Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) refers to an increasingly diagnosed condition involving triglyceride accumulation into hepatocytes resulting in a broad spectrum of liver injury. The progression of NAFLD, a relatively benign condition, to nonalcoholic steatohepatitis (NASH) involves the hepatic infiltration of inflammatory cells and subsequent hepatocellular injury. Ischemia/reperfusion (I/R) injury of the liver is a major complication of liver resection, hepatic trauma, and liver transplantation. To date, there have been no studies that have evaluated the effects of hepatic I/R on models of NASH.
Objective
Evaluate the effects of hepatic I/R on a mouse model of NASH.
Methods
A mouse model of progressive NASH was developed and evaluated using C57BL/6 mice fed a methionine choline deficient diet for 3, 6, 9, and 12 wk. Mice subsequently underwent 90 min of partial hepatic ischemia with reperfusion of 1, 4, and 8 h. Mice were sacrificed after the indicated periods, and blood and liver samples were taken for analysis.
Results
Mice fed the MCD diet showed a rapid induction of hepatic steatosis, inflammation, and fibrosis by 3 wk that persisted over the 12-wk period of diet, as demonstrated by histologic examination, alanine aminotransferase (ALT), and liver content of myeloperoxidase (MPO). The response to I/R in livers with progressive NASH fed MCD diet for 3, 6, 9, and 12 wk showed marked neutrophil recruitment and hepatocyte necrosis.
Conclusion
These data suggest the inflammatory response from I/R is augmented in livers with NASH histopathology compared with normal liver.
Keywords: fatty liver disease, NAFLD, NASH, hepatic steatosis, ischemia reperfusion injury, inflammation, fibrosis, hepatic ischemia, necroinflammation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) refers to an increasingly diagnosed condition involving triglyceride accumulation into hepatocytes resulting in a broad spectrum of liver injury. The progression of NAFLD, a relatively benign condition, to nonalcoholic steatohepatitis (NASH) involves the hepatic infiltration of inflammatory cells and subsequent hepatocellular injury.
Ischemia/reperfusion (I/R) injury of the liver is a major complication of liver resection, hepatic trauma, and liver transplantation. Multiple experimental models have shown that the degree of steatosis in NAFLD livers negatively impacts liver recovery from I/R injury [1–3]. In addition the presence of hepatic steatosis in donors for liver transplantation has been shown to be a known risk factor for primary graft nonfunction and primary delayed function [4–6]. Several theories have been proposed to explain the mechanisms by which steatosis augments liver injury after I/R. These include impairment of hepatic microcirculation [7–9] and alterations in cytokine and chemokine pathways [10]. All of these studies indicate that models with known liver steatosis have increased liver injury after I/R [8, 11–16]. The models used in these studies included leptin-knockout (ob/ob), leptin-deficient (db/db), or high fat diet-induced steatosis. Despite the presence of profound fatty liver, obesity, and diabetes, these models do not develop significant liver inflammation and fibrosis and, therefore, are not representative of NASH [17, 18]. To date, there have been no studies that have evaluated the effects of hepatic I/R on models of NASH. A methionine/choline-deficient (MCD) diet produces hepatic macrosteatosis with significant hepatic inflammation, which is similar to the histopathologic changes that occur in NASH in humans [19]. In the present study, we sought to determine the effects of warm I/R injury in a murine model of NASH induced by a MCD diet to evaluate whether there are significant differences in the injury response during progressive fatty liver disease.
MATERIALS AND METHODS
Experimental Animals
C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Male mice 8–10 wk of age were used in all experiments. To induce NASH, mice were fed either control diet (n “ 6; cat no. 960441; ICN, Aurora, OH) or MCD diet (n “ 30; cat no. 960439; ICN, Aurora, OH) for 3, 6, 9, and 12 wk. This project was approved by the University of Cincinnati Animal Care and Use Committee.
Model of Hepatic Ischemia-Reperfusion Injury
Partial hepatic ischemia was induced as described previously [20]. Briefly, mice were anaesthetized with pentobarbital sodium (60 mg/kg i.p.). A midline laparotomy was performed, and an atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. After 90 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Hepatic reperfusion was performed for 1, 4, and 8 h. Sham control mice underwent the same protocol without vascular occlusion. Mice were killed after the indicated periods of reperfusion, and blood and liver samples were taken for analysis.
Blood and Tissue Analysis
Blood was obtained via cardiac puncture for analysis of serum alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit via bio-assay (Wiener Laboratories, Rosario, Argentina).
MPO Assay
Liver MPO content was assessed by methods similar to those of Schierwagen et al. [21]. Liver tissue (100 mg) was homogenized in 2 mL of buffer A (3.4 mM KH2PO4, 16 mM Na2HPO4, pH 7.4). After centrifugation for 20 min at 10,000 g, the pellet was resuspended in 10 volumes of buffer B (43.2 mM KH2PO4, 6.5 mM Na2PO4, 10 mM EDTA, 0.5% hexadecyltrimethylammonium, pH 6.0) and sonicated for 10 s. After being heated for 2 h at 60°C, the supernatant was reacted with 3,3′,5,5′-tetramethylbenzidine (Sigma), and optical density was determined at 655 nm.
Histologic Examination
A single blinded hepatopathologist reviewed all liver sections for pathologic review and staging. Formalin-fixed liver tissue was processed and paraffin sections were stained with hematoxylin and eosin (H and E) for general histology, Masson’s trichrome for fibrosis, and Sirius Red for steatosis. All sections were examined in a blinded fashion for steatosis, inflammation, and fibrosis. Hepatic steatosis was graded according to the percentage of lipid-laden hepatocytes as 0: 0%, 1: 0%–33%, 2: 33%–67%, 3: 67%–100%. Necroinflammation was graded as 0: none, 1: mild, 2: moderate, 3: severe based on the number and size of the necroinflammatory lesions in representative liver sections.
Statistical Analysis
All data are expressed as means ± SE. Data were analyzed with a one-way ANOVA with subsequent Student-Newman-Keuls test. Differences were considered significant when P < 0.05.
RESULTS
Development of NASH
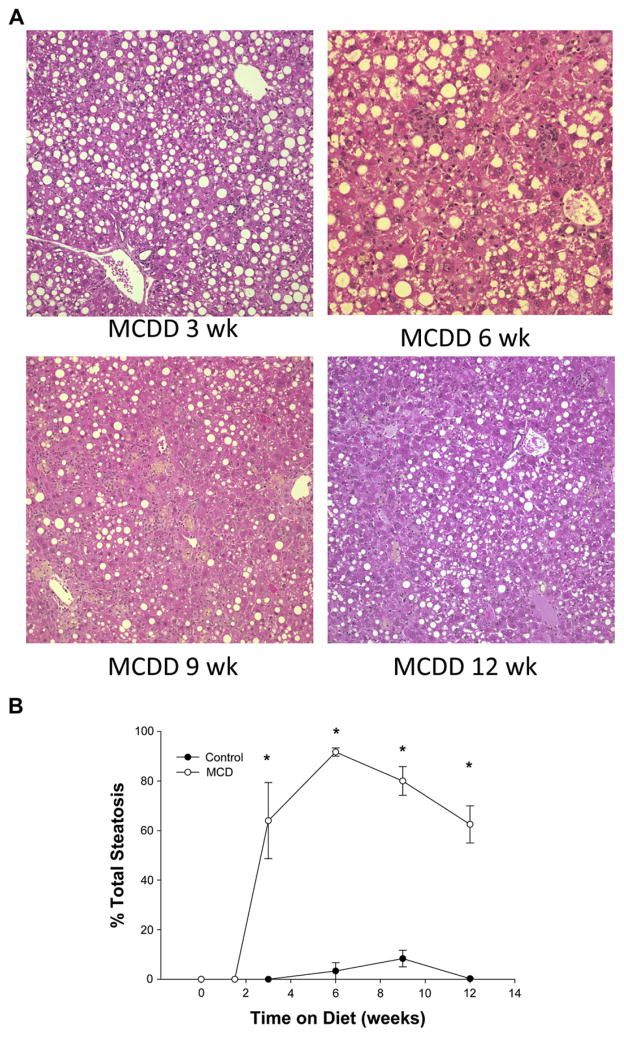
In order to evaluate the effects of I/R on NASH at various stages of progression of the inflammation and fibrosis, we first needed to determine the progressive nature of MCD diet-induced NASH. Mice were fed the MCD diet for up to 12 wk. The MCD diet resulted in 20%–50% weight loss (data not shown) as previously described [22, 23]. After 3, 6, 9, or 12 wk, mice were sacrificed to measure the progression of NASH. Steatosis was measured histologically. Mice fed the MCD diet showed a rapid induction of hepatic steatosis by 3 wk, which persisted over the 12-wk period of diet (Fig. 1A). Quantitation of percent steatosis showed >60% steatosis by 3 wk, approximately 89% steatosis at 6 wk, and >60% steatosis after 9 or 12 wk on diet (Fig. 1B). There were no significant differences amongst the different durations of diet.
FIG. 1.
(A) Hematoxylin and eosin staining of liver specimens at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD) demonstrates early severe steatosis at 3 wk. The steatosis remains significantly elevated at all time points. (B) Percentage of steatosis as graded by a blinded hepatopathologist demonstrates statistically significant steatosis on MCDD compared with wild type. Steatosis was >60% at all time points and peaked at 6 wk.
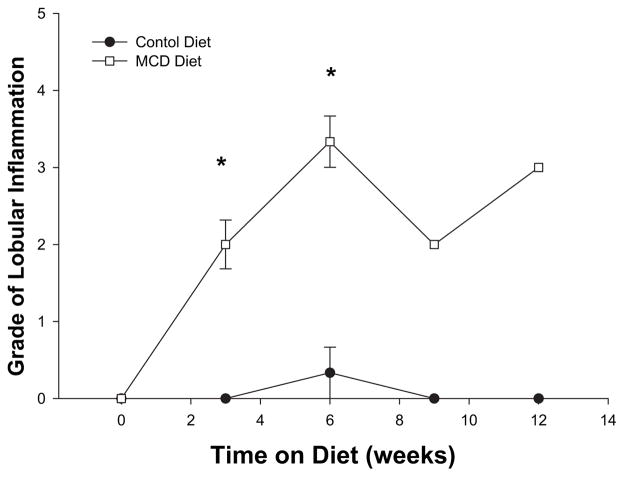
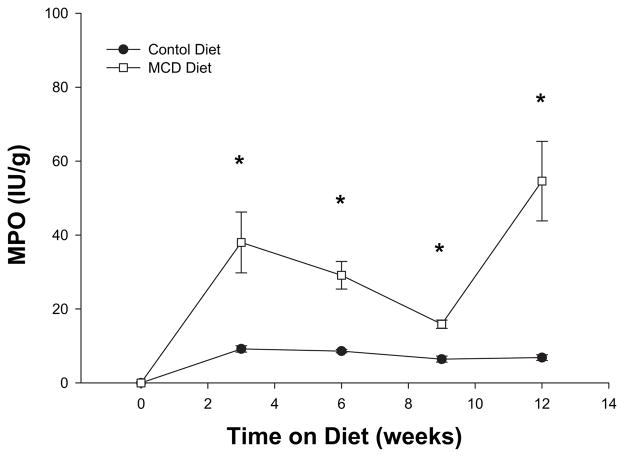
The presence of hepatic inflammation is required to differentiate benign simple steatosis from NASH. Examination of lobular inflammation, determined by scaled grading, indicated that the MCD diet resulted in marked lobular inflammation at 3 wk and progression of lobular inflammatory changes at 12 wk (Fig. 2). Since neutrophils are one of the first inflammatory cells to infiltrate the steatotic liver progressing to NASH, we next evaluated the amount of neutrophil accumulation by measuring liver content of myeloperoxidase (MPO). In mice fed an MCD diet, significant liver neutrophil accumulation was noted after 3 wk on the diet (Fig. 3). Liver MPO content remained increased throughout the 12-wk time course.
FIG. 2.
Grade of lobular inflammation as graded by a blinded hepatopathologist on hematoxylin and eosin staining of liver specimens at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD). There was significantly increased grade of lobular inflammation at all time points on MCDD compared with wild type.
FIG. 3.
Myeloperoxidase (MPO) at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD). There was noted to be significantly increased MPO at all time points on MCDD compared with wild type. The peak of MPO was at 12 wk of MCDD.
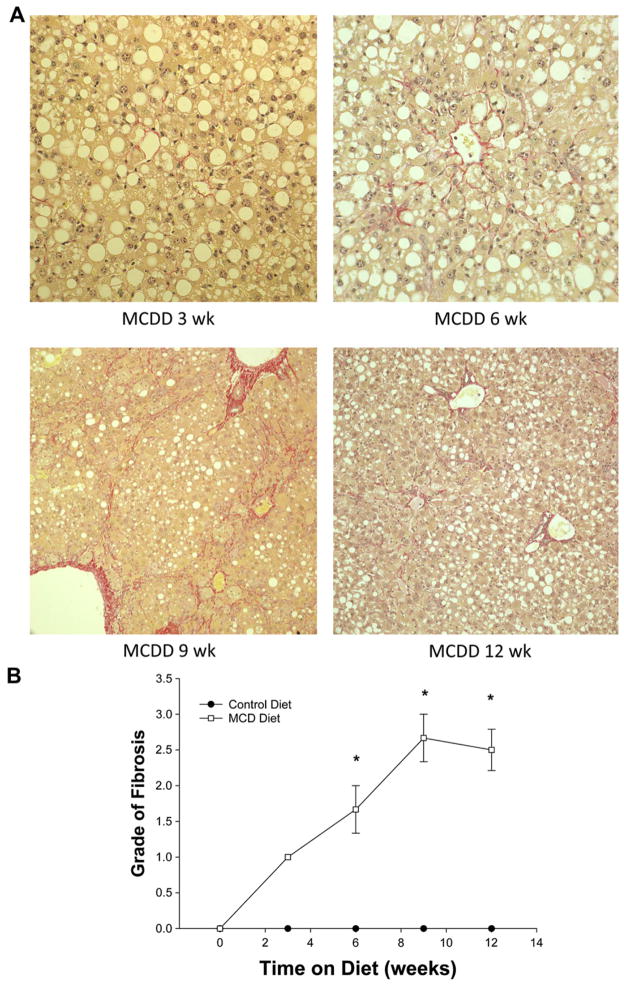
Fibrosis is a component of NASH leading to end-stage liver disease requiring liver transplantation. The MCD diet induced significant hepatic fibrosis within 3 wk as determined by Trichrome staining (Fig. 4A). With increased time on the diet, there was significant progression of diffuse fibrosis, with early fibrosis noted after 3 wk on diet and bridging fibrosis seen in mice after 9 and 12 wk. The degree of fibrosis observed increased progressively with increased time on the MCD diet and peaked at 9 wk (Fig. 4B).
FIG. 4.
(A) Trichrome staining of liver specimens at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD) demonstrates early fibrosis as early as 3 wk. The grade of fibrosis steadily increased as weeks on diet progressed. (B) Fibrosis grade of liver specimens at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD) demonstrates grade 1 fibrosis at 3 wk. The grade of fibrosis steadily increased as weeks on diet progressed. There was no noted fibrotic change in liver specimens of control mice.
The pathologic changes noted above were associated with increased hepatocellular injury as measured by serum levels of alanine aminotransferase (ALT). Increased levels of ALT were obsereved in all mice fed the MCD diet (Fig. 5). Interestingly, ALT levels were highest during the first 9 wk of the diet. By 12 wk on the MCD diet, serum ALT levels had deceased 3-fold, although still significanlty increased over mice fed the control diet (Fig. 5).
FIG. 5.
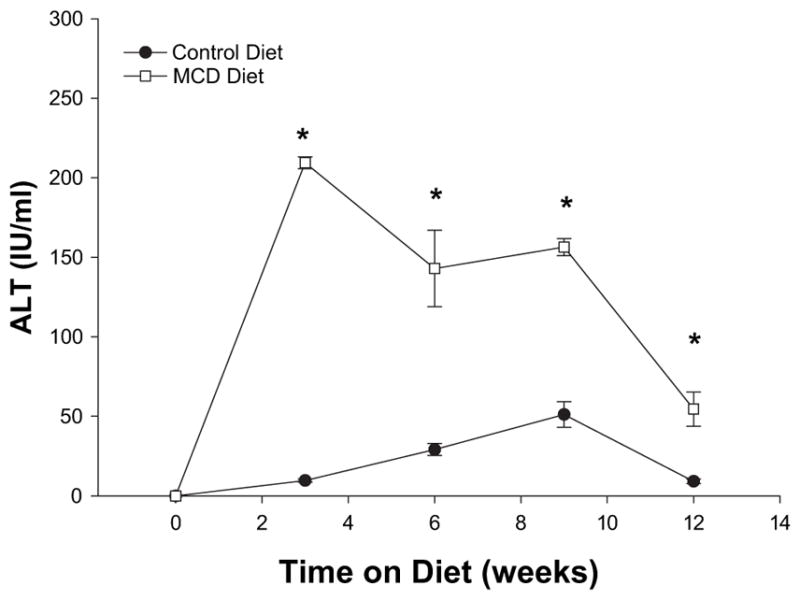
Serum ALT at 3, 6, 9, and 12 wk of methionine choline deficient diet (MCDD) demonstrates significant hepatocyte injury at 3 wk. The degree of hepatocyte injury peaked at 3 wk of injury and steadily decreased at 6, 9, and 12 wk. This is likely due to the progressive fibrosis of the liver with MCDD.
Ischemia/Reperfusion Injury in NASH
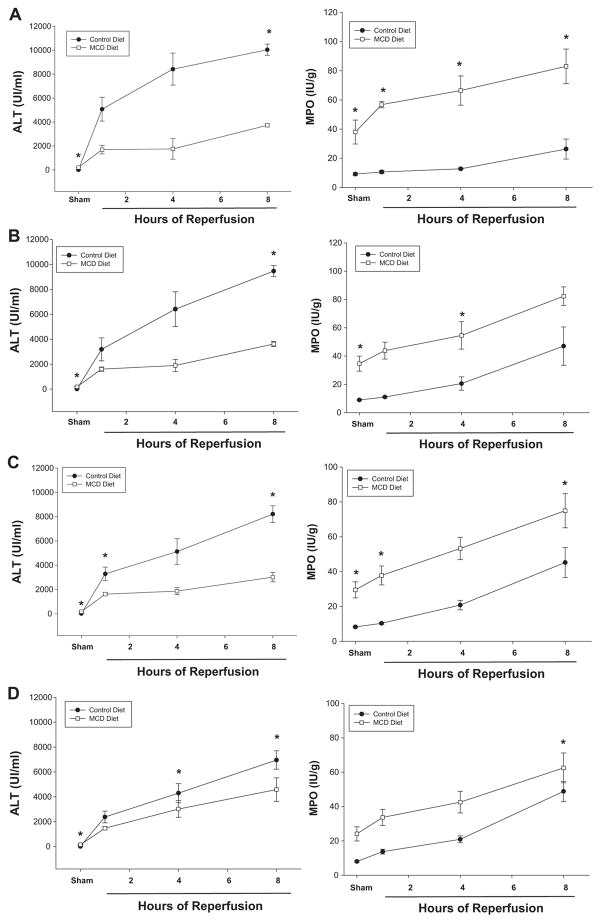
I/R of the liver has been shown to result in an augmented injury response in steatotic liver compared with normal liver [1–3]. To determine the response to I/R in livers with NASH, we evaluated the effects of hepatic I/R on mice fed the MCD diet for 3, 6, 9, or 12 wk. Liver histopathology in the two diet groups did not change with duration on diet. As shown in Fig. 6A, mice fed a normal diet for 3 wk displayed the typical pattern of hemorrhagic hepatocyte necrosis and neutrophil recruitment observed after I/R. All other durations on diet displayed similar results (data not shown). Interestingly, serum levels of ALT were significantly lower in mice fed the MCD diet compared with those fed the control diet after I/R (Fig. 7A–D). This may be due to the fact that there were fewer healthy hepatocytes in the NASH livers compared with control livers. As observed in the histologic assessment, I/R induced significantly more neutrophil recruitment in the livers of mice fed the MCD diet than those fed the control diet (Fig. 7A–D). These data suggest the inflammatory response is augmented in NASH livers compared to normal livers.
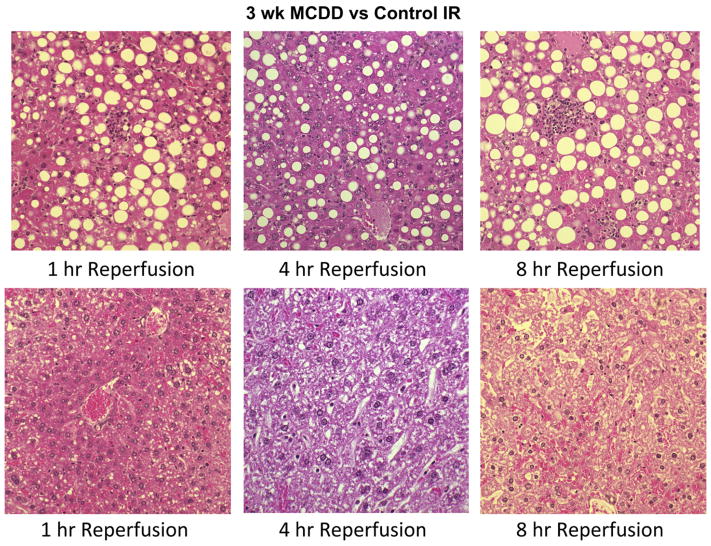
FIG. 6.
(A) Hematoxylin and eosin staining of liver specimens at 3 wk of methionine choline deficient diet (MCDD) and controls after 1, 4, and 8 h of ischemia reperfusion injury to the liver. As demonstrated by pathology, maximal hepatocyte injury with warm partial ischemia was noted at the point of maximal inflammation and fibrosis (12 wk MCDD) with 8 h of reperfusion. This was noted with neutrophil recruitment and hemorrhagic hepatocyte necrosis. Weeks 6, 9, and 12 showed similar patterns and are not shown.
FIG. 7.
(A–D) ALT and MPO at 0, 1, 4, and 8 h of reperfusion after warm partial IR liver injury or MCDD and control diet mice at 3, 6, 9, and 12 wk. At all time points of increasing inflammation and fibrosis with progressive weeks on MCDD, there was increased MPO as weeks progressed and as hours of reperfusion increased. Serum levels of ALT were significantly lower in mice fed the MCD diet compared with those fed the control diet after IR injury.
DISCUSSION
To the best of our knowledge, the present study is the first to evaluate the effects of warm I/R injury in a murine model of progressive NASH induced by MCD diet. There are multiple murine models of NAFLD that have demonstrated increased liver injury in steatotic liver [1–3]. Although these models provide an important characterization of IR injury in a murine NAFLD model, they employ the use of a genetic ob/ob mouse, which demonstrates only simple hepatic steatosis. We used a MCD diet-induced model of NASH as it combines inflammation and steatosis, which more accurately replicates the human experience of NASH. As weeks on diet advanced, we did note a progression of inflammation and hepatic fibrosis, despite a relatively constant level of hepatic steatosis. This characterization of the progression of NASH from simple steatosis to inflammation to bridging fibrosis seen at 9 and 12 wk on the MCD diet replicated the wide range of inflammatory and fibrotic changes seen in human NASH patients and provided a solid foundation for our examination of the effects of warm I/R on different points of this spectrum.
An interesting finding in our characterization of the progression of NASH seen in mice fed an MCD diet was the change in the hepatocyte injury marker, ALT, with progressive inflammation and fibrosis. During the initial 9 wk of the MCD diet, there was a steady increase in serum ALT levels. However, at 12 wk on the MCD diet, there was a sudden decrease of ALT, despite increased inflammation and fibrosis. One plausible explanation would be a reduced number of viable hepatocytes after 12 wk on the MCD diet due to progressive inflammation and fibrosis. Another theory centers around resistance of functional hepatocytes with fibrosis to further insult, as they are in a state of chronic inflammation.
Furthermore, in our characterization of the progression of NASH in the MCD model, we noted a significant influx of neutrophils, as measured by MPO content, into the liver. This is as expected as the increased inflammatory state seen in this model of NASH. Interestingly, in warm I/R injury, the peak time of neutrophil accumulation occurred at 3 wk on MCD diet after 8 h of reperfusion. Although the neutrophil involvement was still significantly higher than control, the amount of accumulation steadily decreased in IR as fibrosis increased with weeks on diet. This result likely parallels the amount of hepatocyte viability and loss of the ability to attract acute phase immune cells in the setting of chronic inflammation.
In warm partial I/R injury, in the setting of progressive NASH, at the time of maximal inflammation and fibrosis (12 wk), we noted maximal hepatocyte injury with 8 h reperfusion. This result was as expected and does parallel the human experience that has been seen in cirrhotic patients undergoing warm I/R injury, which results in substantial hepatocyte injury and poor patient outcomes.
In summary, this study shows interesting findings of the effects of warm I/R injury in a model of progressive NASH. The results demonstrate peak hepatocyte injury at a maximum time point of overall steatotic liver fibrosis and inflammation. This confirms clinical findings of increased liver injury seen in IR in cirrhosis. In addition, live donor and cadaveric liver grafts are generally not used for liver transplantation with biopsy evidence of steatosis >30%. This animal model does highlight the importance of ensuring that the pathologic findings of the graft do not show inflammation or early fibrosis, or any of the findings of NASH. In addition, hepatic resection in patients with NASH should be performed with caution and without warm IR, if possible, to avoid overwhelming hepatocyte injury as we noted in this study.
References
- 1.Hong F, Radaeva S, Pan HN, et al. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 2.Wan CD, Wang CY, Liu T, et al. Alleviation of ischemia/reperfusion injury in ob/ob mice by inhibiting UCP-2 expression in fatty liver. World J Gastroenterol. 2008;14:590. doi: 10.3748/wjg.14.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa T, Ito Y, Wijeweera J, et al. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1385. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Reynes M, Johann M, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23(1 Pt 2):1538. [PubMed] [Google Scholar]
- 5.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Ploeg RJ, D’Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation–a multivariate analysis. Transplantation. 1993;55:807. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 8.El-Badry AM, Moritz W, Contaldo C, et al. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology. 2007;45:855. doi: 10.1002/hep.21625. [DOI] [PubMed] [Google Scholar]
- 9.Ijaz S, Yang W, Winslet MC, et al. Impairment of hepatic micro-circulation in fatty liver. Microcirculation. 2003;10:447. doi: 10.1038/sj.mn.7800206. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Xu W, Zhang X, et al. Expression of the CXC chemokine receptor 3 and its ligands in ischemia-reperfusion injury of liver in rats. Transplant Proc. 2008;40:1300. doi: 10.1016/j.transproceed.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 11.Serafin A, Rosello-Catafau J, Prats N, et al. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 12.Serafin A, Rosello-Catafau J, Prats N, et al. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorini RN, Donovan JL, Rodwell D, et al. Short-term administration of (−)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005;11:298. doi: 10.1002/lt.20348. [DOI] [PubMed] [Google Scholar]
- 14.Ellett JD, Evans ZP, Fiorini JH, et al. The use of the Papworth cocktail is detrimental to steatotic livers after ischemia-reperfusion injury. Transplantation. 2008;86:286. doi: 10.1097/TP.0b013e31817b900f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans ZP, Ellett JD, Fariss MW, et al. Vitamin E succinate reduces ischemia/reperfusion injury in steatotic livers. Transplant Proc. 2008;40:3327. doi: 10.1016/j.transproceed.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans ZP, Ellett JD, Schmidt MG, et al. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283:8573. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 18.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;(Suppl 1):S73. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 19.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance [see comment] J Hepatol. 2004;40:47. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Lentsch AB, Yoshidome H, Cheadle WG, et al. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: Roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 21.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 22.Vetelainen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526. doi: 10.1111/j.1440-1746.2006.04701.x. [DOI] [PubMed] [Google Scholar]
- 23.Rinella ME, Elias MS, Smolak RR, et al. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]