in HCV-2/3 compared to HCV-4 regardless of EVR status.
-
▸
SVR was similar in HCV-1 compared to HCV-4 regardless of EVR status.
How might it impact on clinical practice in the foreseeable future?
-
▸
With PEG-IFN+RBV, SVR rates of approximately 50% in HCV-4, 40% in HCV-1, and 70% in HCV-2/3 can be expected.
-
▸
Given the high cost of direct-acting agents, our data on patients treated with PEGIFN+RBV may help guide therapy in those who will only have access to IFN-based therapies.
Background
Hepatitis C virus (HCV) is a worldwide health burden affecting approximately 170 million patients globally.1–3 In about 40 000 patients each year, chronic infection leads to progressive liver scarring, end-stage liver disease or hepatocellular carcinoma.4 5 These disease outcomes as well as response to therapy are influenced by HCV genotype.
There are six known HCV genotypes, which are geographically distributed. HCV-1 is the most prevalent worldwide, especially in the USA and Northern Europe, and is responsible for approximately 70% of the global chronic hepatitis C (CHC) population.6 In contrast, HCV-4 is more prominent in Africa and the Middle East, comprising up to 80% of the CHC burden in this region.7
Most registration trials with interferon-based therapies have been conducted in Western countries where HCV-1, 2, and 3 are prevalent, but data on other genotypes, especially HCV-4, is limited.8 9 The goal of HCV treatment is to achieve sustained virological response (SVR), defined as undetectable HCV RNA at 24 weeks after cessation of therapy. While SVR rates have been firmly established in HCV-1, 2 and 3 by landmark clinical trials, the rate of SVR in HCV-4 has been wide-ranging from 28% to 71% based on smaller studies with heterogeneous designs mostly conducted in Africa and Eastern Mediterranean countries.7 10–59
Guidelines recommend the same 48-week treatment duration with PEG-IFN+RBV for HCV-4 and HCV-1, based on the assumption that these genotypes have similar SVR rates. While some studies comparing HCV-4 and HCV-1 have shown no difference in SVR rates between these genotypes,42 43 46 others have shown a trend favouring higher SVR rates for HCV-4 patients compared to HCV-1 patients.14 32 Additional research is needed to better our understanding of HCV-4 and HCV-1 since these two genotypes may be considered as separate entities and ultimately require different treatment considerations.
The aim of our study is to systematically and qualitatively assess treatment predictors and outcomes in studies directly comparing patients with HCV-4 and HCV-1, 2, and/or 3 who were treated with PEG-IFN+RBV.
Methods
Data sources and searches
In November 2013, we performed a literature search in PubMed filtered for MEDLINE-indexed articles with the search term: (‘genotype 4’). Studies in non-English languages were included. We also performed a literature search in EMBASE with the search term: ‘hepatitis c’/exp, and conducted a manual review of abstracts using the search term ‘genotype 4’ for all recent international gastroenterology and liver society meetings held between 2012 and 2013, which included the American Association for the Study of Liver Diseases (AASLD), Asian Pacific Study of the Liver (APASL), Digestive Disease Week (DDW) and European Association for the Study of the Liver (EASL).
Study selection
Inclusion criteria were original studies with a minimum sample size of ≥25 treatment-naïve, HCV-4 and comparison treatment arm of HCV-1, 2, and/or 3 patients, all of whom received treatment with PEG-IFN+RBV. Both prospective controlled trials and retrospective cohort reports were eligible for inclusion. Exclusion criteria were patients coinfected with hepatitis B or D, HIV or other liver diseases. Two of the study authors (BEY and BZ) evaluated the studies independently, and a third author (MHN) re-reviewed these articles. Any discrepancies were resolved by consensus.
Data extraction
The study team developed a data abstraction form for this meta-analysis. Information collected from studies were the following: (1) study characteristics including year published, country of origin, study design, study type (randomised-controlled trial vs observational), practice setting (university or community), and intention-to-treat (ITT) analysis; (2) patient characteristics including age, gender, ethnicity, degree of fibrosis, viral load, and ALT level; (3) treatment predictors including length of treatment (24-weeks compared to 48-weeks), rates of rapid virological response (RVR, defined as undetectable HCV RNA at week 4 of treatment) and early virological response (EVR, defined as at least 2-log 10 reduction of HCV RNA from baseline at week 12 of treatment); (4) rates of SVR (SVR, defined as undetectable HCV RNA at 24 weeks after cessation of treatment).
Statistical analysis
Statistical analyses were performed using random effects modelling (DerSimonian and Laird method) and inverse variance method60 to present pooled event rates (overall SVR rate) with corresponding 95% CIs. Study heterogeneity was assessed using χ2-based Cochrane Q-statistic with p≤0.10 and I2≥50% as per the standards of quality for reporting meta-analysis from the Cochrane handbook.60 For subgroup analyses, ORs and corresponding 95% CIs were performed. Funnel plots of ln[OR] against SE were performed to evaluate for publication bias. One-study removed influence analysis was conducted to identify potential outliers contributing to our pooled estimates. A fixed value of ‘0.5’ was added to all cells of study results tables in studies with zero-cell counts.60 Statistical tests were all two sided. All statistical tests were performed using Comprehensive Meta-Analysis, V.2 (Biostat, Englewood, New Jersey, USA).
Results
Literature search
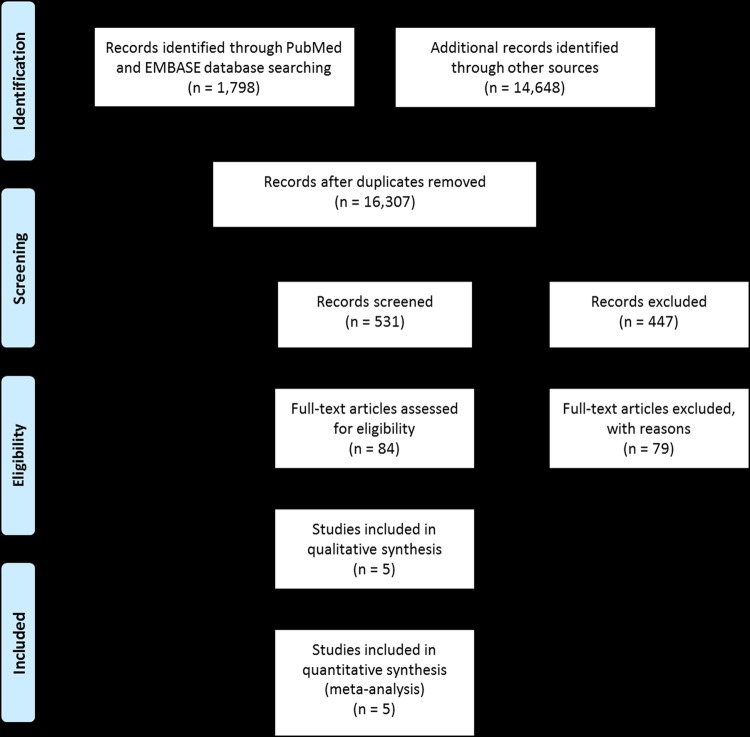
As shown in figure 1, a comprehensive literature review of PubMed and EMBASE identified 1798 studies. Review of scientific conferences held in the past 2 years identified 14 648 abstracts. Based on abstract and article titles, a total of 16 446 studies were not relevant and excluded prior to screening. Eighty-four studies were closely reviewed.7 10–59 61–93 A total of 79 studies were excluded for the following reasons: 45 studies did not have direct comparison arms of HCV-1, 2, and/or 3;7 10–13 15–31 33–39 41 44 45 47–59 14 studies did not have accessible treatment outcomes data;61 62 67 70 72 76 78 79 82 84 85 87 92 93 6 studies were redundant;71 73 75 80 86 91 4 studies were not relevant;63 68 77 88 3 studies included patients coinfected with other conditions, including hepatitis B virus, HIV or other liver diseases;69 81 83 3 studies did not assess treatment-naïve patients;64 65 89 2 studies did not contain original data;74 90 1 study did not meet our minimum sample size requirement of at least 25 HCV patients;66 1 study did not include patients treated for 48 weeks.40 A total of five studies met all eligibility criteria and were included in the primary analysis.14 32 42 43 46
Figure 1.
PRISMA flow diagram of articles identified and screened for inclusion.
Characteristics of included studies and patients
Five full-length articles with a total of 20 014 patients (899 HCV-4; 12 033 HCV-1; and 7082 HCV-2/3 patients) were included in this meta-analysis (table 1). All were observational or non-randomised. Four studies were prospective32 42 43 46 while one was retrospective in design.14 Four of the five studies analysed SVR rates according to ITT.14 32 42 43 Study origins included two from Kuwait,14 32 one from Germany43 and one from Cameroon.46 One study was conducted in 19 countries.42 The majority of patients were male. Mean age ranged from 44.5 to 54.3 years for HCV-4; 47.4 to 53 years for HCV-1; and 46.3 to 51.4 years for HCV-2/3. This analysis only included patients treated with PEG-IFN+RBV.
Table 1.
Characteristics of studies included in primary analysis
| First author, year | Country of origin | Study design | HCV-4 |
HCV-1 |
HCV-2/3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (%) | Age (years) | N | Male (%) | Age (years) | N | Male (%) | Age (years) | N | |||
| Marcellin P et al, 201242 | International (19 countries) | Prospective | 68 | 44.5 | 282 | 52 | 47.4 | 4119 | 60 | 46.3 | 1976 |
| Mauss S et al, 201243 | Germany | Prospective | 76 | Median 41 | 474 | 60 | Median 44 | 7835 | 66 | Not reported | 5062 |
| Al-Enzi SA et al, 201114 | Kuwait | Retrospective | Not reported | Not reported | 51 | Not reported | Not reported | 30 | Not reported | Not reported | 27 |
| Njouom R et al, 200846 | Cameroon | Prospective | 71 | 54.3 | 26 | 82 | 53 | 29 | 86 | 51.4 | 17 |
| Hasan F et al, 200432 | Kuwait | Prospective | 73 | 45 | 66 | 35 | 48 | 20 | Not reported | Not reported | Not reported |
HCV, hepatitis C virus.
SVR rates by genotype
Based on five studies, pooled SVR rate for HCV-4 was 52.7% (CI 43.4% to 61.9%) (Q-statistic=21.04, p<0.001, I2=80.99%) (table 2). Corresponding pooled SVR rates for HCV-1 and HCV-2/3 were 43.7% (CI 40.3% to 47.1%) (Q-statistic=17.696, p=0.001, I2=77.40%) and 72.9% (CI 58.5% to 83.7%) (Q-statistic=190.997, p<0.001, I2=98.43%), respectively. Statistically significant heterogeneity was found in the analysis of each genotype and this may be attributed to variation in the patient characteristics and methodologies among the included studies.
Table 2.
Treatment response in HCV-4 compared to HCV-1 and HCV-2/3
| Treatment response | HCV-4 (n=899) | HCV-1 (n=12 033) | HCV-2/3 (n=7082) |
|---|---|---|---|
| SVR | 53% (CI 43% to 62%) | 44% (CI 40% to 47%) | 73% (CI 58% to 84%) |
| RVR | 39% (CI 35% to 44%) | 25% (CI 24% to 56%) | 76% (CI 71% to 80%) |
| EVR | 72% (CI 64% to 81%) | 59% (CI 58% to 61%) | 91% (CI 89% to 93%) |
| +EVR/+SVR | 75% (CI 61% to 86%) | 64% (CI 46% to 79%) | 85% (CI 71% to 93%) |
| −EVR/+SVR | 10% (CI 6% to 17%) | 13% (CI 12% to 15%) | 23% (CI 16% to 33%) |
EVR, early virological response; HCV, hepatitis C virus; RVR, rapid virological response; SVR, sustained virological response.
SVR rates in HCV-4 and HCV-1 were comparable, detecting no statistically significant difference, OR 1.16 (CI 0.92 to 1.48, p=0.21) (Q-statistic=6.264, p=0.18, I2=36.14%). In contrast, the rate of SVR in HCV-2/3 was higher than HCV-4, OR 2.74 (CI 1.55 to 4.85, p=0.01) (Q-statistic=21.046, p<0.001, I2=85.75%) as well as HCV-1, OR 3.33 (CI 1.89 to 5.87, p<0.001) (Q-statistic=90.944, p<0.001, I2=96.70%).
Treatment predictors of SVR by genotype
Rapid virological response
Two studies provided data on RVR for a total of 12 982 patients.42 43 Pooled rates of RVR were 39.3% (CI 35.3% to 43.5%) (Q-statistic=0.452, p=0.501, I2=0.00%) in 552 patients with HCV-4; 24.8% (CI 23.9% to 25.8%) (Q-statistic=0.131, p=0.717, I2=0.00%) in 8173 patients with HCV-1; and 75.9% (CI 71.2% to 80.0%) (Q-statistic=11.735, p=0.001, I2=91.48%) in 4257 patients with HCV-2/3.
Direct comparison of RVR rates detected statistically significant differences favouring HCV-2/3 over HCV-4, OR 4.85 (CI 3.40 to 6.94, p<0.001) (Q-statistic=3.732, p=0.053, I2=73.21%), and HCV-4 over HCV-1, OR 1.96 (CI 1.64 to 2.35, p<0.001) (Q-statistic=0.295, p=0.59, I2=0.00%).
Early virological response
In four studies, pooled rates of EVR were 72.8% (CI 63.5% to 80.5%) for 695 patients with HCV-4 and 91.4% (CI 88.8% to 93.4%) for 5568 patients with HCV-2/3.14 42 43 46 In three studies, pooled rate of EVR was 59.4% (CI 57.9% to 60.9%) for 4178 patients with HCV-1.14 42 46
Direct comparison of EVR rates detected a statistically significant difference favouring HCV-2/3 over HCV-4, OR 3.53 (CI 1.81 to 6.87, p<0.001) (Q-statistic=17.820, p<0.001, I2=83.16%), but did not detect any statistically significant difference between HCV-4 and HCV-1, OR 1.46 (CI 0.88 to 2.43) (Q-statistic=3.119, p=0.21, I2=35.88%).
SVR in patients who achieved EVR
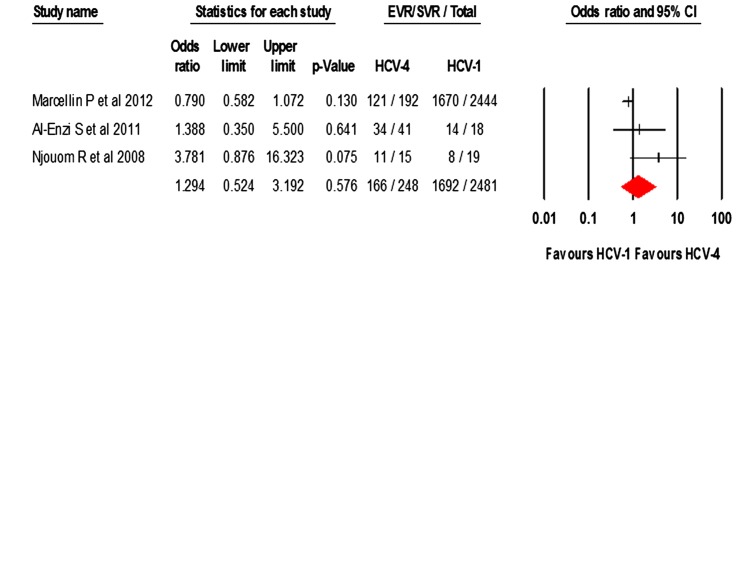
Regarding the rate of SVR in patients who achieved EVR, three studies14 42 46 provided data on HCV-1 and HCV-2/3 while four studies14 32 42 46 provided data on HCV-4. The pooled rates of SVR in those who achieved EVR were 75.4% (CI 61.4% to 85.6%) in 300 HCV-4 patients; 64% (CI 46.4% to 78.6%) in 2481 HCV-1 patients; and 85.2% (CI 71.8% to 92.9%) in 1876 HCV-2/3 patients.
As shown in figure 2, direct comparison of SVR rates detected a statistically significant difference favouring HCV-2/3 over HCV-4 in patients who achieved EVR, OR 2.33 (CI 1.71 to 3.16, p<0.001) (Q-statistic=0.442, p=0.802, I2=0.00%). No statistically significant difference was found between HCV-4 and HCV-1 patients who reached EVR, OR 1.29 (CI 0.52 to 3.19) (Q-statistic=4.701, p=0.095, I2=57.45%).
Figure 2.
Odds of SVR with EVR in HCV-4 compared to (A) HCV-1 or (B) HCV-2/3. EVR, early virological response; HCV, hepatitis C virus; SVR, sustained virological response.
SVR in patients who did not reach EVR
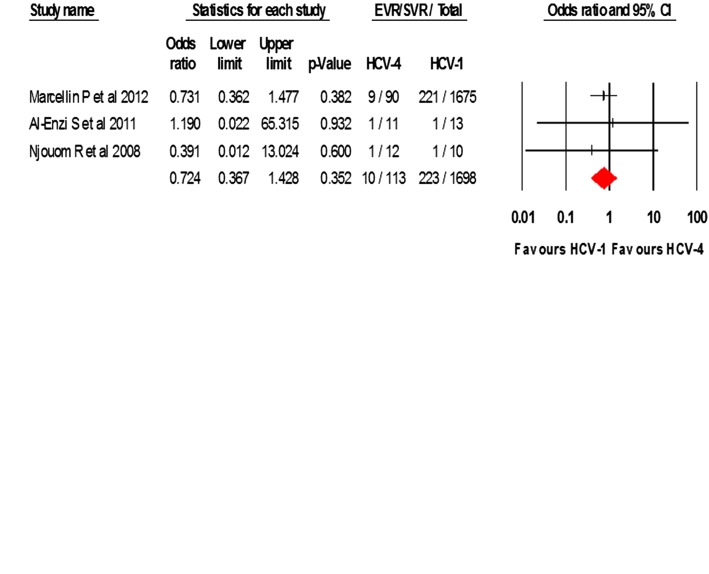
Regarding the rate of SVR in patients who did not reach EVR, four studies14 32 42 46 provided data on HCV-4 while three studies provided data on HCV-1 and HCV-2/3.14 42 46 The pooled rates of SVR in those who did not reach EVR were 10% (CI 5.7% to 16.6%) in 127 HCV-4 patients; 13.1% (CI 11.6% to 14.8%) in 1698 HCV-1 patients; and 22.3% (CI 16.6% to 30.2%) in 146 HCV-2/3 patients.
As shown in figure 3, direct comparison of SVR rates detected a statistically significant difference favouring HCV-2/3 over HCV-4 in patients who did not reach EVR, OR 2.75 (CI 1.28 to 5.92, p=0.01) (Q-statistic=0.64, p=0.969, I2=0.00%). No statistically significant difference was found between HCV-4 and HCV-1 patients who did not reach EVR, OR 0.72 (CI 0.37 to 1.43) (Q-statistic=0.178, p=0.915, I2=0.00%).
Figure 3.
Odds of SVR without EVR in HCV-4 compared to (A) HCV-1 or (B) HCV-2/3. EVR, early virological response; HCV, hepatitis C virus; SVR, sustained virological response.
Discussion
In our primary analysis, we included five studies with a total of 20 014 patients (899 HCV-4; 12 033 HCV-1; and 7082 HCV-2/3). We observed pooled SVR rates of 53%, 44%, and 73% in patients with HCV-4, HCV-1 and HCV-2/3, respectively. While SVR rates with HCV-2/3 patients were significantly higher than HCV-4, we found no statistically significant difference between SVR rates with HCV-1 patients compared to HCV-4.
Prior guidelines from EASL in 201394 and AASLD in 20095 recommended dual therapy with PEG-IFN+RBV for HCV-4 carriers. Both societies’ recommendations for response guided therapy combined recommendations for HCV-4 with HCV-1. Beginning in 2011, telaprevir and boceprevir were the first new direct-acting antivirals (DAA) licensed for use in HCV-1. Currently there are several other DAAs available, including sofosbuvir, simeprevir, sofosbuvir/ledipasvir, and paritaprevir/ritonavir/ombitasvir, which are approved for HCV-1 and HCV-4.95–97 With shorter treatment duration and higher potency, triple therapy has significantly improved virological response rates for many HCV-infected individuals. However, this therapeutic option may remain elusive for patients in developing or under-resourced regions who lack access to DAAs. Therefore, dual therapy with PEG-IFN+RBV will likely remain the mainstay of treatment for many CHC patients in developing countries and is still a treatment option in the WHO guidelines.98
Although societies have grouped HCV-4 with HCV-1, there has been conflicting data as some studies showed a trend towards higher SVR rates in HCV-4 compared to HCV-1,14 32 whereas other studies have not demonstrated any significant differences.42 43 46 In our meta-analysis of studies directly comparing HCV-4 and HCV-1 patients, HCV-4 patients had significantly higher rates of RVR (OR 1.96, CI 1.64 to 2.35, p<0.001), but no statistically significant difference in SVR rates (53% vs 44%, OR 1.16 (CI 0.92 to 1.48, p=0.21)). Additionally, when compared to patients with HCV-2/3, patients with HCV-4 and HCV-1 both had lower rates of RVR, EVR and SVR.
Our findings are similar to results from large randomised controlled trials of PEG-IFN+RBV treatment.8 9 However, the generalisability of these previous trials has been limited due to the paucity of HCV-4, which represented less than 41 patients or 3% of the total subjects randomised to treatment with PEG-IFN+RBV. In contrast, the current meta-analysis includes 899 HCV-4 patients from studies, which also provided comparison data for other treated genotype(s). To our knowledge, this is the first meta-analysis comparing virological response in HCV-4 to HCV-1 and HCV-2/3 patients treated with PEG-IFN+RBV. Subgroup analysis included only observational or non-randomised studies since no large RCTs with sufficient numbers of HCV-4, HCV-1 and/or HCV-2/3 patients have been performed. In the absence of any large RCTs comparing these genotypes, this meta-analysis provides the largest sample of HCV-4, HCV-1 and HCV-2/3 patients with a direct comparison of their SVR rates.
In the secondary analysis of treatment predictors, RVR rates were 39.3% in HCV-4, 24.8% in HCV-1 and 75.9% in HCV-2/3. Prior estimates of RVR in all genotypes have ranged widely: 15%–60% in HCV-4,7 16 17 24 34 38 42–44 58 65 99 20%–45% in HCV-1,99–103 and 60%–95% in HCV-2/3,99 101 102 104–107 which may be due in part to demographic or epidemiological factors as well as the distribution of advantageous IL28B phenotypes, which were not assessed by the studies included in this analysis. In direct comparison, RVR was favoured in HCV-2/3 over HCV-4, OR 4.85 (CI 3.40 to 6.94, p<0.001) and HCV-4 over HCV-1, OR 1.96 (CI 1.64 to 2.35, p<0.001), a finding previously reported in the current literature.
With both AASLD and EASL guidelines, EVR is especially important for response-guided therapy as failure to achieve EVR is used to recommend discontinuation of therapy at week 12 of therapy. In our study, overall EVR rates were 72.8% in HCV-4, 59.4% in HCV-1, and 91.4% in HCV-2/3. SVR rates in those who achieved EVR were 75.4% in HCV-4, 64% in HCV-1 and 85.2% in HCV-2/3. In contrast, SVR rates in those who did not reach EVR were 10% in HCV-4, 13.1% in HCV-1, and 22.3% in HCV-2/3. Failure to achieve EVR was a negative predictor of response to treatment for all genotypes.
As with HCV-1, lack of EVR is a good stopping rule for HCV-4 given the low SVR rate in those without EVR in the current meta-analysis and supports the societal recommendations that group HCV-4 with HCV-1. In addition, continuing therapy in HCV-4 patients who achieve EVR is also important as approximately three-quarter of HCV-4 patients treated with PEG-IFN+RBV achieved EVR and of those patients, three-quarters achieved SVR.
Although our meta-analysis is the first to quantitatively evaluate treatment predictors and outcomes in such a large population of patients with HCV-4, HCV-1, or HCV-2/3, this study was not without its limitations. Data on newer, all-oral regimens was not included. Additionally, only a small number of studies with a significant amount of heterogeneity were available for this analysis, which limited our ability to perform any additional subgroup analyses or detect publication bias. Our comprehensive literature search yielded only observational or non-randomised studies. Although randomised controlled trials are the reference standard, the studies included in this analysis may be more generalisable to routine clinic settings of heterogeneous patient populations.
In summary, in this meta-analysis of PEG-IFN+RBV treated patients, we observed a higher SVR rate in HCV-2/3 (∼70%) and comparable SVR rates in HCV-4 (∼50%) and HCV-1 (∼45%). As in HCV-1, failure to achieve EVR may be a good stopping rule for patients with HCV-4. Considering the lower SVR rates in HCV-4 and HCV-1, HCV-4 patients infected with these genotypes may significantly benefit from the recently FDA-approved triple therapies, where available. In more resource limited regions, given the higher rate of RVR (39%) and EVR in HCV-4 patients (73%) compared to HCV-1 patients (25% and 59%, respectively) and high SVR in those with EVR (75%), a response-guided approach using PEG IFN+RBV is probably still a reasonable option for the majority of patients. As hepatitis C treatment rapidly evolves, future trials may benefit from use of more diverse patient populations to improve the representation of less common genotypes.
Footnotes
Contributors: MHN was guarantor of the article. BEY was involved in the study design, data collection, data analysis and interpretation and drafting of the manuscript. BZ and NHN were involved in the study design, data collection, data analysis and interpretation and participation in the drafting of the manuscript. PV and CRW were involved in the data collection and critical review of the manuscript. DL and GAL were involved in the data interpretation and critical review of the manuscript. MHN was involved in the study design, data collection, data analysis and interpretation, and critical revision of the manuscript. All authors identified above have critically reviewed the paper and approve the final version of this paper, including the authorship statement.
Funding: This study was funded in part by the NIH National Centre for Research Resources, TL1 training grants, 1TL1RR03197, to Nghia H. Nguyen and Bing Zhang.
Competing interests: MHN has served as a consultant and an advisory board member for Gilead Sciences Inc., Bristol-Myers Squibb, Novartis, and Bayer.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 1999;6:35–47. doi:10.1046/j.1365-2893.1999.6120139.x [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558–67. doi:10.1016/S1473-3099(05)70216-4 [DOI] [PubMed] [Google Scholar]
- 3.Wantuck JM, Ahmed A, Nguyen MH. Review article: the epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment Pharmacol Ther 2014;39:137–47. doi:10.1111/apt.12551 [DOI] [PubMed] [Google Scholar]
- 4.Wise M, Bialek S, Finelli L, et al. . Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology 2008;47:1128–35. doi:10.1002/hep.22165 [DOI] [PubMed] [Google Scholar]
- 5.Ghany MG, Strader DB, Thomas DL, et al. . Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335–74. doi:10.1002/hep.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hnatyszyn HJ. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir Ther 2005;10:1–11. [PubMed] [Google Scholar]
- 7.Kamal SM, Ahmed A, Mahmoud S, et al. . Enhanced efficacy of pegylated interferon alpha-2a over pegylated interferon and ribavirin in chronic hepatitis C genotype 4A randomized trial and quality of life analysis. Liver Int 2011;31:401–11. doi:10.1111/j.1478-3231.2010.02435.x [DOI] [PubMed] [Google Scholar]
- 8.Fried MW, Shiffman ML, Reddy KR, et al. . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–82. doi:10.1056/NEJMoa020047 [DOI] [PubMed] [Google Scholar]
- 9.Manns MP, McHutchison JG, Gordon SC, et al. . Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–65. doi:10.1016/S0140-6736(01)06102-5 [DOI] [PubMed] [Google Scholar]
- 10.Abdo AA, Al-Ahdal MN, Khalid SS, et al. . IL28B polymorphisms predict the virological response to standard therapy in patients with chronic hepatitis C virus genotype 4 infection. Hepatol Int 2013;7:533–8. doi:10.1007/s12072-013-9421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afifi MT, El-Gohary A. Evaluation of the efficacy and safety of pegylated interferon (alpha)2a 160 mg (reiferon retard) and ribavirin combination in chronic HCV genotype 4 patients. J Gastroenterol Hepatol 2010;25(Suppl 2):A95 doi:10.1111/j.1440-1746.2009.06211.x [Google Scholar]
- 12.Ahmed MM, Abdel-Salam OME, Mohammed NA, et al. . Oxidative status and the response to pegylated-interferon alpha2a plus ribavirin in chronic genotype 4 HCV hepatitis. EXCLI J 2013;12:605–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ashgar HI, Khan MQ, Helmy A, et al. . Relationship of interferon-gamma-inducible protein-10 kDa with viral response in patients with various heterogeneities of hepatitis C virus genotype-4. Eur J Gastroenterol Hepatol 2013;25:404–10. [DOI] [PubMed] [Google Scholar]
- 14.Al-Enzi SA, Ismail WA, Alsurayei SA, et al. . Peginterferon alfa-2b and ribavirin therapy in Kuwaiti patients with chronic hepatitis C virus infection. East Mediterr Health J 2011;17:669–78. [PubMed] [Google Scholar]
- 15.Antaki N, Bibert S, Kebbewar K, et al. . IL28B polymorphisms predict response to therapy among chronic hepatitis C patients with HCV genotype 4. J Viral Hepat 2013;20:59–64. doi:10.1111/j.1365-2893.2012.01621.x [DOI] [PubMed] [Google Scholar]
- 16.Asselah T, De Muynck S, Broet P, et al. . IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol 2012;56:527–32. doi:10.1016/j.jhep.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 17.De Nicola S, Aghemo A, Rumi MG, et al. . Interleukin 28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis C genotype 4. Hepatology 2012;55:336–42. doi:10.1002/hep.24683 [DOI] [PubMed] [Google Scholar]
- 18.Derbala M, Amer A, Bener A, et al. . Pegylated interferon-alpha 2b-ribavirin combination in Egyptian patients with genotype 4 chronic hepatitis. J Viral Hepat 2005;12:380–5. doi:10.1111/j.1365-2893.2005.00604.x [DOI] [PubMed] [Google Scholar]
- 19.Derbala M, Rizk N, Shebl F, et al. . Interleukin-28 and hepatitis C virus genotype-4: treatment-induced clearance and liver fibrosis. World J Gastroenterol 2012;18:7003–8. doi:10.3748/wjg.v18.i47.7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derbala M, Rizk NM, Al-Kaabi S, et al. . The predictive value of IL28B rs12979860, rs11881222 and rs8099917 polymorphisms and IP-10 in the therapeutic response of Egyptian genotype 4 patients. Virology 2013;444:292–300. doi:10.1016/j.virol.2013.06.025 [DOI] [PubMed] [Google Scholar]
- 21.Derbala MF, Amer AM, Almohanadi M, et al. . Hepatitis C virus genotype 4 with normal transaminases: histological changes, schistosomiasis and response to treatment. J Viral Hepat 2011;18:e258–262. doi:10.1111/j.1365-2893.2010.01403.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derbala MF, El Dweik NZ, Al Kaabi SR, et al. . Viral kinetic of HCV genotype-4 during pegylated interferon alpha 2a: ribavirin therapy. J Viral Hepat 2008;15:591–9. doi:10.1111/j.1365-2893.2008.00988.x [DOI] [PubMed] [Google Scholar]
- 23.El Khayat HR, Fouad YM, Ahmad EA, et al. . Hepatitis C virus (genotype 4)-associated mixed cryoglobulinemia vasculitis: effects of antiviral treatment. Hepatol Int 2012;6:606–12. [DOI] [PubMed] [Google Scholar]
- 24.El Khayat HR, Fouad YM, El Amin H, et al. . A randomized trial of 24 versus 48 weeks of peginterferon alpha-2a plus ribavirin in Egyptian patients with hepatitis C virus genotype 4 and rapid viral response. Trop Gastroenterol 2012;33:112–17. doi:10.7869/tg.2012.27 [DOI] [PubMed] [Google Scholar]
- 25.El Makhzangy H, Esmat G, Said M, et al. . Response to pegylated interferon alfa-2a and ribavirin in chronic hepatitis C genotype 4. J Med Virol 2009;81:1576–83. doi:10.1002/jmv.21570 [DOI] [PubMed] [Google Scholar]
- 26.El Raziky M, Fathalah WF, El-Akel WA, et al. . The Effect of Peginterferon Alpha-2a vs. Peginterferon Alpha-2b in Treatment of Naive Chronic HCV Genotype-4 Patients: A Single Centre Egyptian Study. Hepat Mon 2013;13:e10069 doi:10.5812/hepatmon.10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Shamy A, Shoji I, El-Akel W, et al. . NS5A sequence heterogeneity of hepatitis C virus genotype 4a predicts clinical outcome of pegylated-interferon-ribavirin therapy in Egyptian patients. J Clin Microbiol 2012;50:3886–92. doi:10.1128/JCM.02109-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskander EF, Abd-Rabou AA, Yahya SM, et al. . Does interferon and ribavirin combination therapy ameliorate growth hormone deficiency in HCV genotype-4 infected patients? Clin Biochem 2012;45:3–6. doi:10.1016/j.clinbiochem.2011.08.1145 [DOI] [PubMed] [Google Scholar]
- 29.Esmat G, Fattah SA. Evaluation of a novel pegylated interferon alpha-2a (Reiferon Retard(registered trademark)) in Egyptian patients with chronic hepatitis C—genotype 4. Dig Liver Dis Suppl 2009;3:17–19. doi:10.1016/S1594-5804(09)60011-5 [Google Scholar]
- 30.Farag RE, Arafa MM, El-Etreby S, et al. . Human leukocyte antigen class I alleles can predict response to pegylated interferon/ribavirin therapy in chronic hepatitis C Egyptian patients. Arch Iran Med 2013;16:68–73. [PubMed] [Google Scholar]
- 31.Gad RR, Males S, El Makhzangy H, et al. . Predictors of a sustained virological response in patients with genotype 4 chronic hepatitis C. Liver Int 2008;28:1112–19. doi:10.1111/j.1478-3231.2008.01750.x [DOI] [PubMed] [Google Scholar]
- 32.Hasan F, Asker H, Al-Khaldi J, et al. . Peginterferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C genotype 4. Am J Gastroenterol 2004;99:1733–7. doi:10.1111/j.1572-0241.2004.40077.x [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim M, Gomaa W, Ibrahim Y, et al. . Nitric oxide levels and sustained virological response to pegylated-interferon alpha2a plus ribavirin in chronic HCV genotype 4 hepatitis: A prospective study. J Gastrointestin Liver Dis 2010;19:387–92. [PubMed] [Google Scholar]
- 34.Kamal SM, El Kamary SS, Shardell MD, et al. . Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic response. Hepatology 2007;46:1732–40. doi:10.1002/hep.21917 [DOI] [PubMed] [Google Scholar]
- 35.Kamal SM, El Tawil AA, Nakano T, et al. . Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut 2005;54:858–66. doi:10.1136/gut.2004.057182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karatapanis S, Dimitroulopoulos D, Papastergiou V, et al. . Hepatitis C genotype 4 response rate to pegylated interferon A2A or A2B and ribavirin is similar between caucasians and egyptian patients. Eur J Intern Med 2011;22(Suppl 1):S47 doi:10.1016/S0953-6205(11)60192-1 [Google Scholar]
- 37.Khairy M, Fouad R, Mabrouk M, et al. . The impact of interleukin 28b gene polymorphism on the virological response to combined pegylated interferon and ribavirin therapy in chronic HCV genotype 4 infected Egyptian patients using data mining analysis. Hepat Mon 2013;13:e10509 doi:10.5812/hepatmon.10509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khattab M, Eslam M, Sharwae MA, et al. . Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol 2010;105:1970–7. doi:10.1038/ajg.2010.110 [DOI] [PubMed] [Google Scholar]
- 39.Khattab MA, Eslam M, Shatat M, et al. . Changes in adipocytokines and insulin sensitivity during and after antiviral therapy for hepatitis C genotype 4. J Gastrointestin Liver Dis 2012;21:59–65. [PubMed] [Google Scholar]
- 40.Lopez-Alonso G, Agreda M, Devesa MJ, et al. . [Results of the treatment of chronic hepatitis C genotype 4—a comparative analysis with genotype 1]. Rev Esp Enferm Dig 2008;100: 208–11. doi:10.4321/S1130-01082008000400003 [DOI] [PubMed] [Google Scholar]
- 41.Mahmoud M, El-Tokhy S, El-Lebedy D, et al. . Peripheral blood lymphocytes’ DNA damage in different treatment outcomes of chronic viral C hepatitis genotype 4 infection. J Med Sci 2013;13:353–9. doi:10.3923/jms.2013.353.359 [Google Scholar]
- 42.Marcellin P, Cheinquer H, Curescu M, et al. . High sustained virologic response rates in rapid virologic response patients in the large real-world PROPHESYS cohort confirm results from randomized clinical trials. Hepatology 2012;56:2039–50. doi:10.1002/hep.25892 [DOI] [PubMed] [Google Scholar]
- 43.Mauss S, Berger F, Vogel M, et al. . Treatment results of chronic hepatitis C genotype 5 and 6 infections in Germany. Z Gastroenterol 2012;50:441–4. doi:10.1055/s-0031-1282072 [DOI] [PubMed] [Google Scholar]
- 44.Monis A, Ali Monis A, Al Swaff R. Virologic response at week 8 of combined treatment as a predictor of sustained virologic response in non rapid virologic response, chronic HCV genotype 4 infected patients. Egypt J Med Hum Genet 2012;13:331–5. doi:10.1016/j.ejmhg.2012.03.002 [Google Scholar]
- 45.Moucari R, Ripault MP, Martinot-Peignoux M, et al. . Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut 2009;58:1662–9. doi:10.1136/gut.2009.185074 [DOI] [PubMed] [Google Scholar]
- 46.Njouom R, Sartre MT, Timba I, et al. . Efficacy and safety of peginterferon alpha-2a/ribavirin in treatment-naive Cameroonian patients with chronic hepatitis C. J Med Virol 2008;80:2079–85. doi:10.1002/jmv.21319 [DOI] [PubMed] [Google Scholar]
- 47.Omran MH, Ibrahim NE, Youssef SS, et al. . Relation of interleukin-1beta gene to treatment response in chronic patients infected with HCV genotype 4. J Infect Dev Ctries 2013;7:851–8. doi:10.3855/jidc.3823 [DOI] [PubMed] [Google Scholar]
- 48.Papastergiou V, Dimitroulopoulos D, Skorda L, et al. . Predictors of sustained virological response in Greek and Egyptian patients with hepatitis C genotype 4: does ethnicity matter? J Med Virol 2012;84:1217–23. doi:10.1002/jmv.23324 [DOI] [PubMed] [Google Scholar]
- 49.Pasha HF, Radwan MI, Hagrass HA, et al. . Cytokines genes polymorphisms in chronic hepatitis C: impact on susceptibility to infection and response to therapy. Cytokine 2013;61:478–84. doi:10.1016/j.cyto.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 50.Ragheb MM, Nemr NA, Kishk RM, et al. . Strong prediction of virological response to combination therapy by IL28B gene variants rs12979860 and rs8099917 in chronic hepatitis C genotype 4. Liver Int 2014;34:890–5. doi:10.1111/liv.12321 [DOI] [PubMed] [Google Scholar]
- 51.Saad Y, Ahmed A, Saleh DA, et al. . Adipokines and insulin resistance, predictors of response to therapy in Egyptian patients with chronic hepatitis C virus genotype 4. Eur J Gastroenterol Hepatol 2013;25:920–5. doi:10.1097/MEG.0b013e32835f2726 [DOI] [PubMed] [Google Scholar]
- 52.Saad Y, Said M, Nassar Y, et al. . Microsomal Triglyceride Transfer Protein (MTP) polymorphisms as marker for prediction of response to antiviral therapy in Egyptian patients with chronic HCV genotype 4. Hepatology 2012;56(Suppl 1):1005A. [Google Scholar]
- 53.Shahin Y, Metwally MA, Shaheen W, et al. . A scoring model for prediction of relapse among chronic HCV genotype 4 patients treated with peg interferon and ribavirin. J Hepatol 2013;58(Suppl 1):S358–9. doi:10.1016/S0168-8278(13)60875-2 [Google Scholar]
- 54.Shaker O, El-Shehaby A, Fayez S, et al. . Osteopontin gene polymorphisms as predictors for the efficacy of interferon therapy in chronic hepatitis C Egyptian patients with genotype 4. Cell Biochem Funct 2013;31:620–5. [DOI] [PubMed] [Google Scholar]
- 55.Shaker OG, Sadik NA. Polymorphisms in interleukin-10 and interleukin-28B genes in Egyptian patients with chronic hepatitis C virus genotype 4 and their effect on the response to pegylated interferon/ribavirin-therapy. J Gastroenterol Hepatol 2012;27: 1842–9. doi:10.1111/j.1440-1746.2012.07273.x [DOI] [PubMed] [Google Scholar]
- 56.Shiha G, El-Etrby S, Zalata K, et al. . Efficacy of new PEG-interferon a-2a (Reiferon Retard(registered trademark)) plus ribavirin in egyption patients with chronic hepatitis C genotyp 4. Hepatol Int 2010;4:187–8. [Google Scholar]
- 57.Stauber RE, Scherzer T, Putz-Bankuti CM, et al. . Baseline vitamin D levels do not influence SVR in patients with chronic HCV genotype 1 or 4 infection undergoing peginterferon/ribavirin treatment. J Hepatol 2011;54(Suppl 1):S468–9. doi:10.1016/S0168-8278(11)61188-4 [Google Scholar]
- 58.Taha AA, El-Ray A, El-Ghannam M, et al. . Efficacy and safety of a novel pegylated interferon alpha-2a in Egyptian patients with genotype 4 chronic hepatitis C. Can J Gastroenterol 2010;24:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urquijo JJ, Diago M, Boadas J, et al. . Safety and efficacy of treatment with pegylated interferon alpha-2a with ribavirin in chronic hepatitis C genotype 4. Ann Hepatol 2013;12:30–5. [PubMed] [Google Scholar]
- 60.Deeks JJ, Higgins JPT, Altman DG, eds. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from www.cochrane-handbook.org [Google Scholar]
- 61.Abdel-Rahman M, Saad Y, El-Raziky M, et al. . Hepatitis C genotype 4 with normal transaminases: correlation with fibrosis and response to treatment, a cohort Egyptian study of 4277 patients. Clin Res Hepatol Gastroenterol 2013;37:479–84. doi:10.1016/j.clinre.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 62.Akbar HO, Al Ghamdi A, Qattan F, et al. . Chronic hepatitis C in Saudi Arabia: three years local experience in a university hospital. Hepat Mon 2012;12:e6178 doi:10.5812/hepatmon.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al Ali J, Owayed S, Al-Qabandi W, et al. . Pegylated interferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C genotype 4 in adolescents. Ann Hepatol 2010;9:156–60. [PubMed] [Google Scholar]
- 64.Al Ashgar H, Helmy A, Khan MQ, et al. . Predictors of sustained virological response to a 48-week course of pegylated interferon alfa-2a and ribavirin in patients infected with hepatitis C virus genotype 4. Ann Saudi Med 2009;29:4–14. doi:10.4103/0256-4947.51816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al Ashgar HI, Khan MQ, Al-Ahdal M, et al. . Hepatitis C genotype 4: genotypic diversity, epidemiological profile, and clinical relevance of subtypes in Saudi Arabia. Saudi J Gastroenterol 2013;19:28–33. doi:10.4103/1319-3767.105920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Ali J, Siddique I, Varghese R, et al. . Pegylated interferon-alpha2b plus ribavirin for the treatment of chronic hepatitis C virus genotype 4 infection in patients with normal serum ALT. Ann Hepatol 2012;11:186–93. [PubMed] [Google Scholar]
- 67.Alfaleh FZ, Alswat K, Helmy A, et al. . The natural history and long-term outcomes in patients with chronic hepatitis C genotype 4 after interferon-based therapy. Liver Int 2013;33:871–83. doi:10.1111/liv.12127 [DOI] [PubMed] [Google Scholar]
- 68.Derbala M, Amer A, Shebl F, et al. . Neutropenia and viral load decline during treatment of hepatitis C virus genotype-4 patients: The paradox of treatment modification. Hepatol Int 2012;6:194. [DOI] [PubMed] [Google Scholar]
- 69.Derbala MF, Al Kaabi SR, El Dweik NZ, et al. . Treatment of hepatitis C virus genotype 4 with peginterferon alfa-2a: impact of bilharziasis and fibrosis stage. World J Gastroenterol 2006;12:5692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diago M, Hassanein T, Rodes J, et al. . Optimized virologic response in hepatitis C virus genotype 4 with peginterferon-alpha2a and ribavirin. Ann Intern Med 2004;140:72–3. doi:10.7326/0003-4819-140-1-200401060-00035 [DOI] [PubMed] [Google Scholar]
- 71.Elefsiniotis IS, Pavlidis C, Dimitroulopoulos D, et al. . Differential viral kinetics in treated genotype 4 chronic hepatitis C patients according to ethnicity. J Viral Hepat 2009;16:738–42. doi:10.1111/j.1365-2893.2009.01134.x [DOI] [PubMed] [Google Scholar]
- 72.Elefsiniotis IS, Pavlidis C, Ketikoglou I, et al. . Patient's age modifies the impact of the proposed predictors of sustained virological response in chronic hepatitis C patients treated with PEG-interferon plus ribavirin. Eur J Intern Med 2008;19:266–70. doi:10.1016/j.ejim.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 73.Elefsiniotis IS, Vezali E, Mihas C, et al. . Predictive value of complete and partial early virological response on sustained virological response rates of genotype-4 chronic hepatitis C patients treated with PEG-interferon plus ribavirin. Intervirology 2009;52:247–51. doi:10.1159/000228548 [DOI] [PubMed] [Google Scholar]
- 74.el-Khattib AA, Abdelhakam SM, Ghoraba DM, et al. . Outcome of antiviral therapy in Egyptian Hepatitis C Virus (HCV) genotype 4 patients with advanced liver fibrosis. Eur J Intern Med 2012;23: e34–5. doi:10.1016/j.ejim.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 75.Fathalla W, El-Akel W, Salama A, et al. . The effect of peginterferon alpha-2a vs. Peginterferon alpha-2B in treatment of naive chronic HCV genotype-4 patients: A cohort egyptian study. Gastroenterology 2012;142(5 Suppl. 1):S939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferenci P, Laferl H, Scherzer TM, et al. . Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology 2008;135:451–8. doi:10.1053/j.gastro.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 77.Elsayed-elbatae H. Could vitamin D supplementation improve response to antiviral treatment for hepatitis C virus genotype 4? Hepatol Int 2013;7(Suppl 1):S343. [Google Scholar]
- 78.Hamdi N, El-Akel W, El-Serafy M, et al. . Transcriptional response of MxA, PKR and SOCS3 to interferon-based therapy in HCV genotype 4-infected patients and contribution of p53 to host antiviral response. Intervirology 2012;55:210–18. doi:10.1159/000327783 [DOI] [PubMed] [Google Scholar]
- 79.Kamal S, Ghoraba D, Nabegh L, et al. . Pegylated interferon alfa-2a vs pegylated interferon alfa-2b, plus ribavirin, for chronic hepatitis C genotype 4 patients: A randomized controlled trial. Hepatology 2009;50(Suppl 4):1025A–6A. [Google Scholar]
- 80.Khattab MA, Abdel-fattah ME, Eslam M, et al. . Hepatic steatosis in genotype 4 chronic hepatitis C patients: implication for therapy. J Clin Gastroenterol 2010;44:707–12. [DOI] [PubMed] [Google Scholar]
- 81.Legrand-Abravanel F, Nicot F, Boulestin A, et al. . Pegylated interferon and ribavirin therapy for chronic hepatitis C virus genotype 4 infection. J Med Virol 2005;77:66–9. doi:10.1002/jmv.20414 [DOI] [PubMed] [Google Scholar]
- 82.Mimidis K, Papadopoulos VP, Elefsiniotis I, et al. . Hepatitis C virus survival curve analysis in naive patients treated with peginterferon alpha-2b plus ribavirin. A randomized controlled trial for induction with high doses of peginterferon and predictability of sustained viral response from early virologic data. J Gastrointestin Liver Dis 2006;15:213–19. [PubMed] [Google Scholar]
- 83.Roulot D, Bourcier V, Grando V, et al. . Epidemiological characteristics and response to peginterferon plus ribavirin treatment of hepatitis C virus genotype 4 infection. J Viral Hepat 2007;14:460–7. doi:10.1111/j.1365-2893.2006.00823.x [DOI] [PubMed] [Google Scholar]
- 84.Shaker O, Ahmed A, Doss W, et al. . MxA expression as marker for assessing the therapeutic response in HCV genotype 4 Egyptian patients. J Viral Hepat 2010;17:794–9. doi:10.1111/j.1365-2893.2009.01241.x [DOI] [PubMed] [Google Scholar]
- 85.Shaker O, Bassiony H, El Raziky M, et al. . Human leukocyte antigen class II alleles (DQB1 and DRB1) as predictors for response to interferon therapy in HCV genotype 4. Mediators Inflamm 2013;2013:392746 doi:10.1155/2013/392746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shaker OG, Eskander EF, Yahya SM, et al. . Genetic variation in BCL-2 and response to interferon in hepatitis C virus type 4 patients. Clin Chim Acta 2011;412:593–8. doi:10.1016/j.cca.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 87.Shiha G, Samir W, Seif S, et al. . Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients with hepatitis C virus genotype 4. Hepatology 2012;56(Suppl 1):1023A. [Google Scholar]
- 88.Taha A, Hasan M, El-Ray A, et al. . Impact of cigarette smoking on the sustained viral response to treatment with pegylated interferon alpha-2a and ribavirin combination in male patients with chronic Hepatitis C Genotype 4. Hepatol Int 2012;6:187. [Google Scholar]
- 89.Velosa J, Serejo F, Bana T, et al. . Chronic hepatitis C treated with peginterferon alfa plus ribavirin in clinical practice. Hepatogastroenterology 2011;58:1260–6. doi:10.5754/hge10239 [DOI] [PubMed] [Google Scholar]
- 90.Zayed N, Awad AB, El-Akel W, et al. . The assessment of data mining for the prediction of therapeutic outcome in 3719 Egyptian patients with chronic hepatitis C. Clin Res Hepatol Gastroenterol 2013;37:254–61. doi:10.1016/j.clinre.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 91.Zayed N, Esmat G, Elakel WA, et al. . Therapeutic outcome in 6198 interferon-naive Egyptian patients with chronic hepatitis C genotype-4: A real experience. Hepatology 2012;56(Suppl 1):1016A. [DOI] [PubMed] [Google Scholar]
- 92.Zeidan A, El-Etreby S, Bahgat M, et al. . Pulmonary changes following the combination therapy of peginterferon alpha-2a and ribavirin for chronic hepatitis C genotype 4 infection. Hepatol Int 2012;6:196. [Google Scholar]
- 93.Zekri AR, Haleem HA, Esmat GE, et al. . Immunomodulators, sFas and Fas-L as potential noninvasive predictors of IFN treatment in patients with HCV genotype-4. J Viral Hepat 2007;14:468–77. doi:10.1111/j.1365-2893.2006.00832.x [DOI] [PubMed] [Google Scholar]
- 94.European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392–420. doi:10.1016/j.jhep.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 95.2013. Sovaldi [package insert] http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf.
- 96.2014. Harvoni [package insert] . http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf.
- 97.2014. Viekira Pak [package insert] http://www.abbvie.com/content/dam/abbviecorp/us/desktop/contentrooms/downloads/ProductFactsheet_ViekiraPak_US.pdf.
- 98.Organization WH. Guidelines for the screening, care, and treatment of persons with hepatitis C infection 2014 [cited 27 Sep 2014]. http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1 [PubMed]
- 99.Rao P, Koshy A, Philip J, et al. . Pegylated interferon alfa-2b plus ribavirin for treatment of chronic hepatitis C. World J Hepatol 2014;6:520–6. doi:10.4254/wjh.v6.i7.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonardi R, Tabone M, Manca A, et al. . Short duration treatment in genotype 1 chronic hepatitis C patients with rapid virologic response to pegylated interferon plus ribavirin. Biomed Pharmacother 2011;65:303–6. doi:10.1016/j.biopha.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 101.Mangia A, Santoro R, Minerva N, et al. . Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med 2005;352:2609–17. doi:10.1056/NEJMoa042608 [DOI] [PubMed] [Google Scholar]
- 102.Yu JW, Wang GQ, Sun LJ, et al. . Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol 2007;22:832–6. doi:10.1111/j.1440-1746.2007.04904.x [DOI] [PubMed] [Google Scholar]
- 103.Yu ML, Dai CY, Huang JF, et al. . Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology 2008;47:1884–93. doi:10.1002/hep.22319 [DOI] [PubMed] [Google Scholar]
- 104.Dalgard O, Bjoro K, Ring-Larsen H, et al. . Pegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology 2008;47:35–42. doi:10.1002/hep.21975 [DOI] [PubMed] [Google Scholar]
- 105.Lagging M, Langeland N, Pedersen C, et al. . Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology 2008;47:1837–45. doi:10.1002/hep.22253 [DOI] [PubMed] [Google Scholar]
- 106.Shiffman ML, Suter F, Bacon BR, et al. . Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med 2007;357:124–34. doi:10.1056/NEJMoa066403 [DOI] [PubMed] [Google Scholar]
- 107.von Wagner M, Huber M, Berg T, et al. . Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology 2005;129:522–7. doi:10.1053/j.gastro.2005.05.008 [DOI] [PubMed] [Google Scholar]