Abstract
Plague is a primarily flea-borne rodent-associated zoonosis that is often fatal in humans. Our study focused on the plague-endemic West Nile region of Uganda where affordable means for the prevention of human plague are currently lacking. Traditional hut construction and food storage practices hinder rodent exclusion efforts, and emphasize the need for an inexpensive but effective host-targeted approach for controlling fleas within the domestic environment. Here we demonstrate the ability of an insecticide delivery tube that is made from inexpensive locally available materials to reduce fleas on domestic rodents. Unbaited tubes were treated with either an insecticide alone (fipronil) or in conjunction with an insect growth regulator [(S)-methoprene], and placed along natural rodent runways within participant huts. Performance was similar for both treatments throughout the course of the study, and showed significant reductions in the proportion of infested rodents relative to controls for at least 100 d posttreatment.
Keywords: plague, flea control, Yersinia pestis, Uganda, topical insecticide
Plague, caused by Yersinia pestis, is a primarily flea-borne, rodent-associated zoonosis that is characterized by long quiescent periods that are disrupted by rapidly spreading epizootics (Eisen and Gage 2009). Humans are most at risk for exposure to plague bacteria during epizootics when infectious fleas abandon their dying hosts and seek bloodmeals from new hosts. Historically, plague caused three global pandemics and claimed millions of human lives. Owing largely to economic development that allowed for improved sanitation that reduced contact rates between humans, rats, and their fleas (e.g., rodent-proof housing construction, availability of insecticides), incidence of plague has declined substantially in most plague-endemic regions (Tikhomirov 1999). In recent decades, the majority of human plague infections have been reported from the less economically developed African region (World Health Organization [WHO] 2004), including the West Nile region of Uganda, which represents an epidemiological focus for plague in that country.
Within the West Nile region, an average of ≈200 suspect plague cases have been reported annually from 1999 to 2011 (Moore et al. 2012). Most residents of this region live on incomes which fall below the poverty line and rely on subsistence agriculture to make a living (Lakwo et al. 2008). Poor housing construction allows easy access to stored foods and hinders efforts to eliminate rodents from the home environment (Eisen et al. 2013, 2014). As a result, recent plague control efforts have focused largely on reducing fleas (both on- and off-host) within huts.
Indoor residual sprays (IRS), identical to those used in malaria control, were found to significantly reduce rodent-associated fleas for at least 100 d (Borchert et al. 2012). This method has the added benefit of controlling nontarget arthropod vectors, thus reducing risks of other vector-borne pathogens. However, IRS is costly (3.00 USD per hut for chemical alone; Borchert et al. 2012), and requires external input of specialized equipment and skilled applicators. Very recently, IRS sprays have been employed on a limited basis in villages throughout the region. However, to date, these sprays have been deployed only as an intervention strategy within villages where human cases have already occurred, or on an experimental basis in response to locally reported rat die-offs (R.J.E., unpublished data). Due to regional funding constraints, it remains unfeasible to apply IRS as a plague prevention strategy before epizootics or human plague cases are identified. Using an alternative host-targeted approach, Borchert et al. attempted flea reduction on domestic rodents in the West Nile region by using oral baits containing a systemic insecticide (imidacloprid). This type of approach was potentially less costly than coordinated IRS sprays and allowed residents to maintain hut-level control over flea-reduction efforts. However, the insecticide used lacked sufficient residual activity (effective duration was <14 d), the system required rebaiting, and relied on the preferential consumption of the treated bait over other readily available food items (Borchert et al. 2010).
In Uganda and elsewhere in East Africa, Rattus rattus (R. rattus) is considered the principal host involved in epizootic transmission of plague bacteria (Hopkins 1949, Gratz 1999, Borchert et al. 2007). This species is highly susceptible to Y. pestis infection, often exhibits high mortality during epizootics, and harbors fleas that readily bite humans and are competent vectors of Y. pestis (Pollitzer 1954, Gratz 1999, Amatre et al. 2009). In the West Nile region, R. rattus are the most common species of rodent infesting rural homes, and represent >90% of in-hut live captures (Amatre et al. 2009, Borchert et al. 2012, Eisen et al. 2014). Here, as in other regions worldwide, R. rattus is highly commensal, and exists in a permanent association with humans and their habitations (Kingdon 1974, Nowak 1999).
In the hut environment, the movements of R. rattus can be readily anticipated, as individual rats share the tendency to colonize thatched rooftops and travel along well-established runways (Hopkins 1949, Kingdon 1974, Delany 1975). The area atop mud walls, locally referred to as the wall plate, is commonly traveled by resident rodents, as evidenced by the presence of grease markings, droppings, and reports from householders (Boegler, unpublished data).
Capitalizing on the behavior of commensal rodents commonly associated with huts in the West Nile, we sought to develop a low-cost and low-maintenance method to reduce fleas on hut-dwelling rodents for the duration of the ~3-mo plague season. Here, we developed and evaluated a locally supplied and constructed insecticide delivery system that was distributed on wall plates along rodent runways. The habitual movements of hut-associated R. rattus along this wall plate offered the ability to apply topical insecticides without the use of baits as attractants. Flea infestation of hut-trapped rodents pre- and posttreatment was used to measure efficacy of this insecticide delivery system.
Materials and Methods
Site Selection, Study Groups, and Enrollment of Study Participants
Our study was conducted between 29 January and 30 May 2013 within Okoro County, in the plague-endemic West Nile Region of northwestern Uganda. Nine villages were chosen, and grouped into three sets of three villages. As described previously (Borchert et al. 2012), each village within a set had similar area, elevation, population size, land use, and housing style. Within each set of villages, individual villages were randomly assigned to one of three treatments (fipronil, fipronil and (S)-methoprene, or control; Fig. 1). To account for the possibility that rodents might travel between huts and impact sample independence, groups were assigned at the village level and not the hut level. Further, to minimize the potential for control and treatment village rodent communities to differ over the study time period in the absence of insecticide delivery tubes (IDTs), villages were clustered spatially; the distance between any two villages included in the study was no >24.2 km and was as little as 1.0 km.
Fig. 1.
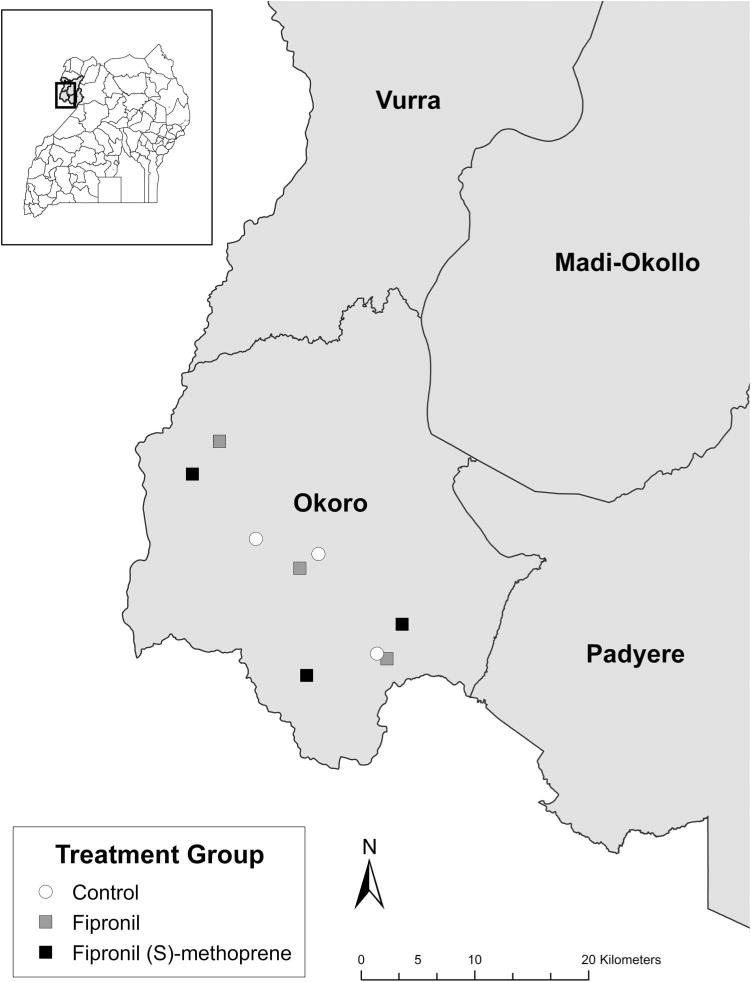
Location of treatment-assigned villages in Okoro county, Uganda.
Within each of the nine villages, 100 huts were selected for participation in the study. Selection of huts was conducted starting from a central location within each village and extending toward the village perimeter until the target number of huts was reached. During the enrollment visit, householders that could not be contacted were not included in the study. Householders of 899 huts chose to participate, while householders of 10 huts declined, resulting in a hut enrollment of 98.9%. Residents of the 10 huts that declined to participate indicated they did so because they did not want rodents rereleased into their homes. Informed consent for participation in the study was obtained from both householders and local village chairpersons in accordance with human-subjects research boards in the United States and Uganda (IRB number 234765 and UG number 23476, respectively). For each participating hut, a unique hut number was assigned and location of the hut was recorded using a handheld global positioning system (GPS) receiver (Trimble Nomad 800 LC, Trimble Navigation, Sunnyvale, CA).
Description of IDTs and Controls
IDTs were constructed using locally available materials (final design, Fig. 2). For each IDT, two 1-liter plastic water bottles were trimmed on both ends and joined at their base to yield a tube ≈8 cm in diameter and 40 cm in length. Black plastic tarp was cut to ≈40 cm and wrapped around the exterior of the tube to protect the insecticide-treated wicks from light. A round 6 mm oil-lamp wick ≈20 cm in length was threaded loosely through the center of the tube, and wick ends were affixed on the exterior of the tube using duct tape.
Fig. 2.
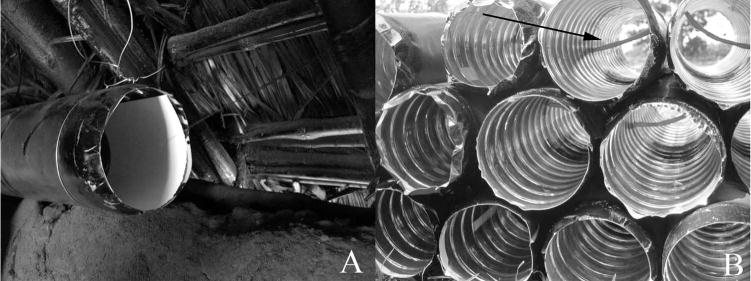
Insecticide delivery tube. (A) Installed on a hut wall plate, with rodent tracking paper. (B) Showing wick placement (arrow) and rodent tracking ink.
Two compounds, fipronil and a fipronil–(S)-methoprene combination, were used within the delivery system, and the ability of these compounds to reduce flea loads on domestic rats was measured against an untreated control group. Fipronil and fipronil–(S)-methoprene, a combination insecticide–insect growth regulator (IGR), have been popularized as spot-on treatments for flea control on domestic dogs and cats; they were selected for evaluation in this study for their demonstrated residual activity against on-host fleas and ability to function by dermal application (Ritzhaupt et al. 2000, Metzger and Rust 2002, Young et al. 2004, Franc et al. 2007). Within the fipronil treatment group, wicks were treated with a 2% weight per volume active ingredient (AI) fipronil solution mixed using a commercially available topical spot-on treatment (SENTRY Fiproguard, Sergeant’s Pet Care Products Inc., Omaha, NE) and locally supplied canola cooking oil. This concentration was based on the effective application rate described previously for controlling ticks on outbred mice in the lab and white-footed mice in the field (Dolan et al. 2004) and weight-adjusted for R. rattus. Based on previous field studies in the West Nile region, an average weight of 80 g was assumed (CDC, unpublished data). The fipronil–(S)-methoprene treatment group received a combination 2% fipronil–1.8% (S)-methoprene “wt:vol” solution mixed in canola oil (SENTRY Fiproguard PLUS, Sergeant’s Pet Care Products Inc.). Huts within the control group received IDTs treated with canola oil only. For each village, insecticide was mixed the day IDTs were placed in the huts. A total of 2.5 ml of treated oil (or oil only) was added by pipette to the wicks within each tube according to the treatment assignment of the village.
For each hut, one IDT was secured using flexible wire to the wall plate beneath the thatched roofline. Finally, to allow for the replacement of lost or damaged treatment wick, multiple extra wicks were treated 7–14 d after the initial placement of rodent tubes. These wicks were affixed inside empty rodent tubes and stored indoors, out of direct sunlight at ambient temperature until use. During each trap placement visit, tubes were inspected and damaged or missing wicks were replaced.
Evaluation of Tube Usage and Effectiveness at Reducing Fleas on Rodents
To evaluate the efficacy of treated IDTs at reducing fleas on domestic rodents, rodents were trapped inside each hut 14 d before the introduction of the tubes, and 20, 40, 60, 80, and 100 d after. At each time point, two Tomahawk live traps (48.3 by 17.1 by 17.1 cm; Tomahawk Trap Co., Tomahawk, WI) were baited using an equal-part mixture of corn, ground nuts, and dried fish, set overnight on the floor of each hut, and retrieved in the morning. Captured rodents were sedated using inhalational halothane, identified to species using a published taxonomic key (Delany 1975), and combed for fleas. To aid in field identifications, physical characteristics of each rodent were recorded, including weight, and lengths of body, tail, right hind foot, and ear. Finally, to identify recaptures, rodents were fitted with uniquely numbered metal ear tags (Hasco Tag Company, Dayton, KY), then released at the point of capture for day 20, 40, 60, and 80. Following capture at day 100, rodents were not released, but humanely euthanized in accordance with approved animal care protocols (Institutional Animal Care and Use Committee, 12-009). All fleas collected from rodents were transferred to 70% ethanol and later counted and identified to species following the taxonomic key by Hopkins (1947).
During the trap placement visits, if access to an individual hut was restricted (e.g., locked doors, householders absent), trapping was not conducted within the hut for that time point. Additionally, during the course of the study, a number of huts were damaged (e.g., demolished by homeowners, destroyed by fire). These huts were excluded from the study beginning at the time following the sampling session when the damage occurred. The total number of huts where traps were successfully placed during each trapping session is listed in Table 2.
Table 2.
Flea infestation prevalence and intensity of live-trap captures and track pad use in combined treatment and control village huts of Okoro county, West Nile, Uganda (January–May)
| Days postplacement of IDT |
Treatment | No. huts |
No. traps set |
No. rodents |
No. Rr |
No. non-Rr |
Percent of rodents infested (n) |
Percent of Rr infested (n) |
Percent of non-Rr infested (n) |
Avg fleas per Rr |
Avg fleas per non-Rr |
Percent Rr recaptured (n) |
Percent non-Rr recaptured (n) |
Trap success (%) |
Track pad use (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −14 | Treatment | 599 | 1,174 | 353 | 341 | 12 | 28.6 (101) | 27.6 (94) | 58.3 (7) | 0.7 | 1.9 | 0 (0) | 0 (0) | 29.1 | 62.3a |
| Control | 300 | 578 | 184 | 175 | 9 | 26.6 (49) | 25.0 (43) | 66.7 (6) | 0.6 | 2.7 | 0 (0) | 0 (0) | 31.8 | 58.8a | |
| 20 | Treatment | 598 | 1,120 | 313 | 300 | 13 | 14.1 (44) | 11.3 (34) | 76.9 (10) | 0.4 | 8.6 | 31.3 (94) | 0 (0) | 26.9 | 72.9 |
| Control | 300 | 570 | 165 | 157 | 8 | 24.8 (41) | 22.9 (36) | 62.5 (5) | 0.4 | 2.8 | 24.2 (38) | 0 (0) | 28.9 | 69.4 | |
| 40 | Treatment | 595 | 1,064 | 301 | 281 | 20 | 13.0 (39) | 10.3 (29) | 50.0 (10) | 0.2 | 2.0 | 48.0 (135) | 5.0 (1) | 26.4 | 77.1 |
| Control | 300 | 570 | 134 | 120 | 14 | 32.8 (44) | 28.0 (33) | 78.6 (11) | 0.6 | 2.1 | 43.3 (52) | 7.1 (1) | 23.5 | 76.7 | |
| 60 | Treatment | 591 | 1,058 | 232 | 214 | 18 | 13.8 (32) | 9.8 (21) | 61.1 (11) | 0.2 | 3.8 | 56.1 (120) | 11.1 (2) | 20.2 | 57.1 |
| Control | 297 | 564 | 120 | 108 | 12 | 34.2 (41) | 29.6 (32) | 75.0 (9) | 0.6 | 4.0 | 50.0 (54) | 16.7 (2) | 21.3 | 63.1 | |
| 80 | Treatment | 586 | 1,104 | 259 | 243 | 16 | 13.5 (35) | 8.6 (21) | 88.0 (14) | 0.2 | 8.9 | 48.6 (118) | 31.2 (5) | 22.1 | 66.5 |
| Control | 297 | 578 | 123 | 107 | 16 | 44.7 (55) | 42.1 (45) | 62.5 (10) | 1.1 | 5.1 | 45.8 (49) | 12.5 (2) | 21.3 | 64.1 | |
| 100 | Treatment | 579 | 1,064 | 227 | 212 | 15 | 15.0 (34) | 10.4 (22) | 80.0 (12) | 0.3 | 5.9 | 58.0 (123) | 20.0 (3) | 19.9 | 63.8 |
| Control | 296 | 564 | 106 | 99 | 7 | 50.0 (53) | 48.5 (48) | 71.4 (5) | 1.0 | 2.6 | 45.5 (45) | 42.9 (3) | 18.8 | 68.1 |
Tracking papers in place for 14 d (until IDTs were placed).
Success of this intervention at a household level was dependent on limited rodent movement. Therefore, to measure the distance traveled by recaptured rodents, hut locations were mapped using Arc GIS software (ArcMap 10.1, ESRI, Redlands, CA), projected to universal transverse Mercator zone 36N WGS 1984, and distances measured using an external software tool (Beyer 2012).
To assess whether IDTs were visited by rodents, a simple ink and paper tracking system was used. Similar to the methodology described for measuring rodent diversity in the field, (Van Apeldoorn et al. 1993, Drennan et al. 1998), two 10-cm strips of heavy card-stock paper (65 lb, Wausau Paper, Mosinee, WI) were cut and fitted into both ends of each IDT (Fig. 2). A tracking ink was then mixed using a 1:4 wt:vol ratio of carbon powder (99+%, Fisher, Pittsburgh, PA) to heavy mineral oil (Fisher) and applied using a paintbrush to the center of each tube, beneath the treatment wick and between the two tracking papers, in a roughly 7 cm square area. As rodents traveled past the treated wick and through the tube, footprints were left on the paper. On day 20, 40, 60, 80, and 100, fresh ink and paper were added to each IDT in the evening, and recovered the next morning. Tracking papers were examined for rodent prints, and their presence or absence was recorded in association with each IDT. The presence of rodent prints was interpreted as a rodent passage through the tube during the nighttime hours. For huts that were inaccessible during the trap placement session, tracking papers were not placed (and not evaluated) for that time point.
Statistical Analysis
To compare infestation prevalence (the proportion of rodents harboring at least one flea) between the two treatments, fipronil and fipronil–(S)-methoprene, a test of equivalence was performed, using the first observed capture of each rodent post IDT placement. Because the treatments were shown to be equivalent, in subsequent analyses the fipronil and fipronil–(S)-methoprene treatment groups were pooled and this pooled treatment group was evaluated against the control group.
We first sought to determine if removal of fleas from hosts had an effect on infestation intensity (average number of fleas per rodent) upon recapture. If previous capture and flea removal did not affect infestation intensity upon recapture, this justified including recaptures in subsequent analyses. To evaluate this, a generalized mixed model assuming a Poisson distribution was fitted to control group observations at all time points following placement of IDTs. Recapture status and trap session were included as main effects and together as an interaction effect, while individual rodent ID was included as a random effect. Captures from treatment villages were not included in the evaluation so as not to confound treatment effect with recapture effect.
To determine if IDTs were effective at reducing infestation prevalence on all rodents or on R. rattus individually, generalized mixed models assuming binomial distributions were fitted. The categorical variables treatment and trap session were included both as main effects and together as an interaction effect while village ID and individual rodent ID were included as random effects. The variance for the random effect, village ID, was allowed to vary by grouping.
To determine if IDTs were effective at reducing the proportion of infested non-Rattus rodents, and to determine if the treatment effect was different for R. rattus and non-Rattus, the fixed effects treatment and rodent species classification were included in a generalized mixed model assuming a binomial distribution. The interaction effect between treatment and the rodent species classification was included, and the random effect individual rodent ID was accounted for. Observations from the preplacement trap session were excluded from this model, and all observations from the 5 time points post IDT-placement were pooled.
To explore the potential for partial treatment by IDTs, we analyzed the average flea loads on infested R. rattus within treatment and control villages. A generalized mixed model assuming a Poisson distribution was fitted to observations where the flea counts are greater than zero at all time points postplacement. The categorical variables treatment and trap session were included both as main effects and together as an interaction effect, while individual rodent ID was included as a random effect.
For all analyses, a significance level of α = 0.05 was employed. For any test of multiple comparisons, Bonferonni adjustments were applied. Standard diagnostics were performed on all models to ensure model assumptions held. All analyses were run in either SAS 9.3 (SAS Institute 2011) or R 3.0.1 (R Core Team 2013). Summary statistics describing rodent and flea collections were performed in JMP 10.0.1 (SAS Institute 2012).
Results
Description of Fleas and Hosts Captured Within Huts
During a total of 10,006 trap nights, a total of 2,517 rodents were captured (25.1 rodents per 100 trap nights). R. rattus was the most commonly observed species and represented the majority (93.6%) of all captures, followed by Arvicanthis niloticus (4.7%). Captures of Mastomys spp., Lophuromys spp., Cricetomys gambianus, and Praomys jacksoni were less frequent, and together they represented <2% of all observations (Table 1). The total number of rodents captured within each of the nine villages was comparable (range: 232–366, summarized in Table 2), suggesting that rodent densities were also similar. From all rodents, a total of 1,749 fleas were collected and identified as Xenopsylla brasiliensis (73.1%), Xenopsylla cheopis (12.1%), Dinopsyllus lypusus (7.7%), Ctenophthalmus spp. (3.6%) and less commonly, Stivalius torvus, Ctenocephalides felis, Echidnophaga gallinacea, and Tungapenetrans (2.7% combined). A small number of fleas were damaged, preventing accurate identification (0.8%; Table 1). When considered individually, the fleainfestation intensity (mean number of fleas per host) for R. rattus was 0.4 while for other species of rodents trapped within huts, infestation intensity was higher (5.5; Table 1). Most often, infested R. rattus harbored Xenopsylla spp. fleas over other types: >90% of fleas collected from hut-associated R. rattus belonged to this genus. The mean weight of all captured rodents was 90.1 g (SD: 41.3 g), while for R. rattus, mean capture weight was 90.4 g (SD: 30.3 g).
Table 1.
Numbers and species of fleas infesting hut-trapped rodents, Okoro county, West Nile, Uganda (January–May)
| Rodent species (n) | No. fleas collected (no. fleas per host)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X. brasiliensisa,b | X. cheopisa,b | D. lypususa,b | Ct. bacopus/Ct. c. cabirusa,b | S. torvusa | C. felisa | E. gallinaceaa | T. penetransa | Unknown | Total | |
| Rattus rattus (2,357) | 728 (0.31) | 211 (0.09) | 46 (0.02) | 19 (0.01) | 10 (<0.01) | 28 (0.01) | 3 (<0.01) | 1 (<0.01) | 7 (<0.01) | 1,053 (0.45) |
| Arvicanthis niloticus (119) | 535 (4.50) | 1 (0.01) | 81 (0.68) | 27 (0.23) | 2 (0.02) | 2 (0.02) | 0 (0) | 0 (0) | 7 (0.06) | 655 (5.50) |
| Mastomys spp. (16) | 7 (0.44) | 0 (0) | 4 (0.25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (0.69) |
| Lophuromys sikapusi (14) | 2 (0.14) | 0 (0) | 4 (0.29) | 12 (0.86) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 18 (1.29) |
| Lophuromys flavopunctatus (8) | 5 (0.63) | 0 (0) | 0 (0) | 5 (0.63) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (1.25) |
| Praomys jacksoni (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cricetomys gambianus (1) | 1 (1.00) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.00) |
| Unknown (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total (2,517) | 1,278 (0.51) | 212 (0.08) | 135 (0.05) | 63 (0.03) | 12 (<0.01) | 30 (0.01) | 3 (<0.01) | 1 (<0.01) | 14 (0.01) | |
Xenopsylla brasiliensis, Xenopsylla cheopis, Dinopsyllus lypusus, Ctenophthalmus bacopus/Ctenophthalmus caleatus cabirus, Stivalius torvus, Ctenocephalides felis, Echidnophaga gallinacea, Tunga penetrans.
Confirmed or suspected vectors of Y. pestis (Devignat 1949, Gratz 1999).
After 12 h of placement, over half of all ink and paper track pads showed evidence of rodent visitation to the IDT at each time point of the study (range: 57.1–72.9%), and the level of IDT usage was comparable for control and treated groups (Table 2). While track pad results indicated a fairly consistent use of IDTs, trap success by treatment group decreased over the time course of the study, ranging from 18.8 to 31.8%, with the greatest proportion of occupied traps at early time points (Table 2).
In contrast with the other rodent species that were collected, R. rattus are often permanent residents within human habitations and are much more commonly trapped within huts. In this study, R. rattus demonstrated capture site fidelity. Recaptures of R. rattus represented between 24.2 and 58.0% of species-specific observations across treatments and time points (Table 2). Of the R. rattus individuals that were captured more than once (541 out of 1,525 total), only roughly a third (164 or 30.3%) showed evidence of movement away from a single hut. Of those that were recaptured from more than one hut, the median minimum distance traveled between trap locations was 15.7 m (range: 1.0–347.0 m). The median maximum distance traveled between two huts for recaptured rodents was 17.0 m (range: 1.0–770.9 m).
Evaluation of the Efficacy of IDTs to Reduce Flea Infestations on Hut-Trapped Rodents
The effect of fipronil versus fipronil–(S)-methoprene treatment on infestation prevalence was found to be equivalent. The confidence interval for the difference in the proportion of uninfested hosts between the two treatments (CI: −0.06, 0.05) fell within the defined equivalence region (CI: −0.1, 0.1; n = 730 rodents). Therefore, “treatment” here includes the combined observations from both chemical formulations.
Because fleas were removed from rodents with each capture, we were interested in determining if infestation intensity among recaptured rodents was different from infestation at first capture. Among rodents trapped within control huts, infestation intensity was not significantly different between first-time capture and recapture rodents at time points post-IDT placement (T range: −0.17 to 0.32; df = 176; P for all comparisons = 1.00; n = 648 rodents). Therefore, recaptures were included in subsequent analyses.
Before IDT placement, observed infestation prevalence of rodents trapped within treatment and control villages was 28.6 and 26.6%, respectively. Following placement of IDTs, the proportion of infested rodents in control villages increased to 50.0% by day 100, while in treatment villages, infestation prevalence decreased to 14.1% by day 20 and remained suppressed for the duration of the analysis period (Table 2). The difference in infestation prevalence within rodents in control huts from those in IDT-treated huts was statistically different at day 40, 60, 80, and 100 compared with the difference detected between the two groups before placement of IDTs (T ≤ −3.9; df = 841; P for all comparisons ≤0.002; n = 2517 rodents). However, at day 20, no statistically significant difference was found (T = −2.4; df = 841; P = 0.085; Fig. 3).
Fig. 3.
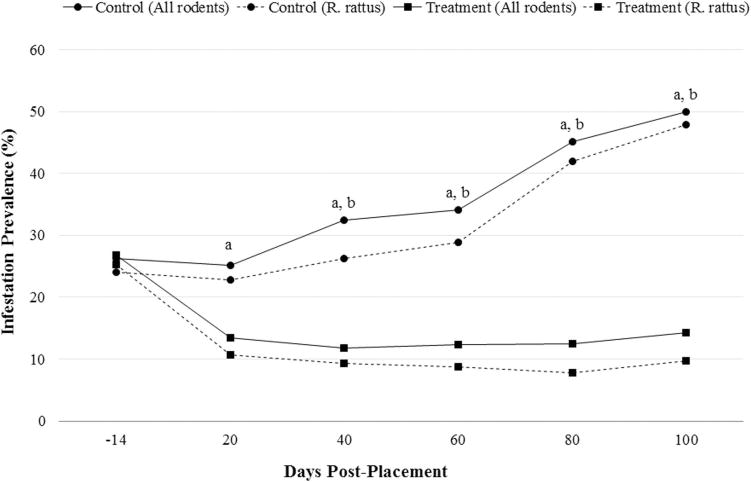
Proportion of infested rodents of all species and R. rattus collected from treatment and control-assigned huts in West Nile Uganda (January–May). Difference of proportions (control versus pooled treatment) significantly different (P < 0.05) than pre-IDT placement for R. rattus (a) and all rodents combined (b).
Because R. rattus was the target species for this intervention, the response of flea infestation for this species to IDT placement was evaluated separately from all other hosts. Similar to the trends reported when all rodent species were evaluated, the observed proportion of infested R. rattus trapped within control huts increased from 25.0% at preplacement to 48.5% by day 100 postplacement. Over the same time period, the observed infestation prevalence of R. rattus in the treatment villages decreased from 27.6 to 10.4% (Table 2). For R. rattus, the difference in infestation prevalence between treatment versus control villages was statistically different at day 20–100 when compared with the difference observed during the preplacement trap session (T≤ −2.8; df = 822; P for all comparisons ≤0.03; n = 2,357 R. rattus; Fig. 3).
There were 1,841 R. rattus captured at time points post IDT placement, while there were only 139 non-Rattus rodents captured post IDT placement. The observed difference in infestation prevalence between control and treatment for R. rattus averaged across all post IDT time points was 24.1% whereas the observed difference for non-R. rattus was −1.2% (T = −3.38; df = 632; P = 0.002; n = 1,980). Not only was the effect of IDT on infestation prevalence statistically different for R. rattus and non-R. rattus, but the IDT was ineffective for non-R. rattus (T = 0.11; df = 632; P = 1.00; n = 1,980).
To explore the reason for failure of treatment on roughly 10% of R. rattus captures at each time point post-IDT placement, the average flealoads on infested rodents following treatment were evaluated. Significantly lower flea infestation intensity within the treatment group as compared with the control group could indicate a partial treatment effect. However, we observed no statistically significant difference between infestation intensity of R. rattus between treatment and control villages for the five time points post-IDT placement (T range: −1.17 to 1.06; df = 36; P for all comparisons = 1.00; n = 321).
Discussion
We demonstrated the ability of an inexpensive, simple insecticide delivery system to control fleas on hut-dwelling rats for at least 100 d. By exploiting the movement patterns and behavioral characteristics of R. rattus, on-host flea control was achieved for this species without baiting or retreatment of the IDTs. Consistent with a previous study from the West Nile region (Amatre et al. 2009), between January and May when our study was conducted, infestation prevalence among rodents trapped in untreated huts increased. Demonstrating efficacy of the IDTs, during the same time frame, infestation prevalence in treated huts remained suppressed compared with pretreatment measures. Although this study was conducted during an interepizootic period, the observed suppression of on-host fleas for many weeks while regional increases were observed suggests it is possible that placement of IDTs before the plague season could prevent local flea populations from increasing during high-risk periods.
As in previous studies from the West Nile region (Amatre et al. 2009, Borchert et al. 2012, Eisen et al. 2014), R. rattus was the most commonly encountered rodent in the domestic setting. Almost exclusively, it harbored Xenopsylla spp. fleas, which are competent vectors of Y. pestis and also willingly bite humans (Burroughs 1947, Gratz 1999). This, coupled with the high susceptibility of R. rattus to Y. pestis, underscores the importance of R. rattus as epizootic hosts and Xenopsylla spp. as bridging vectors to humans in the hut setting. Although a significant overall reduction of infested R. rattus was observed following introduction of IDTs, roughly 10% of rats at each capture were not completely cleared of fleas. Failure of treatment in these individuals could have resulted from noncontact with treated wicks, or from rodents which contacted IDT chemicals, but were not fully treated. We assumed an average R. rattus weight of 80 g in this study, and formulated chemical concentrations for rodents of this size. Therefore, it was possible, given that the observed average weight of R. rattus was 90.1 g, that some individuals might have received a subeffective dose. However, this idea was not supported by our analysis, which indicated that infestation intensity remained comparable between treatment and control group captures following the placement of IDTs. Therefore, it seems unlikely that increasing the concentration of chemicals would significantly increase the efficacy of IDTs. Similarly, because infestation prevalence did not decline substantially below 10% and later rebound, it is unlikely that retreatment of IDTs would improve efficacy. Although the remaining percentage of infested R. rattus was slightly higher in our study compared with flea reduction using IRS (range: 0–10.2% by treated village at day 100), the residual activity of at least 100 d was similar between IDTs and IRS (Borchert et al. 2012). Because of its greater efficacy, IRS may be preferred during interventions aimed at reducing flea loads during plague epizootics, but IDTs may be valuable as a less costly tool to prevent epizootic transmission and lower the risk of subsequent human cases by suppressing flea infestations of rats.
During the 100 d following placement of the IDTs, we did not observe any significant differences between the insecticide (fipronil) and insecticide–IGR combination (fipronil–(S)-methoprene) treatments in terms of reducing the numbers of flea-infested rodents in huts. Because IGRs function on the immature stages of flea development, the combination treatment was expected to exhibit a delayed or extended period of flea suppression, but this was not detected during the 100 d of the study. Therefore either might be used within the delivery system. Fipronil and (S)-methoprene employ modes of action distinct from chemical compounds recommended for indoor residual spraying (WHO 2013); thus the risk of introducing selection pressures contrary to emergency interventions is reduced. Likewise, the use of fipronil-based IDTs for flea reduction on rodents may be of interest in areas where resistance to insecticides used in plague interventions has been reported (Chanteau et al. 1998, Shyamal et al. 2008, Ames 2011). However, cross-resistance of fipronil and related GABA-gated chloride channel antagonists (cyclodienes) has been observed in other insect vectors (Kristensen et al. 2005, Wondji et al. 2011), and resistance-associated genetic mutations of the Rdl gene have been identified in at least one species of flea (Bass et al. 2004). Therefore, in areas with a history of cyclodiene use, the potential for resistance in flea populations should be evaluated.
The observed reduction of flea-infested rodents was independent of the effect of physical removal of fleas upon capture, suggesting that mechanical elimination of fleas on these hosts would be an ineffective control strategy. Fleas are periodic ectoparasites, and nest-associated flea loads can be greater than those of their associated rodent host (Krasnov et al. 2004). For untreated rodents in this study, reinfestation by these nest-associated fleas seems likely following capture. However, for treated rodents, visits to the nest may confer additional protection against nidiculous fleas. Direct contact with treated rodents may be necessary for effective control of these fleas, while passive transfer of chemical to bedding seems less effective (Metzger and Rust 2002). Therefore, the treatment status of the rodent currently colonizing the nest is probably more important than that of previous occupants.
In single-application trials of fipronil (S)–methoprene on domestic animals, residual activity (adult flea control of ≥90%) has been demonstrated for 4–6 wk (Young et al. 2004, Schnieder et al. 2008). Inhibition of flea emergence (of ≥90%) was demonstrated for longer periods: 12 wk as observed by Young et al. (2004) and at least 10 wk under a severe challenge experiment (Franc et al. 2007), but none reported residual activity for 100 d or more. It is likely, given the frequent rodent usage of IDTs that we observed, that the extended activity (>3 mo) of insecticides applied via IDTs on the infestation prevalence of hut-associated rodents in this study could have resulted from retreatments of individual rodents. During 12 h of tracking paper placement at each time point, over two-thirds of all IDTs showed evidence of rodent movement. Further, relatively high recapture rates and short distances between recapture events suggest that most R. rattus maintain close associations with a single hut or groups of huts, increasing the likelihood that an individual rodent might encounter an IDT (and potentially be treated) more than once. In addition, a number of wicks were replaced in this study following damage by resident rodents. These wicks were generated 7–14 d following the initial IDT placement; therefore, it is possible that for some huts, the length of IDT efficacy could be overestimated by as much as 14 d.
IDT treatment was much more effective for controlling flea infestations of R. rattus than for other rodents captured inside huts. Infestation prevalence was higher for non-Rattus rodents before IDT placement, and was not significantly reduced at time points following placement. In contrast to R. rattus, which are highly commensal, Arvicanthis niloticus, Mastomys spp., and others are not thought to be permanent residents of huts in the West Nile. Though Mastomys was once the predominant commensal rodent within the region, it has largely been displaced by R. rattus (Hopkins 1949). Currently, both A. niloticus and Mastomys are most commonly trapped in sylvatic and peridomestic environments away from the huts (Amatre et al. 2009, Eisen et al. 2014) and tend to nest in burrows and underbrush at ground level (Delany 1975, Nowak 1999). Given these differences in habitat preference and behavior, non-Rattus individuals are probably less likely to encounter IDTs placed on the wall plate, and therefore less likely to receive treatment. Finally, although non-Rattus rodents represented only a small proportion of in-hut captures during this study (6.2%), their contribution to the overall number of fleas collected was disproportionally large (39.8% of total fleas collected), and was composed primarily of known or suspected Y. pestis vectors (Tables 1 and 2). Therefore, these rodents may deserve greater scrutiny in future evaluations of plague prevention and control strategies, and may require an alternate method of targeted flea control.
Because household-level and governmental funding for such interventions are limited, the relative expense of plague prevention tools for the West Nile region is a necessary consideration. Insecticide delivery tubes were constructed of locally supplied materials and cost ~US$0.83 to produce and US$0.69 to treat. However, with simple modifications to IDT design (using a single 1 liter bottle, omitting plastic covering) the cost of an individual tube could be reduced to roughly US$0.44. In this evaluation, hut-associated flea control was achieved without baiting or retreatment visits. In practical terms, this would reduce labor costs for deployment of the intervention, as IDTs could be treated and placed during a single visit. Further inquiry is necessary to determine the value local householders and health officials place on hut-level plague control methods, including IDTs. However, when IDT treatment and placement were offered without cost, householders of nearly all (98.9%) of the selected huts chose to participate, suggesting that this type of flea control is acceptable to local communities.
Acknowledgments
We thank Cyrus Mungujakisa, Emmanuel Tibo, Ezekiel Kajik, Fred Wuuna, Victor Olowo, Mukobi Yafesi, Robert Kibenga Banjo, Tom Stanley Asaku (Uganda Virus Research Institute, Arua, Uganda), Jeff N. Borchert (CDC, Entebbe, Uganda), and Marc Dolan (CDC, Fort Collins, CO) for their valuable input and assistance with this study, as well as Sarah Bruhn and Alvin Bronstein (Rocky Mountain Poison and Drug Center, Denver, CO) for their helpful advice. We also thank the study participants of Okorro County for kindly allowing us into their villages and homes. This research was supported through fellowship with Oak Ridge Institute for Science and Education (ORISE) administered by Oak Ridge Associated Universities (ORAU) and funded by the Centers for Disease Control and Prevention (CDC).
References Cited
- Amatre G, Babi N, Enscore RE, Ogen-Odoi A, Atiku LA, Akol A, Gage KL, Eisen RJ. Flea diversity and infestation prevalence on rodents in a plague-endemic region of Uganda. Am J Trop Med Hyg. 2009;81:718–724. doi: 10.4269/ajtmh.2009.09-0104. [DOI] [PubMed] [Google Scholar]
- Ames AD. DDT and pyrethroid resistance in Xenopsylla cheopis (Rothschild), the oriental rat flea in northern Uganda. PhD, Colorado State University; Fort Collins, CO: 2011. [Google Scholar]
- Bass C, Schroeder I, Turberg A, Field LM, Williamson MS. Identification of the Rdl mutation in laboratory and field strains of the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae) Pest Manag Sci. 2004;60:1157–1162. doi: 10.1002/ps.937. [DOI] [PubMed] [Google Scholar]
- Beyer HL. Geospatial Modelling Environment, version 0.7.2.1. 2012 http://www.spatialecology.com/gme.
- Borchert JN, Mach JJ, Linder TJ, Ogen-Odoi A, Angualia S. Invasive rats and bubonic plague in northwest Uganda. In: Witmer GW, Pitt WC, Fagerstone A, editors. Managing vertebrate invasive species: proceedings of an international symposium. U.S. Department of Agriculture/APHIS Wildlife Services, National Wildlife Research Center; Fort Collins, CO: 2007. pp. 283–293. [Google Scholar]
- Borchert JN, Enscore RE, Eisen RJ, Atiku LA, Owor N, Acayo S, Babi N, Montenieri JA, Gage KL. Evaluation of rodent bait containing imidacloprid for the control of fleas on commensal rodents in a plague-endemic region of northwest Uganda. J Med Entomol. 2010;47:842–850. doi: 10.1603/me09221. [DOI] [PubMed] [Google Scholar]
- Borchert JN, Eisen RJ, Atiku LA, Delorey MJ, Mpanga JT, Babi N, Enscore RE, Gage KL. Efficacy of indoor residual spraying using lambda-cyhalothrin for controlling nontarget vector fleas (Siphonaptera) on commensal rats in a plague endemic region of northwestern Uganda. J Med Entomol. 2012;49:1027–1034. doi: 10.1603/me11230. [DOI] [PubMed] [Google Scholar]
- Burroughs AL. Sylvatic plague studies: the vector efficiency of nine species of fleas compared with Xenopsylla cheopis. J Hyg. 1947;45:371–396. doi: 10.1017/s0022172400014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanteau S, Ratsifasoamanana L, Rasoamanana B, Rahalison L, Randriambelosoa J, Roux J, Rabeson D. Plague, a reemerging disease in Madagascar. Emerg Infect Dis. 1998;4:101–104. doi: 10.3201/eid0401.980114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany MJ. The rodents of Uganda. Trustees of the British Museum; London, England: 1975. [Google Scholar]
- Devignat R. Epidemiologie de la peste au Lac Albert 1944–1945–1946. Ann Soc Belg Med Trop. 1949;29:277–305. [PubMed] [Google Scholar]
- Dolan MC, Maupin GO, Schneider BS, Denatale C, Hamon N, Cole C, Zeidner NS, Stafford KC., 3rd Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol. 2004;41:1043–1054. doi: 10.1603/0022-2585-41.6.1043. [DOI] [PubMed] [Google Scholar]
- Drennan JE, Beier P, Dodd NL. Use of track stations to index abundance of sciurids. J Mammal. 1998;79:352–359. [Google Scholar]
- Eisen RJ, Gage KL. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet Res. 2009;40:1. doi: 10.1051/vetres:2008039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Enscore RE, Atiku LA, Zielinski-Gutierrez E, Mpanga JT, Kajik E, Andama V, Mungujakisa C, Tibo E, MacMillan K, et al. Evidence that rodent control strategies ought to be improved to enhance food security and reduce the risk of rodent-borne illnesses within subsistence farming villages in the plague-endemic West Nile region, Uganda. Int J Pest Manag. 2013;59:259–270. doi: 10.1080/09670874.2013.845321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, MacMillan K, Atiku LA, Mpanga JT, Zielinski-Gutierrez E, Graham CB, Boegler KA, Enscore RE, Gage KL. Identification of risk factors for plague in the West Nile region of Uganda. Am J Trop Med Hyg. 2014;90:1047–1058. doi: 10.4269/ajtmh.14-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc M, Beugnet F, Vermot S. Efficacy of fipronil-(S)-methoprene on fleas, flea egg collection, and flea egg development following transplantation of gravid fleas onto treated cats. Vet Ther. 2007;8:285–292. [PubMed] [Google Scholar]
- Gratz NG. Rodent reservoirs and flea vectors of natural foci of plague. In: Dennis DT, Gage KL, Gratz NG, Poland JD, Tikhomirov E, editors. Plague manual: epidemiology, distribution, surveillance and control. Vol. 74. World Health Organization; Geneva, Switzerland: 1999. pp. 63–96. [Google Scholar]
- Hopkins GHE. Annotated and illustrated keys to the known fleas of East Africa. Ugandan J (Sci Suppl) 1947;11:133–190. [Google Scholar]
- Hopkins GHE. Report on rats, fleas and plague in Uganda. East African Standard, Ltd., Government Printer of Uganda; Uganda: 1949. [Google Scholar]
- Kingdon J. East African mammals. 2B. University of Chicago Press; Chicago, IL: 1974. [Google Scholar]
- Krasnov BR, Khokhlova IS, Shenbrot GI. Sampling fleas: the reliability of host infestation data. Med Vet Entomol. 2004;18:232–240. doi: 10.1111/j.0269-283X.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Hansen KK, Jensen KMV. Cross-resistance between dieldrin and fipronil in German cockroach (Dictyoptera: Blattellidae) J Econ Entomol. 2005;98:1305–1310. doi: 10.1603/0022-0493-98.4.1305. [DOI] [PubMed] [Google Scholar]
- Lakwo A, Cwinyaai W, Abdallay O. West Nile profiling report. Agency for Accelerated Regional Development; Nebbi, Uganda: 2008. [Google Scholar]
- Metzger ME, Rust MK. Laboratory evaluation of fipronil and imidacloprid topical insecticides for control of the plague vector Oropsylla montana (Siphonaptera: Ceratophyllidae) on California ground squirrels (Rodentia: Sciuridae) J Med Entomol. 2002;39:152–161. doi: 10.1603/0022-2585-39.1.152. [DOI] [PubMed] [Google Scholar]
- Moore SM, Monaghan A, Griffith KS, Apangu T, Mead PS, Eisen RJ. Improvement of disease prediction and modeling through the use of meteorological ensembles: human plague in Uganda. PloS ONE. 2012;7:e44431. doi: 10.1371/journal.pone.0044431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RM. Walker’s mammals of the world. 6th. Vol. 2. Johns Hopkins University Press; Baltimore, MD: 1999. [Google Scholar]
- Pollitzer. Plague. Monogr Ser World Health Organ. 1954;22:1–698. [Google Scholar]
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Ritzhaupt LK, Rowan TG, Jones RL. Evaluation of efficacy of selamectin and fipronil against Ctenocephalides felis in cats. J Am Vet Med Assoc. 2000;217:1666–1668. doi: 10.2460/javma.2000.217.1666. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT 9.3 user’s guide: the GLIMMIX procedure. SAS Institute; Cary, NC: 2011. [Google Scholar]
- SAS Institute. Using JMP 10. SAS Institute; Cary, NC: 2012. [Google Scholar]
- Schnieder T, Wolken S, Mencke N. Comparative efficacy of imidacloprid, selamectin, fipronil-(S)-methoprene, and metaflumizone against cats experimentally infested with Ctenocephalides felis. Vet Ther. 2008;9:176–183. [PubMed] [Google Scholar]
- Shyamal B, Kumar R Ravi, Sohan L, Balakrishnan N, Veena M, Shiv L. Present susceptibility status of rat flea Xenopsylla cheopis (Siphonaptera: Pulicidae), vector of plague against organochlorine, organophosphate and synthetic pyrethroids 1. The Nilgiris district, Tamil Nadu, India. J Comm Dis. 2008;40:41–45. [PubMed] [Google Scholar]
- Tikhomirov E. Epidemiology and distribution of plague. In: Dennis DT, Gage KL, Gratz NG, Poland JD, Tikhomirov E, editors. Plague manual: epidemiology, distribution, surveillance and control. Vol. 74. World Health Organization; Geneva, Switzerland: 1999. pp. 11–41. [Google Scholar]
- Van Apeldoorn R, El Daem M, Hawley K, Kozakiewicz M, Merriam G, Nieuwenhuizen W, Wegner J. Footprints of small mammals: a field method of sampling data for different species. Mammalia. 1993;57:407–422. [Google Scholar]
- (WHO) World Health Organization. Human plague in 2002 and 2003. Wkly Epidemiol Rec. 2004;79:301–306. [PubMed] [Google Scholar]
- (WHO) World Health Organization. WHO recommended insecticides for indoor residual spraying against malaria vectors: WHO Pesticides Evaluation Scheme (WHOPES) World Health Organization; Geneva, Switzerland: 2013. http://www.who.int/whopes/Insecticides_IRS_Malaria_25_Oct_2013.pdf. [Google Scholar]
- Wondji CS, Dabire RK, Tukur Z, Irving H, Djouaka R, Morgan JC. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem Molec Biol. 2011;41:484–491. doi: 10.1016/j.ibmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DR, Jeannin PC, Boeckh A. Efficacy of fipronil/(S)-methoprene combination spot-on for dogs against shed eggs, emerging and existing adult cat fleas (Ctenocephalides felis, Bouche) Vet Parasitol. 2004;125:397–407. doi: 10.1016/j.vetpar.2004.07.021. [DOI] [PubMed] [Google Scholar]


