Abstract
Background
Breast cancer is one of the leading causes of cancer-related deaths for women. Numerous studies have shown that single-nucleotide polymorphisms (SNPs) on the ESR1 gene are associated to this disease. However, data and conclusions are inconsistent and controversial.
Material/Methods
To investigate the association between PvuII (rs2234693), XbaI (rs9340799) and P325P (rs1801132) polymorphisms of ESR1 gene with the risk of breast cancer under different population categorizations, we searched multiple databases for data collection, and performed the meta-analysis on a total of 25 case-control studies. Three different comparison models – dominant model, recessive model, and homozygote comparison model – were applied to evaluate the association.
Results
Our results indicated that people with TT+TC or TT genotype were at a greater risk of developing breast cancer than those with CC genotype in the PvuII polymorphism. While for XbaI and P325P polymorphisms, no significance was found using any of the 3 models. Furthermore, the data were also stratified into different subgroups according to the ethnicity (white or Asian) and source of controls (hospital-based or population-based), and separate analyses were conducted to assess the association. The ethnicity subgroup assessment showed that the higher risk of breast cancer for TT genotype of PvuII polymorphism than CC genotype only occurred in Asian people, but not in white populations. For the source-stratified subgroup analysis, significant association suggested that people with TT + TC genotype were at a greater risk of developing breast cancer than those with CC genotype in the hospital-based subgroup.
Conclusions
Thus, this meta-analysis clarified the inconsistent conclusions from previous studies, conducted analyses for the entire population as well as for different subgroups using diverse population categorization strategies, and has the potential to help provide a personalized risk estimate for breast cancer susceptibility.
MeSH Keywords: Estrogen Receptor Modulators; Meta-Analysis; Polymorphism, Genetic
Background
Breast cancer (BC) is the most common malignant tumor for women worldwide [1]. Similar to other cancer types, genetic factors play a central role in the development and progression of breast cancer [2]. Studies show that excessive estrogen from the exogenous source can have pathological consequences in human cell, and result in the alteration of tumors, including the occurrence of breast cancer [3]. Two major types of estrogen receptors (ESRs), named as ESR1 and ESR2, act as the key regulators in controlling the actions of estrogen. The ESR1 gene encodes a transcription factor with an estrogen-binding domain, an activation domain, and an estrogen response element (ERE) DNA-binding domain. By regulating the cell proliferation and differentiation via paracrine mechanism, ESR1 is believed to be tightly associated with breast cancer [4]. Therefore, genetic variations in the ESR1 gene, which can lead to disordered estrogen activity, become a potential risk for breast cancer. Single-nucleotide polymorphisms (SNPs) of ESR1 have been studied in numerous clinical studies. Many association studies on this gene have been confined to 2 SNPs (originally detected with the restriction enzymes PvuII and XbaI [5]), which are located in the first intron of ESR1. The ESR1 PvuII and XbaI polymorphisms have been associated to tumorigenesis and many other diseases [6], involving heterogeneous conclusions. The meta-analysis conducted by Li et al. concluded that the PvuII polymorphism of ESR1 was a risk factor for prostate cancer development [7], while the meta-analysis conducted by Gu et al. found no association between frequencies of the PvuII (C>T) polymorphism and prostate cancer susceptibility, but found a positive correlation between XbaI (A>G) polymorphism and the risk of prostate cancer [8]. A recent study showed that the ESR1 PvuII CC/CT and XbaI GG/GA genotypes could increase susceptibility to systemic lupus erythematosus (SLE) [9]. Several other meta-analyses suggested that the PvuII variant, instead of XbaI, was negatively associated with Alzheimer’s disease (AD) in white populations, especially in southern European people, but not in Asian populations [7,10]. The risk of idiopathic scoliosis was not obviously associated with the ESR1 PvuII or XbaI polymorphism [11]. It has been also frequently reported that the PvuII and XbaI polymorphisms of the ESR1 gene are related to breast cancer [12,13]. Li and Xu reported that ESR1 PvuII (C>T) polymorphism placed pre-menopausal women at risk for breast cancer, but XbaI (A>G) polymorphism is not associated with the risk of breast cancer [14]. P325P polymorphism in the exon 4 of ESR1 gene has been found to be associated with bone mineral density in post-menopausal women [15]. Korean women carrying both the ESR1 P325P CC and CDK7 Ex2-28C>T (rs2972388) TT genotypes have been shown to be at increased breast cancer risk [16]. However, because of the heterogeneous of data sources and analysis methods, the conclusions in many of these studies were inconsistent and controversial. Although 2 studies have been conducted on this issue, both of them have some drawbacks. Specifically, Li et al. narrowed the population to Asian women [14]. Hu et al. focused on some of SNPs in ESR1, but SNPs like P325P, which is also associated with the risk of breast cancer, was not included in their articles [17]. In this study, we performed an updated meta-analysis by involving as many data as possible from published studies, to provide a more precise estimation of the potential association between ESR1 PvuII, XbaI, and P325P polymorphisms and the risk of breast cancer. We collected all related studies from online databases to assess the association between 3 SNPs on ESR1 and breast cancer susceptibility. In addition, the analyses were conducted for the entire population, as well as for different subgroups using diverse population categorization strategies.
Material and Methods
Search strategy
We performed an online search of PubMed, Elsevier, Science Direct, Karger, Web of Science, Wiley Online Library, and Springer databases for eligible studies on the association between ESR1 PvuII, XbaI, and P325P polymorphisms with breast cancer susceptibility. The related terms, including“ESR1”, “rs2234693”, “rs9340799”, “rs1801132”, “polymorphism”,“breast cancer” and “BC” were used for searching. The literature search was updated on September 2014.
Data collection
A total of 91 results were found in the literature search. Among these studies, only ones which meet the following criteria were included in our meta-analysis: (i) case-control study that focused on breast cancer and ESR1 gene polymorphisms; (ii) ethnicity and source information was available for case and control; (iii) the diagnosis of breast cancer was confirmed by pathological or histological examination; (v) were published in English language. Studies were excluded when they were: (i) irrelevant articles, duplicated articles; (ii) not case-control study; (iii) genotype frequency information was not accessible; and (iv) meta-analysis, letters, reviews, or editorial articles. As a result, 25 articles were eventually included in the meta-analysis. In our data collection procedure we restricted the time frame from Jan. 2000 to Sept. 2014. Since there was no eligible study prior to 2003, all included studies were published later than 2003. For each article, the following data were collected: the first author’s last name, year of publication, country of origin, ethnicity, source of controls, and the number and frequency of ESR1 PvuII, XbaI, and P325P polymorphisms of cases or controls.
Statistical methods
We used STATA software (version 12.0) for all analyses. The strength of the association between ESR1 polymorphisms and breast cancer susceptibility was assessed using all databases by pooled odds ratios (ORs) with 95% confidence intervals (CIs). Three models were used to evaluate the association: dominant model, recessive model, and homozygote comparison model. We also performed subgroup analyses by ethnicity (white or Asian) and source of controls (hospital-based or population-based). The heterogeneity assumption was assessed by I2 index. Higher I2 indicates more significant heterogeneity. I2=50% represents the dividing point between low and high heterogeneity. When I2≤50%, we assumed that there was no significant heterogeneity between pooled data. Correspondingly, I2>50 was treated as significant heterogeneity. Moreover, based on the I2 index, we chose a different model in analysis: Mantel-Haenszel (M-H) fixed-effects model was used to analyze datasets without significant heterogeneity and DerSimonian and Laird (D-L) random-effects model was used to analyze datasets showing obvious heterogeneity. In our meta-analysis, we used M-H fixed-effects model to test the heterogeneity first, and then chose different models based on the testing results. ORs were calculated with each model within 95% confidence intervals. Forest plots were generated to summarize the results. Potential publication bias was assessed by the Begg’s funnel plots and the Egger’s test. All reported P values were for a two-tailed test.
Results
We performed an online search of multiple databases for eligible studies on the association between ESR1 polymorphisms and breast cancer susceptibility. The procedure of article collection is shown in Figure 1. By excluding irrelevant articles, duplicated articles, and articles not focused on ESR1 polymorphisms and breast cancer, we found a total of 25 case-control studies covering 24 740 cases, and 38 866 controls were eligible [12,13,16–38], main characteristics of which are shown in Table 1. For the ethnicity distribution, there were 8 studies of Asians and 15 studies of whites. For the source of controls, 14 studies used population-based controls and 11 studies used hospital-based controls.
Figure 1.
Flow diagram of studies included in the meta-analysis.
Table 1.
Characteristics of literatures included in the meta-analysis.
| Author | Year | Case | Control | Country | Ethnicity | Source* | Age | Genotyping method | Premeno-pausal proportion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PvuII | CC | CT | TT | Total | CC | CT | TT | Total | |||||||
| Madeira | 2014 | 9 | 49 | 6 | 64 | 8 | 39 | 25 | 72 | Brazil | Caucasian | HB | Median: 55 | PCR-RFLP | Mixed |
| Chattpoadhyay | 2014 | 39 | 164 | 157 | 360 | 62 | 162 | 136 | 360 | India | Caucasian | PB | <50: 44% | PCR-RFLP | 49% |
| Tang | 2013 | 127 | 374 | 293 | 875 | 136 | 375 | 334 | 886 | China | Asian | HB | Mean: 49 | MALDI-TOF | 50% |
| Lu | 2013 | 57 | 228 | 227 | 542 | 137 | 454 | 425 | 1016 | China | Asian | PB | Mean: 49 | PCR-RFLP | N/A |
| Sakoda | 2011 | 93 | 290 | 229 | 612 | 120 | 427 | 327 | 874 | China | Asian | PB | <50: 51.7% | SNaPshot assays | 55% |
| Han | 2011 | 107 | 399 | 353 | 859 | 151 | 402 | 324 | 877 | China | Asian | HB | Mean: 51 | TaqMan | 48% |
| Sonestedt | 2009 | 108 | 273 | 158 | 539 | 218 | 539 | 316 | 1073 | Sweden | Caucasian | PB | Mean: 57 | SEQUENOM | N/A |
| Dunning | 2009 | 938 | 2164 | 1260 | 4362 | 934 | 2296 | 1318 | 4548 | UK | Caucasian | PB | PCR-RFLP | ||
| Ladd | 2008 | 24 | 94 | 72 | 190 | 453 | 1648 | 1602 | 3703 | Netherlands | Caucasian | PB | Mean: 70 | N/A | 0% |
| Gonzalez-Mancha | 2008 | 82 | 209 | 153 | 444 | 150 | 361 | 193 | 704 | Spain | Caucasian | HB | Mean: 58 | PCR-RFLP | |
| Wang | 2007 | 87 | 188 | 117 | 392 | 176 | 393 | 214 | 783 | USA | Caucasian | PB | PCR-MPLA | ||
| Kjaergaard | 2007 | 245 | 613 | 398 | 1256 | 537 | 1225 | 727 | 2489 | Denmark | Caucasian | HB | TaqMan | 25% | |
| Hu | 2007 | 16 | 58 | 39 | 113 | 19 | 45 | 49 | 113 | China | Asian | HB | <50: 73% | PCR-RFLP | 72% |
| Shen | 2006 | 29 | 120 | 98 | 247 | 43 | 124 | 107 | 274 | China | Asian | PB | <50: 79% | PCR-RFLP | |
| Onland-Moret | 2005 | 69 | 150 | 89 | 308 | 96 | 153 | 88 | 337 | Netherlands | Caucasian | PB | Mean: 57 | PCR-RFLP | |
| Modugno | 2005 | 80 | 115 | 53 | 248 | 1272 | 1810 | 819 | 3901 | USA | Caucasian | PB | Mean: 71 | PCR-MPLA | |
| Wedren | 2004 | 268 | 634 | 390 | 1292 | 313 | 651 | 384 | 1348 | Sweden | Caucasian | PB | 50–74 | PCR–RFLP | 0% |
| Shin | 2003 | 35 | 91 | 75 | 201 | 26 | 103 | 61 | 190 | Korea | Asian | HB | PCR-RFLP | ||
| Cai | 2003 | 138 | 516 | 415 | 1069 | 190 | 546 | 430 | 1166 | China | Asian | PB | Mean: 47 | PCR-RFLP | 64% |
| Xbal | GG | GA | AA | Total | GG | GA | AA | Total | |||||||
| Madeira | 2014 | 12 | 47 | 5 | 64 | 14 | 58 | 0 | 72 | Brazil | Caucasian | HB | Median: 55 | PCR-RFLP | Mixed |
| Sakoda | 2011 | 22 | 197 | 395 | 614 | 30 | 277 | 569 | 876 | China | Asian | PB | <50: 51.7% | SNaPshot assays | 55% |
| Dunning | 2009 | 521 | 1967 | 1682 | 4170 | 526 | 2048 | 1873 | 4447 | UK | Caucasian | PB | PCR-RFLP | ||
| Wang | 2007 | 19 | 137 | 237 | 393 | 29 | 299 | 461 | 789 | USA | Caucasian | PB | PCR-MPLA | ||
| Slattery | 2007 | 52 | 235 | 287 | 574 | 61 | 313 | 351 | 725 | USA | Caucasian | PB | PCR-RFLP | ||
| Shen | 2006 | 14 | 84 | 149 | 247 | 21 | 87 | 168 | 276 | China | Asian | PB | <50: 79% | PCR–RFLP | |
| Cai | 2003 | 36 | 497 | 536 | 1069 | 49 | 507 | 610 | 1166 | China | Asian | PB | Mean: 47 | PCR-RFLP | 64% |
| P325P | CC | CG | GG | Total | CC | CG | GG | Total | |||||||
| Han | 2011 | 208 | 441 | 216 | 865 | 232 | 452 | 201 | 885 | China | Asian | HB | Mean: 51 | TaqMan | 48% |
| Ding | 2010 | 241 | 468 | 225 | 934 | 402 | 751 | 391 | 1544 | China | Asian | HB | Taqman | ||
| Jeon | 2009 | 218 | 311 | 217 | 746 | 182 | 288 | 185 | 655 | Korea | Asian | HB | Mean: 47 | MALDI-TOF | |
| Sidding | 2008 | 55 | 23 | 1 | 79 | 56 | 27 | 2 | 85 | Sudan | Caucasian | HB | Mean: 46 | PCR-SSCP | 67% |
| Wang | 2007 | 237 | 137 | 19 | 393 | 461 | 299 | 29 | 789 | USA | Caucasian | PB | PCR-MPLA | ||
| Gallicchio | 2006 | 52 | 31 | 7 | 90 | 794 | 440 | 64 | 1298 | USA | Caucasian | PB | Mean: 54 | TaqMan | 26.2% |
| Fernandez | 2006 | 355 | 156 | 18 | 529 | 356 | 167 | 22 | 545 | Spain | Caucasian | HB | <50: 27% | Taqman | 15% |
HB – hospital-based; PB – population-based.
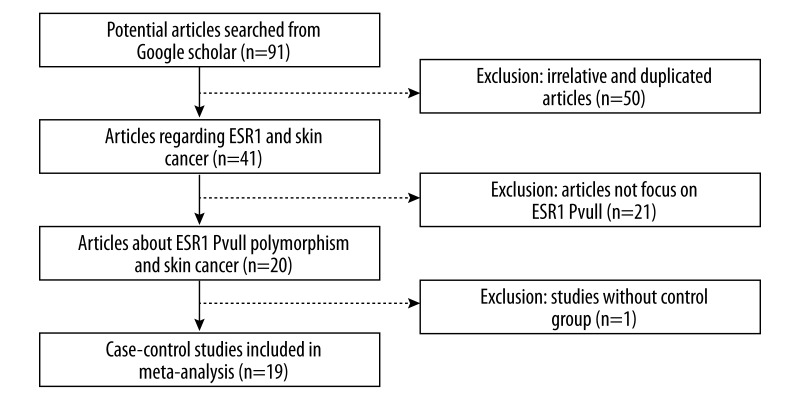
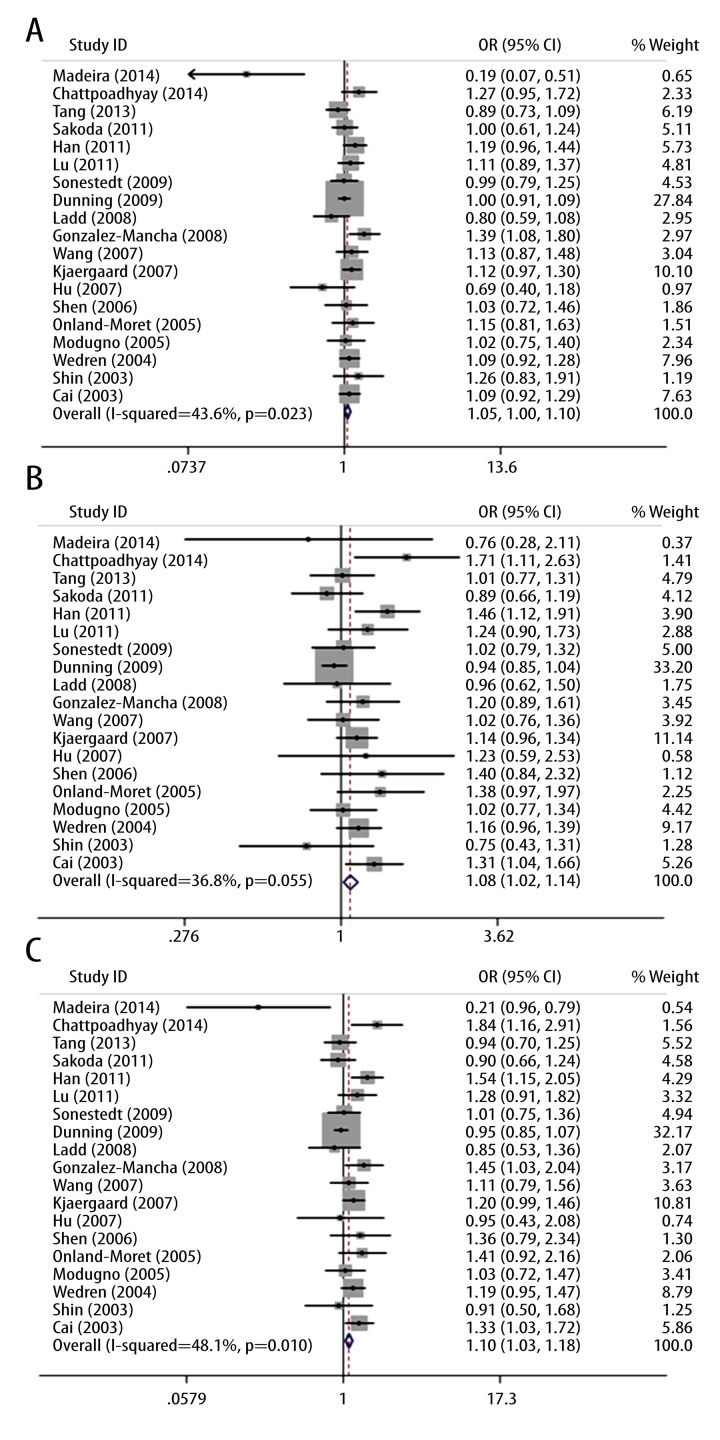
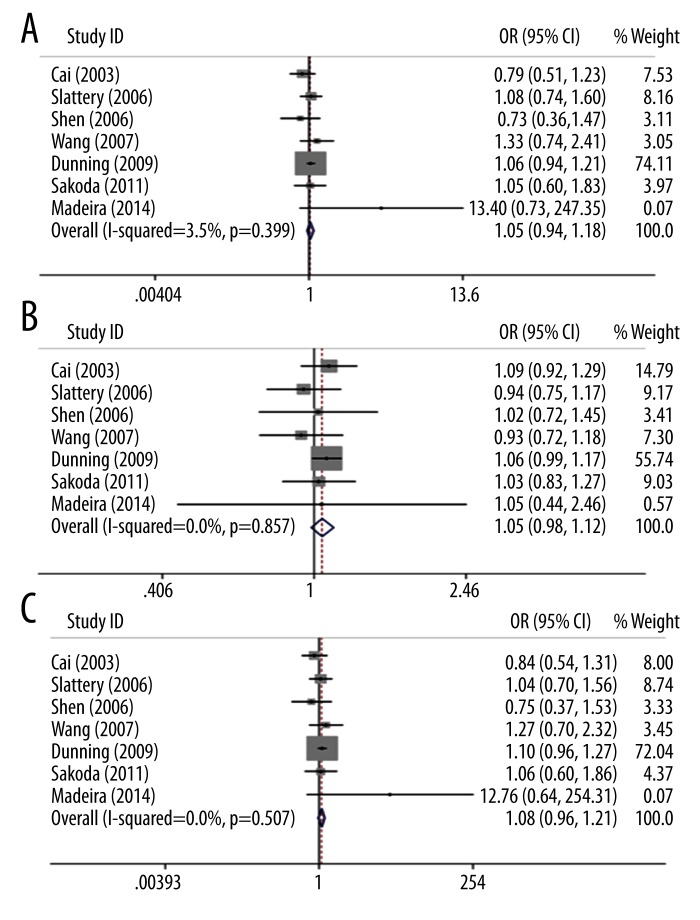
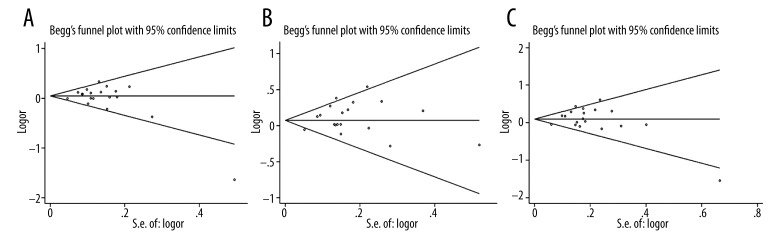
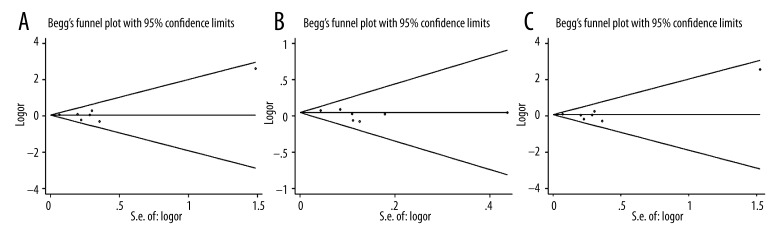
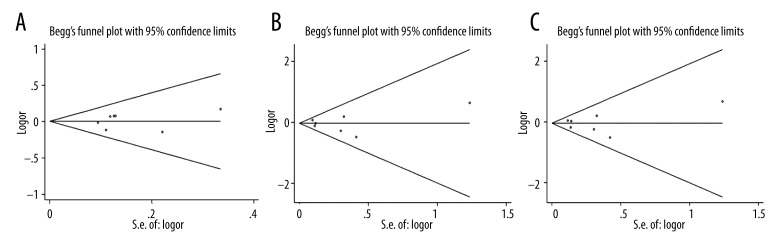
To choose a proper model for the study, we first used the I2 indexes to evaluate the heterogeneity of the data for all 3 SNPs. As shown in Table 2, for PvuII, the I2 indexes ranged from 36% to 48%, and for XbaI and P325P, the I2 values were mostly equal to 0% in all 3 tested genetic models. Statistically significant heterogeneities were only observed for PvuII in dominant model TT vs. (TC+CC) and homozygote model (TT vs. CC). The PvuII polymorphism showed a relative higher I2 index than the other 2 SNPs mainly because more studies were included in the PvuII analysis. Nevertheless, all of the I2 indexes were smaller than 50%, which can be still considered as non-significant heterogeneity. Therefore, the statistical power was still acceptable in our study. Since the I2 indexes were smaller than 50%, M-H fixed-effects models were used for all of the 3 SNPs. The forest plots for PvuII, XbaI, and P325P are shown in Figures 2–4, respectively. Overall, we found significant associations between ESR1 PvuII polymorphism and breast cancer susceptibility in both recessive model ((TT+TC) vs. CC: OR=1.08, 95% CI (1.02–1.14), p=0.01, Figure 2B) and homozygote model (TT vs. CC: OR=1.10, 95% CI (1.03–1.18), p=0.03, Figure 2C), but not in dominant model (TT vs. (TC+CC): OR=1.05, 95% CI (1.00–1.10), p=0.05, Figure 2A). These results indicated that the people with TT or TC genotype were at a greater risk of developing breast cancer than those with CC genotype in the ESR1 PvuII polymorphism. On the other hand, for XbaI and P325P, no significance was found for all 3 models (GG vs. GA+AA: OR=1.05, 95% CI (0.94–1.18), p=0.37, Figure 3A; GG+GA vs. AA: OR=1.05, 95% CI (0.98–1.12), p=0.15, Figure 3B; GG vs. AA: OR=1.08, 95% CI (0.96–1.21), p=0.22, Figure 3C; CC vs. CG+GG: OR=1.01, 95% CI (0.91–1.11), p=0.90, Figure 4A; CC+CG vs. GG: OR=0.97, 95% CI (0.86–1.09), p=0.60, Figure 4B; CC vs. GG: OR=0.96, 95% CI (0.84–1.10), p=0.56, Figure 4C). We found that there was no significant publication bias based on funnel plot for all 3 SNPs (Figures 5–7). Egger’s and Begg’s tests also indicated that there was no obvious bias for publications investigating the relationship of ESR1 polymorphisms with breast cancer risk, as shown in Table 2.
Table 2.
Meta-analysis for all population with Dominant model, Recessive model and homozygote comparison.
| Analysis model | Analysis method | Heterogeneity | OR | Publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | Overall | Lower | Upper | p-value | Begg | Egger | ||
| Pvull | |||||||||
| TT vs. TC+CC | Fixed | 43.6 | 0.02 | 1.05 | 1.00 | 1.10 | 0.05 | 0.48 | 0.47 |
| TT+TC vs. CC | Fixed | 36.8 | 0.06 | 1.08 | 1.02 | 1.14 | 0.01 | 0.94 | 0.15 |
| TT vs. CC | Fixed | 48.1 | 0.01 | 1.10 | 1.03 | 1.18 | 0.03 | 0.68 | 0.62 |
| Xbal | |||||||||
| GG vs. GA+AA | Fixed | 3.5 | 0.40 | 1.05 | 0.94 | 1.18 | 0.37 | 0.76 | 0.73 |
| GG+GA vs. AA | Fixed | 0.0 | 0.86 | 1.05 | 0.98 | 1.12 | 0.15 | 0.55 | 0.19 |
| GG vs. AA | Fixed | 0.0 | 0.51 | 1.08 | 0.96 | 1.21 | 0.22 | 0.76 | 0.87 |
| P325P | |||||||||
| CC vs. CG+GG | Fixed | 0.0 | 0.82 | 1.01 | 0.91 | 1.11 | 0.90 | 0.76 | 0.74 |
| CC+CG vs. GG | Fixed | 0.0 | 0.63 | 0.97 | 0.86 | 1.09 | 0.60 | 0.76 | 0.68 |
| CC vs. GG | Fixed | 0.0 | 0.64 | 0.96 | 0.84 | 1.10 | 0.56 | 1.00 | 0.83 |
Figure 2.
Forest plot of the association between breast cancer risk and ESR1 PvuII polymorphism in all population with respect to (A) dominant model (TT vs. TC+CC), (B) recessive model (TT+TC vs. CC), and (C) homozygote model (TT vs. CC).
Figure 3.
Forest plot of the association between breast cancer risk and ESR1 XbaI polymorphism in all population with respect to (A) dominant model (GG vs. GA+AA), (B) recessive model (GG+GA vs. AA) and (C) homozygote model (GG vs. AA).
Figure 4.
Forest plot of the association between breast cancer risk and ESR1 P325P polymorphism in all population with respect to (A) dominant model (CC vs. CG+GG), (B) recessive model (CC+CG vs. GG) and (C) homozygote model (CC vs. GG).
Figure 5.
Funnel plot of the association between breast cancer risk and ESR1 PvuII polymorphism in all population with respect to (A) dominant model (TT vs. TC+CC), (B) recessive model (TT+TC vs. CC) and (C) homozygote model (TT vs. CC).
Figure 6.
Funnel plot of the association between breast cancer risk and ESR1 XbaI polymorphism in all populations with respect to (A) dominant model (GG vs. GA+AA), (B) recessive model (GG+GA vs. AA), and (C) homozygote model (GG vs. AA).
Figure 7.
Funnel plot of the association between breast cancer risk and ESR1 P325P polymorphism in all populations with respect to (A) dominant model (CC vs. CG+GG), (B) recessive model (CC+CG vs. GG), and (C) homozygote model (CC vs. GG).
Furthermore, we performed subgroup analysis, and results are shown in Tables 3–5. For the subgroup analysis by ethnicity, the I2 indexes for PvuII were larger than 50% in both dominant model and homozygote model for white subgroups, indicating a high heterogeneity in these 2 genetic models (Table 3). Correspondingly, we used the random-effects model for assessing the association in these high-heterogeneity cases, and used the fixed-effects model in other cases. Although the above analysis showed that TT genotype of PvuII had higher risk of breast cancer than CC genotype in all populations, further subgroup assessment demonstrated that only Asians followed this trend (TT vs. CC: OR=1.18, 95% CI (1.04–1.33), p=0.01), while whites did not (TT vs. CC: OR=1.13, 95% CI (0.98–1.29), p=0.09). For the source-stratified subgroup analysis, significant association was observed in the recessive model of hospital-based subgroup (TT+TC vs. CC: OR=1.15, 95% CI (1.03–1.28), p=0.02), suggesting that the people with TT + TC genotype were at a greater risk of developing breast cancer than those with CC genotype in the hospital-based subgroup. On the other hand, similar with the results obtained by using the entire population, analysis on XbaI (Table 4) and P325P polymorphisms (Table 5) showed that there was almost no heterogeneity for any of the subgroup cases, with I2 being equal to 0 for all tests except for XbaI in the white group. In addition, no statistical significant association was found between XbaI and P325P polymorphisms and breast cancer susceptibility in any of the subgroups. Given these results, we conclude that only TT genotype in PvuII was associated with the risk of breast cancer for Asians, and polymorphisms in the other 2 SNPs in ESR1 had little influence on breast cancer.
Table 3.
Subgroup meta-analysis of the association between ESR1 PvuIIpolymorphisms and breast cancer risk.
| Subgroup | TT vs. TC+CC | TT+TC vs. CC | TT vs. CC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | |
| Ethnicity | ||||||||||||
| Caucasian | 58.5 | 0.01 | 1.06 (0.95–1.18) | 0.28 | 31.9 | 0.14 | 1.05 (0.98–1.12) | 0.16 | 56.1 | 0.01 | 1.13 (0.98–1.29) | 0.09 |
| Asian | 10.0 | 0.35 | 1.05 (0.97–1.14) | 0.24 | 38.0 | 0.01 | 1.17 (1.04–1.31) | 0.12 | 33.8 | 0.16 | 1.18 (1.04–1.33) | 0.01 |
| Source | ||||||||||||
| HB | 74.6 | <0.01 | 1.02 (0.83–1.26) | 0.83 | 15.0 | 0.32 | 1.15 (1.03–1.28) | 0.02 | 58.9 | 0.02 | 1.13 (0.90–1.43) | 0.28 |
| PB | 0.0 | 0.77 | 1.04 (0.98–1.10) | 0.23 | 44.2 | 0.05 | 1.05 (0.99–1.12) | 0.13 | 81.3 | <0.01 | 0.78 (0.64–0.94) | 0.01 |
P-value from heterogeneity test;
P-value from OR test.
Table 4.
Subgroup meta-analysis of the association between ESR1 Xbalpolymorphisms and breast cancer risk.
| Subgroup | GG vs. GA+AA | GG+GA vs. AA | GG vs. AA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | |
| Ethnicity | ||||||||||||
| Caucasian | 11.9 | 0.33 | 1.09 (0.96–1.22) | 0.17 | 0.0 | 0.51 | 1.04 (0.97–1.13) | 0.27 | 0.0 | 0.41 | 1.11 (0.98–1.26) | 0.10 |
| Asian | 0.0 | 0.67 | 0.85 (0.62–1.16) | 0.30 | 0.0 | 0.89 | 1.06 (0.94–1.20) | 0.34 | 0.0 | 0.73 | 0.88 (0.64–1.20) | 0.42 |
| Source | ||||||||||||
| PB | 0.0 | 0.66 | 1.04 (0.93–1.17) | 0.46 | 0.0 | 0.76 | 1.05 (0.98–1.12) | 0.15 | 0.0 | 0.75 | 1.07 (0.95–1.20) | 0.27 |
P-value from heterogeneity test;
P-value from OR test;
Analysis on HB is not performed due to the lack of study.
Table 5.
Subgroup meta-analysis of the association between ESR1 P325Ppolymorphisms and breast cancer risk.
| Subgroup | CC vs. CG+GG | CC+CG vs. GG | CC vs. GG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | I2 (%) | ph# | OR (95%CI) | pOR* | |
| Ethnicity | ||||||||||||
| Caucasian | 0.0 | 0.81 | 1.06 (0.90–1.24) | 0.50 | 0.0 | 0.51 | 0.88 (0.60–1.29) | 0.52 | 0.0 | 0.50 | 0.90 (0.61–1.33) | 0.60 |
| Asian | 0.0 | 0.51 | 0.98 (0.87–1.10) | 0.70 | 0.0 | 0.43 | 0.98 (0.87–1.11) | 0.73 | 0.0 | 0.42 | 0.97 (0.84–1.12) | 0.67 |
| Source | ||||||||||||
| HB | 0.0 | 0.72 | 1.00 (0.90–1.12) | 0.98 | 0.0 | 0.67 | 0.99 (0.88–1.11) | 0.83 | 0.0 | 0.64 | 0.98 (0.85–1.13) | 0.81 |
| PB | 0.0 | 0.39 | 1.03 (0.83–1.27) | 0.82 | 0.0 | 0.70 | 0.71 (0.44–1.14) | 0.16 | 0.0 | 0.60 | 0.72 (0.44–1.18) | 0.19 |
P-value from heterogeneity test;
P-value from OR test.
Discussion
In recent years, the association of genetic susceptibility to cancers has drawn more and more attention to the study of polymorphisms of genes involved in tumorigenesis and other diseases. Numerous studies have been conducted to investigate the association between breast cancer susceptibility with 3 SNPs on ESR1: PvuII, XbaI, and P325P. However, because of the heterogeneous of data and methods, the conclusions in these studies are inconsistent and controversial. For example, some studies concluded that the PvuII CC and CT genotype significantly increased the risk of breast cancer [12,13]. Some studies claimed that T allele of PvuII conferred a higher risk of breast cancer [18,24,32]. Other studies showed that ESR1 PvuII polymorphism did not have any significant effect on breast cancer [19,21,25,27,28]. Given these results, it is necessary to perform a meta-analysis to clarify this issue, which can rapidly and effectively increase sample size by combining data of association studies, thus enhancing the statistical power of analysis to estimate the genetic effects. Pooling data from different studies also has the advantage of reducing random errors. With the accumulation of the studies over the years, we performed an updated meta-analysis, by including 3 SNPs of ESR1 and by involving as many data as possible from published studies, to provide a more comprehensive and reliable estimation of the potential association correlation between ESR1 PvuII, XbaI, and P325P polymorphisms and the risk of breast cancer. In the present study, our results showed that genotype TT+TC or TT in ESR1 PvuII were significantly associated with increased breast cancer risk in overall population compared with CC genotype. The ESR1 PvuII polymorphism is intronic and possibly affects receptor function by changing ESR1 expression levels or altering its pre-mRNA splicing. Herrington et al. found that the C allele of PvuII produced a functional binding site for a transcription factor B-Myb, which resulted in significantly increasing transcription of a downstream reporter construct compared to the T allele [39]. This indicates that CC genotype correlates with a higher ESR1 transcriptional level and may explain our observation that TT+TC or TT genotypes were associated with higher breast cancer risk than was CC genotype, but further functional studies are needed to investigate the functions of these alleles.
It is likely that the tumorigenesis of breast cancer is affected by many factors such as age, ethnicity, environment, and other variables. We therefore performed subgroup analysis based on ethnicity of samples. We found only Asians with TT genotype of ESR1 PvuII polymorphism had a higher risk of breast cancer than people with CC genotype, while whites did not show this trend. This may be attributable to genetic heterogeneity among different populations. We could not rule out the possibility of gene-gene interactions or the possibility of linkage disequilibrium between polymorphisms. Further studies of multiple polymorphisms in ESR1 [40,41] or different genes or gene regulators such as microRNAs [42–44] are needed to address this question. In addition, it is also possible that differences in environment and lifestyle between different populations may affect the tumorigenesis of breast cancer.
The heterogeneity between studies could also be from the heterogeneous controls. Therefore, we also conducted a source-stratified subgroup analysis on 14 studies of population-based controls and 11 studies of hospital-based controls, and found significant association in the recessive model of the hospital-based subgroup. Interestingly, we also noticed that TT genotype of ESR1 PvuII polymorphism in the population-based subgroup decreased the risk of breast cancer more than CC genotype. The inconsistent results between different subgroups could come from the possible non-differential misclassification bias because the hospital-based controls might develop more breast cancer than healthy populations in subsequent years. For P325P, only 2 studies were included in subgroup analysis for PB. Given this small sample size, the statistical power is limited. More studies should be conducted to provide a more precise result.
Conclusions
Our study provided a systematic review and updated meta-analysis of genetic association between ESR1 PvuII, XbaI and P325P polymorphisms and the risk of human breast cancer. Using 3 models (dominant model, recessive model, and homozygote comparison model), we confirmed that only PvuII polymorphism was a risk factor for breast cancer susceptibility in the overall population, but not XbaI and P325P SNPs. Moreover, our results suggest that subgroup assessment by ethnicity of samples and source of controls yields results that are different from those using the overall population. Thus, we believe our study clarifies the inconsistent conclusions from previous studies, and will shed some light on future breast cancer-related research.
Footnotes
Source of support: Self financing
Conflict of interest statement
No conflict of interest.
References
- 1.Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet. 2003;33(Suppl):238–44. doi: 10.1038/ng1107. [DOI] [PubMed] [Google Scholar]
- 2.Nathanson KL, Wooster R, Weber BL. Breast cancer genetics: what we know and what we need. Nat Med. 2001;7(5):552–56. doi: 10.1038/87876. [DOI] [PubMed] [Google Scholar]
- 3.Crooke PS, Justenhoven C, Brauch H, et al. Estrogen metabolism and exposure in a genotypic-phenotypic model for breast cancer risk prediction. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1502–15. doi: 10.1158/1055-9965.EPI-11-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103(7):2196–201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill SM, Fuqua SA, Chamness GC, et al. Estrogen receptor expression in human breast cancer associated with an estrogen receptor gene restriction fragment length polymorphism. Cancer Res. 1989;49(1):145–48. [PubMed] [Google Scholar]
- 6.Sun H, Deng Q, Pan Y, et al. Association between estrogen receptor 1 (ESR1) genetic variations and cancer risk: a meta-analysis. J BUON. 2015;20(1):296–308. [PubMed] [Google Scholar]
- 7.Cheng D, Liang B, Hao Y, Zhou W. Estrogen receptor alpha gene polymorphisms and risk of Alzheimer’s disease: evidence from a meta-analysis. Clin Interv Aging. 2014;9:1031–38. doi: 10.2147/CIA.S65921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Z, Wang G, Chen W. Estrogen receptor alpha gene polymorphisms and risk of prostate cancer: a meta-analysis involving 18 studies. Tumour Biol. 2014;35(6):5921–30. doi: 10.1007/s13277-014-1785-4. [DOI] [PubMed] [Google Scholar]
- 9.Cai L, Zhang JW, Xue XX, et al. Meta-analysis of associations of IL1 receptor antagonist and estrogen receptor gene polymorphisms with systemic lupus erythematosus susceptibility. PloS One. 2014;9(10):e109712. doi: 10.1371/journal.pone.0109712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T. Meta-analysis of PvuII, XbaI variants in ESR1 gene and the risk of Alzheimer’s disease: the regional European difference. Neurosci Lett. 2014;574:41–46. doi: 10.1016/j.neulet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Li C, Li M. The estrogen receptor alpha gene (XbaI, PvuII) polymorphisms and susceptibility to idiopathic scoliosis: a meta-analysis. J Orthop Sci. 2014;19(5):713–21. doi: 10.1007/s00776-014-0597-0. [DOI] [PubMed] [Google Scholar]
- 12.Tang LY, Chen LJ, Qi ML, et al. Effects of passive smoking on breast cancer risk in pre/post-menopausal women as modified by polymorphisms of PARP1 and ESR1. Gene. 2013;524(2):84–89. doi: 10.1016/j.gene.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Madeira KP, Daltoe RD, Sirtoli GM, et al. Estrogen receptor alpha (ERS1) SNPs c454-397T>C (PvuII) and c454-351A>G (XbaI) are risk biomarkers for breast cancer development. Mol Biol Rep. 2014;41(8):5459–66. doi: 10.1007/s11033-014-3419-8. [DOI] [PubMed] [Google Scholar]
- 14.Li LW, Xu L. Menopausal status modifies breast cancer risk associated with ESR1 PvuII and XbaI polymorphisms in Asian women: a HuGE review and meta-analysis. Asian Pac J Cancer Prev. 2012;13(10):5105–11. doi: 10.7314/apjcp.2012.13.10.5105. [DOI] [PubMed] [Google Scholar]
- 15.Jurada S, Marc J, Prezelj J, et al. Codon 325 sequence polymorphism of the estrogen receptor alpha gene and bone mineral density in postmenopausal women. J Steroid Biochem Mol Biol. 2001;78(1):15–20. doi: 10.1016/s0960-0760(01)00069-3. [DOI] [PubMed] [Google Scholar]
- 16.Jeon S, Choi JY, Lee KM, et al. Combined genetic effect of CDK7 and ESR1 polymorphisms on breast cancer. Breast cancer research and treatment. Breast Cancer Res Treat. 2010;121(3):737–42. doi: 10.1007/s10549-009-0640-6. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Song CG, Lu JS, et al. A multigenic study on breast cancer risk associated with genetic polymorphisms of ER Alpha, COMT and CYP19 gene in BRCA1/BRCA2 negative Shanghai women with early onset breast cancer or affected relatives. J Cancer Res Clin Oncol. 2007;133(12):969–78. doi: 10.1007/s00432-007-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chattopadhyay S, Siddiqui S, Akhtar MS, et al. Genetic polymorphisms of ESR1, ESR2, CYP17A1, and CYP19A1 and the risk of breast cancer: a case control study from North India. Tumour Biol. 2014;35(5):4517–27. doi: 10.1007/s13277-013-1594-1. [DOI] [PubMed] [Google Scholar]
- 19.Dunning AM, Healey CS, Baynes C, et al. Association of ESR1 gene tagging SNPs with breast cancer risk. Hum Mol Genet. 2009;18(6):1131–39. doi: 10.1093/hmg/ddn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Zuloeta Ladd AM, Vasquez AA, Rivadeneira F, et al. Estrogen receptor alpha polymorphisms and postmenopausal breast cancer risk. Breast Cancer Res Treat. 2008;107(3):415–19. doi: 10.1007/s10549-007-9562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Jiang T, Bai H, et al. Genetic variants of 6q25 and breast cancer susceptibility: a two-stage fine mapping study in a Chinese population. Breast Cancer Res Treat. 2011;129(3):901–7. doi: 10.1007/s10549-011-1527-x. [DOI] [PubMed] [Google Scholar]
- 22.Kjaergaard AD, Ellervik C, Tybjaerg-Hansen A, et al. Estrogen receptor alpha polymorphism and risk of cardiovascular disease, cancer, and hip fracture: cross-sectional, cohort, and case-control studies and a meta-analysis. Circulation. 2007;115(7):861–71. doi: 10.1161/CIRCULATIONAHA.106.615567. [DOI] [PubMed] [Google Scholar]
- 23.Modugno F, Zmuda JM, Potter D, et al. Association of estrogen receptor alpha polymorphisms with breast cancer risk in older Caucasian women. Int J Cancer. 2005;116(6):984–91. doi: 10.1002/ijc.21105. [DOI] [PubMed] [Google Scholar]
- 24.Onland-Moret NC, van Gils CH, Roest M, et al. The estrogen receptor alpha gene and breast cancer risk (The Netherlands) Cancer Causes Control. 2005;16(10):1195–202. doi: 10.1007/s10552-005-0307-5. [DOI] [PubMed] [Google Scholar]
- 25.Sakoda LC, Blackston CR, Doherty JA, et al. Selected estrogen receptor 1 and androgen receptor gene polymorphisms in relation to risk of breast cancer and fibrocystic breast conditions among Chinese women. Cancer Epidemiol. 2011;35(1):48–55. doi: 10.1016/j.canep.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y, Li DK, Wu J, et al. Joint effects of the CYP1A1 MspI, ERalpha PvuII, and ERalpha XbaI polymorphisms on the risk of breast cancer: results from a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2006;15(2):342–47. doi: 10.1158/1055-9965.EPI-05-0485. [DOI] [PubMed] [Google Scholar]
- 27.Sonestedt E, Ivarsson MI, Harlid S, et al. The protective association of high plasma enterolactone with breast cancer is reasonably robust in women with polymorphisms in the estrogen receptor alpha and beta genes. J Nutr. 2009;139(5):993–1001. doi: 10.3945/jn.108.101691. [DOI] [PubMed] [Google Scholar]
- 28.Rajaraman P, Wang SS, Rothman N, et al. Polymorphisms in apoptosis and cell cycle control genes and risk of brain tumors in adults. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1655–61. doi: 10.1158/1055-9965.EPI-07-0314. [DOI] [PubMed] [Google Scholar]
- 29.Wedren S, Lovmar L, Humphreys K, et al. Oestrogen receptor alpha gene haplotype and postmenopausal breast cancer risk: a case control study. Breast Cancer Res. 2004;6(4):R437–49. doi: 10.1186/bcr811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Q, Shu XO, Jin F, et al. Genetic polymorphisms in the estrogen receptor alpha gene and risk of breast cancer: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12(9):853–59. [PubMed] [Google Scholar]
- 31.Shin A, Kang D, Nishio H, et al. Estrogen receptor alpha gene polymorphisms and breast cancer risk. Breast Cancer Res Treat. 2003;80(1):127–31. doi: 10.1023/a:1024439202528. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Mancha R, Galan JJ, Crespo C, et al. Analysis of the ERalpha germline PvuII marker in breast cancer risk. Med Sci Monit. 2008;14(3):CR136–43. [PubMed] [Google Scholar]
- 33.Lu H, Chen D, Hu L-P, et al. Estrogen receptor alpha gene polymorphisms and breast cancer risk: a case-control study with meta-analysis combined. Asian Pac J Cancer Prev. 2013;14(11):6743–49. doi: 10.7314/apjcp.2013.14.11.6743. [DOI] [PubMed] [Google Scholar]
- 34.Siddig A, Mohamed AO, Awad S, et al. Estrogen receptor alpha gene polymorphism and breast cancer. Ann NY Acad Sci. 2008;1138:95–107. doi: 10.1196/annals.1414.015. [DOI] [PubMed] [Google Scholar]
- 35.Slattery ML, Sweeney C, Herrick J, et al. ESR1, AR, body size, and breast cancer risk in Hispanic and non-Hispanic white women living in the Southwestern United States. Breast Cancer Res Treat. 2007;105(3):327–35. doi: 10.1007/s10549-006-9453-z. [DOI] [PubMed] [Google Scholar]
- 36.Ding SL, Yu JC, Chen ST, et al. Diverse associations between ESR1 polymorphism and breast cancer development and progression. Clin Cancer Res. 2010;16(13):3473–84. doi: 10.1158/1078-0432.CCR-09-3092. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez LP, Milne RL, Barroso E, et al. Estrogen and progesterone receptor gene polymorphisms and sporadic breast cancer risk: a Spanish case-control study. Int J Cancer. 2006;119(2):467–71. doi: 10.1002/ijc.21847. [DOI] [PubMed] [Google Scholar]
- 38.Gallicchio L, Berndt SI, McSorley MA, et al. Polymorphisms in estrogen-metabolizing and estrogen receptor genes and the risk of developing breast cancer among a cohort of women with benign breast disease. BMC Cancer. 2006;6:173. doi: 10.1186/1471-2407-6-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrington DM, Howard TD, Brosnihan KB, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105(16):1879–82. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 40.Son BH, Kim MK, Yun YM, et al. Genetic polymorphism of ESR1 rs2881766 increases breast cancer risk in Korean women. J Cancer Res Clin Oncol. 2015;141(4):633–45. doi: 10.1007/s00432-014-1849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, He Y, Qin Z, et al. Evaluation of functional genetic variants at 6q25.1 and risk of breast cancer in a Chinese population. Breast Cancer Res. 2014;16(4):422. doi: 10.1186/s13058-014-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Lu X, Fang Y, et al. Association between miR34b/c polymorphism rs4938723 and cancer risk: a meta-analysis of 11 studies including 6169 cases and 6337 controls. Med Sci Monit. 2014;20:1977–82. doi: 10.12659/MSM.892350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren HT, Wang XJ, Kang HF, et al. Associations between C1772T polymorphism in hypoxia-inducible factor-1alpha gene and breast cancer: a meta-analysis. Med Sci Monit. 2014;20:2578–83. doi: 10.12659/MSM.892374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Pu Z, Ge J, et al. Association of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C polymorphisms and overall survival of breast cancer patients after tamoxifen therapy. Med Sci Monit. 2015;21:563–69. doi: 10.12659/MSM.893473. [DOI] [PMC free article] [PubMed] [Google Scholar]