Abstract
Cocaine addiction is a life-long relapsing disorder that results from long-term adaptations within the brain. We find that Activin-receptor signaling, including the transcription factor Smad3, is upregulated in the rat nucleus accumbens shell following withdrawal from cocaine. Direct genetic and pharmacological manipulations of this pathway bidirectionally alter cocaine seeking, while governing morphological plasticity in nucleus accumbens neurons. These findings reveal that Activin/Smad3 signaling is induced following withdrawal from cocaine, and such regulation may be a key molecular mechanism underlying behavioral and cellular plasticity in the brain following cocaine self-administration.
Addiction is a life-long affliction manifested by episodes of relapse despite prolonged abstinence. It is thought that the neuroadaptations that result from drug exposure represent a neurobiological mechanism for long-term behavioral changes, highlighting the need to more fully understand the long-term molecular changes mediating drug craving and relapse1.
Activin, a member of the Transforming Growth Factor-β superfamily, signals via serine/threonine kinase receptors, type II and type I, which then phosphorylate Smad3 and induce translocation into the nucleus to regulate gene expression2. Activin signaling governs cellular and morphological plasticity associated with psychiatric disorders through both a canonical transcriptional pathway and a more direct mediation of mechanisms associated with structural plasticity3,4. Thus, we hypothesized that Activin represents an intracellular bridge between proximal mediators of cellular and structural plasticity and long-term sustained transcriptional events, which may drive drug-taking behaviors.
To determine if Activin signaling is altered by cocaine exposure, rats were trained to self-administer cocaine (1 mg/kg/inf) or saline (Supplementary Fig. 1a). Tissue punches were taken from the nucleus accumbens (NAc) shell region 24 hours (1 d withdrawal; 1WD) or 7 d (7WD) following the last cocaine exposure (Fig 1a). Activin receptor 2a (AcvR2a) mRNA and protein levels (but not AcvR1b, saline: 1.000 ± 0.0573, cocaine: 0.9758 ± 0.0646; t-test: t(14) = 0.2813, P > 0.05, [n = 8/group]) were increased following 7WD (Fig. 1b,c; full length blots presented in Supplementary Fig. 6). These changes were not observed following 1WD from cocaine when compared to saline controls. Smad3 phosphorylation, but not total protein level, was increased following 7WD from cocaine (Fig. 1d; full length blots presented in Supplementary Fig. 6). The upregulation of Activin-receptor/Smad3 signaling was specific to the NAc, as there was no change in the caudate putamen (CPu) (Supplementary Fig. 2a,b; full length blots presented in Supplementary Fig. 7). However in the NAc core subregion, a similar increase in AcvR2a protein expression was observed, without a significant change in phosphorylated (p) Smad3 (Supplementary Fig 2c,d; full length blots presented in Supplementary Fig. 7).
Figure 1. Cocaine self-administration activates Activin-Receptor/Smad3 signaling.
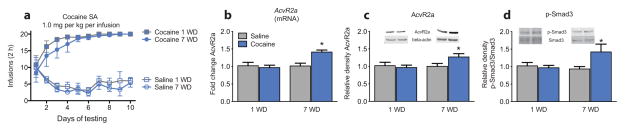
(a) Mean number of infusions per day in rats before undergoing 1 or 7 d of withdrawal (1WD and 7WD, respectively) (Two-way repeated measures ANOVA: drug × withdrawal: F27,240 = 4.697, P < 0.01). Rats self-administered significantly more infusions of cocaine than saline. Number of infusions were the same between withdrawal groups. (b) Relative Activin receptor 2a (AcvR2a) mRNA (F1,20 = 8.648, P < 0.01 [n = 6/group]), and (c) protein expression (F1,18 = 7.157, P < 0.01, [n = 5–6/group]:), and (d) relative ratio of phosphorylated (p-Smad3) to total Smad3 protein expression (drug: F1,18 = 4.864, withdrawal: F1,18 = 4.805, P < 0.05, [n = 5–6/group]) in the nucleus accumbens after 1WD or 7WD (b–d: All Two-way ANOVAs). Data are expressed as mean ± SEM; *P < 0.05 vs. saline.
Additionally, these pathways were not altered acutely (1 h) following the final cocaine self-administration session (Supplementary Fig. 3a,b), but remained elevated following re-exposure to cocaine self-administration following 7WD (Supplementary Fig. 3c–e; full length blots presented in Supplementary Fig. 7), suggesting withdrawal from cocaine is required to initiate these adaptations that endure through future exposures to cocaine, which may represent a mechanism by which cocaine-taking remains stable following longer periods of abstinence5.
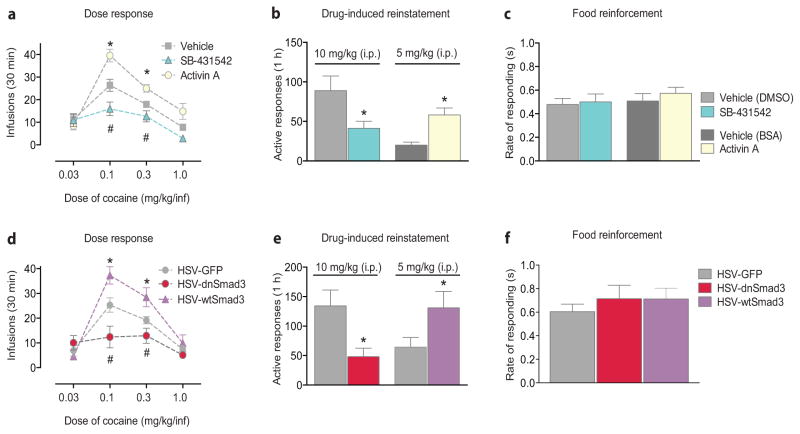
Using a within-session dose-response self-administration paradigm (Supplementary Fig. 1b), pharmacological activation of Activin-receptor signaling by intra-accumbal microinjections of Activin A caused a vertical shift in cocaine self-administration, a model thought to reflect addiction-like vulnerability6, whereas rats receiving microinjections of the Activin receptor antagonist (SB-431542) self-administered less cocaine (Fig. 2a). In a separate group of animals (Supplementary Fig. 1c), microinjections of SB-431542 decreased active responses during drug-induced reinstatement (10 mg/kg cocaine, i.p.), whereas microinjections of Activin A increased active responses (5 mg/kg cocaine, i.p.; Fig. 2b) compared to vehicle. Neither Activin A nor SB-431542 altered the rate of responding for a food reinforcer (Fig. 2c), number of food reinforcers earned, or locomotor activity (Supplementary Figs. 1d, 4a–d).
Figure 2. Manipulating Activin-Receptor/Smad3 signaling alters drug-related responding.
(a) Within-session cocaine self-administration dose-response following microinjections of Activin A, Activin receptor antagonist (SB-431542), or vehicle into the nucleus accumbens (Two-way repeated measures ANOVA: F6,120 = 3.858, P < 0.01, [n = 7–9/group]). (b) Active responses during drug-induced reinstatement following microinjection of SB-431542 (t14 = 2.316, P < 0.05, [n = 8/group]) or Activin A (t16 = 4.013, P < 0.01, [n = 9/group]; t-test). (c) Rate of responding for a food reinforcer following microinjection of SB-431542, Activin A, or vehicle (One-way ANOVA: F3,24 = 0.4735, P = 0.70, [n = 7/group]). (d) Within-session cocaine self-administration dose-response following viral-mediated overexpression into the nucleus accumbens of either dominant-negative Smad3 (HSV-dnSmad3) or wild-type Smad3 (HSV-wtSmad3), or control (HSV-GFP) (Two-way repeated measures ANOVA: dose: F3,144 = 32.57, virus: F2,144 = 11.42, interaction: F6,144 = 5.007, all Ps < 0.01, [n = 9–11/group]). (e) Active responses during drug-induced reinstatement with overexpression of HSV-dnSmad3 (t17 = 2.742, P < 0.05, [n = 9–10/group]) or HSV-wtSmad3 (t19 = 2.115, P < 0.05, [n = 10–11/group]; t-test). (f) Rate of responding for a food reinforcer following viral overexpression of HSV-dnSmad3, HSV-wtSmad3, or HSV-GFP (One-way ANOVA: F2,19 = 0.4493, P = 0.6447, [n = 7–8/group]). Data are expressed as mean ± SEM; #,*P < 0.05 vs. vehicle/HSV-GFP.
Viral (herpes simplex virus, HSV)-mediated overexpression in the NAc shell of a dominant-negative Smad3 (HSV-dnSmad3), in which the c-terminus serines are mutated to alanines (SAXA)7 (Supplementary Fig. 5a,b), decreased cocaine self-administration at 0.1 and 0.3 mg/kg/inf compared to the HSV-GFP control (Fig. 2d; Supplementary Fig. 1b). Overexpression of HSV-dnSmad3 also blocked drug-induced reinstatement (10 mg/kg cocaine, i.p.; Fig. 2e; Supplementary Fig 1c). Overexpression of the wild-type (HSV-wtSmad3) increased the number of infusions self-administered at 0.1 and 0.3 mg/kg/inf (Fig. 2d) and increased active responding during drug-induced reinstatement (5 mg/kg cocaine, i.p.) when compared to HSV-GFP (Fig. 2e), though no differences were observed in rate of responding for a food reinforcer (Fig. 2f), the number of food reinforcers earned, or locomotor activity (Supplementary Figs. 1d, 4e,f).
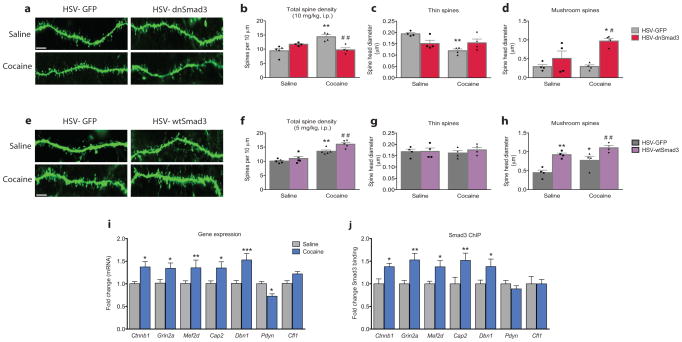
Morphological evaluation of drug-induced (10 mg/kg cocaine, i.p.) reinstatement of cocaine self-administration showed an increase in the density of dendritic spines on medium spiny neurons (MSNs) in the NAc compared to saline (Fig. 3a,b), an effect that was completely blocked by HSV-dnSmad3. Conversely, overexpression of HSV-wtSmad3 potentiated the cocaine-induced increase in spine density (Fig. 3e,f), which suggests that the modulation of the nascent spines governs reinstatement behavior. Furthermore, spine head diameters as a function of spine subtype were compared, which change following cocaine exposure and subsequent reinstatement8,9. Drug-induced reinstatement (10 mg/kg cocaine, i.p.) of cocaine self-administration reduced the head diameter of thin spines, an effect that was blocked by HSV-dnSmad3 (Fig. 3c). Although cocaine had no effect on mushroom spines at this dose, HSV-dnSmad3 overexpression increased the head diameter of these more stable spines (Fig. 3d). At the lower priming dose of cocaine (5 mg/kg, i.p.), there was no change in head diameter of thin spines (Fig. 3g), however, the head diameter of mushroom type spines was increased, an effect that was mimicked and potentiated by HSV-wtSmad3 overexpression (Fig. 3h).
Figure 3. Smad3 mediates cocaine-induced dendritic spine morphology of neurons in the NAc shell.
(a) Dendritic spine images of viral overexpression of HSV-dnSmad3 or HSV-GFP with following drug-induced reinstatement (10 mg/kg, i.p.) cocaine (scale bar = 5 μm). (b) Total spine density (F1,13 = 23.92, P < 0.01, [n = 4–5/group]), and spine head diameters of (c) thin (drug × virus: F1,12 = 10.35, P < 0.01, [n = 4/group]) and (d) mushroom (drug: F1,12 = 4.398, virus: F1,12 = 16.28, Ps < 0.05, [n = 4/group]) dendritic spines on medium spiny neurons in the nucleus accumbens with HSV-dnSmad3 (b–d: All Two-way ANOVAs). (e) Dendritic spine images of viral overexpression of HSV-wtSmad3 or HSV-GFP with following drug-induced reinstatement (5 mg/kg, i.p.) cocaine (scale bar = 5 μm). (f) Total spine density (drug: F1,16 = 62.85, virus: F1,16 = 10.01, Ps < 0.01, [n = 5/group]) and spine head diameters of (g) thin (P > 0.05 [n = 4/group]) and (h) mushroom (drug: F1,12 = 11.82, virus: F1,12 = 30.01, Ps < 0.01, [n = 4/group]) dendritic spines on medium spiny neurons in the nucleus accumbens with HSV-wtSmad3 (f–h: All Two-way ANOVAs). (i) Relative β-catenin (Ctnnb1), NR2A (Grin2a), myocyte enhancer factor 2D (Mef2D), adenylyl cyclase-associated protein 2 (Cap2), drebrin (Dbn1), prodynorphin (Pdyn), and cofilin (Cfl1) mRNA expression in the nucleus accumbens following 7 d withdrawal from saline or cocaine self-administration (F13,66 = 5.523, P < 0.01, [n = 5–6/group]). (j) Upregulated binding of Smad3 to Ctnnb1, Grin2a, Mef2D, Cap2, and Dbn1 in the nucleus accumbens as measured by chromatin immunoprecipitation (ChIP) following 7 WD from saline or cocaine self-administration (F13,66 = 3.897, P < 0.01, [n = 5–7/group]) (i–j: Both Two-way ANOVAs). Data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, *** P < 0.001 vs. saline or saline HSV-GFP, #P < 0.05, ##P < 0.01 vs. cocaine HSV-GFP.
Activin-receptor signaling regulation of transcription occurs via binding of Smad3 to Smad-binding elements on DNA or to coactivators with other transcriptional regulators2,10. We thus hypothesized that upregulation of Activin/Smad3 signaling in the NAc by withdrawal from self-administration mediates cocaine’s ability to regulate MSN structural plasticity. We identified several putative Smad3 gene targets in the NAc based on Smad3 DNA consensus binding sites (β-catenin, NR2A, myocyte enhancer factor 2D, adenylyl cyclase-associated protein 2, drebrin, prodynorphin; Fig. 3i; Primer list Supplementary Table 1), each of which has previously been implicated in actin dynamics and cocaine-induced plasticity11–14. Chromatin immunoprecipitation (ChIP) assays with Smad3 revealed that Smad3 binding was significantly increased with cocaine self-administration at several of these same gene promoters (β-catenin, NR2A, Myocyte enhancer factor 2D, Adenylyl cyclase-associated protein 2, Drebrin; Fig. 3j; ChIP Primer list Supplementary Table 2), suggesting that Activin/Smad3 activity may transcriptionally regulate key modulators of cocaine-induced plasticity in the NAc.
Together, these findings demonstrate a novel pathway in the regulation of cocaine-induced behavioral, cellular, and morphological plasticity. These data establish that upregulation of Activin/Smad3 activity induced by withdrawal from cocaine self-administration is both necessary and sufficient for a cocaine-mediated increase in drug-seeking/taking in a bidirectional manner.
The endurance of these cocaine-seeking behaviors is thought to result from a functional “rewiring” of the brain, involving the alteration of dendritic spine density in the NAc. Recruitment of Activin-receptor signaling following 7 d withdrawal, but not acutely after cocaine self-administration, suggests the adaptations are independent of direct actions of cocaine exposure, but instead may be through long-term transcriptional and epigenetic mechanisms1. Psychostimulant-induced structural plasticity exists along a dynamic continuum, which represents functional changes in synaptic connectivity, strength (i.e., long-term depression and potentiation), and postsynaptic glutamate receptor composition15. Immediately following re-exposure to cocaine, there is a reversion of spine type from the more stable mushroom to the thin type, correlating with changes in synaptic strength that ultimately mediate relapse15–17.
Cocaine-induced spine density changes represent a functional reconfiguration of neural circuits between the NAc and other areas of the brain (e.g., amygdala and prefrontal cortex) that are critical in mediating future drug-related behaviors8,16,18. The increase in mushroom spines, which are thought to be more mature stable synapses with greater synaptic strength, may represent a relative increase in synaptic connectivity onto NAc MSNs and would further promote or attenuate drug-seeking behaviors in a cell type- and afferent-dependent fashion19,20. Future studies will distinguish how Activin-receptor signaling may be differentially regulated in D1- or D2-containing MSNs, or mediate synaptic efficacy of divergent NAc inputs, thus controlling synaptic and behavioral plasticity.
The findings of this study provide a new insight into the molecular basis by which cocaine induces persistent cocaine seeking. The Activin/Smad3 signaling cascade represents a possible mechanism for the long-term behavioral and cellular plasticity that govern relapse behaviors, and provides new directions for the development of novel therapies for cocaine addiction.
Online Methods
Subjects
Naïve male Sprague-Dawley rats (250–275 g) were allowed to habituate to the colony room for 2 d upon arrival. Rats had ad libitum access to food and water and were singly housed following surgery and for the duration of the self-administration phase of the experiments in order to protect the catheter/harness assembly. Behavioral testing took place 7 d/wk during the dark phase of the 12 h light-dark cycle. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications21–23. This study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Self-administration test chambers
Twenty-four standard experimental test chambers (MED Associates, Inc., St. Albans, VT) were used. The intelligence panel had two snout-poke holes located on one wall of the test chamber. Two stimulus lights were mounted above the snout-poke holes, and a house light was mounted in the middle of the back wall of the test chamber. Snout pokes were monitored with infrared detectors. All test chambers were housed in isolation, which mitigates all external light sources and sounds, including the motors for syringe infusion pumps that were located outside of the sound-attenuating chamber. Test chambers were computer controlled through a MED Associates interface with MED-PC. The temporal resolution of the system is 0.01 s.
Drugs
Cocaine hydrochloride, gifted by NIDA, was dissolved in sterile 0.9% saline. Cocaine solutions (acquisition: 4.5 mg/mL; dose-response testing: 2.0 mg/mL) were prepared on a weekly basis. Cocaine was delivered via a syringe pump, and pump durations/injection volumes were adjusted according to body weight on a daily basis in order to deliver the correct dose of drug (acquisition: 1.0 mg/kg/infusion; dose-response testing: 0.03, 0.10, 0.30, 1.00 mg/kg/inf). Systemic injections used in tests of reinstatement were administered at a constant injection volume of 1.0 mL/kg.
Jugular catheterization and patency testing
Rats were implanted with chronic indwelling jugular catheters. The details of this procedure have been described previously24,25. Rats were allowed 7 d to recover following surgery. The catheters were flushed daily with 0.2 mL of a solution of enrofloxacin (4 mg/mL) in heparinized saline (50 IU/mL in 0.9% sterile saline) to preserve catheter patency. At the end of behavioral testing, each animal received an i.v. infusion of ketamine hydrochloride (0.5 mg/kg in 0.05 mL) and the behavioral response was observed to verify catheter patency. Loss of muscle tone and righting reflexes served as behavioral indicators of patency. Only rats with patent catheters were used in data analyses (< 10 % of animals tested for self-administration were excluded from the experiment due to loss of patency).
Regulation of Activin/Smad3 signaling following withdrawal from cocaine self-administration
One week after jugular catheter surgery, the rats were assigned to self-administer either saline or 1.0 mg/kg/inf cocaine. Rats were tested for self-administration for ten test sessions, during which responses to the active alternative resulted in i.v. injections of cocaine (or saline) according to a fixed ratio 1 (FR1) schedule of reinforcement followed by a 30 s timeout period. Infusions were accompanied by a 5 s illumination of the stimulus light above the active snout-poke hole, and the house light was extinguished for the duration of the time-out period. Snout-poke responses to the inactive alternative resulted in no programmed consequences. Session durations were 2 h. Following testing, catheters were flushed and rats were returned to the colony room. The criterion for acquisition of cocaine self-administration was an average of ten infusions per day (< 10 % of animals tested for self-administration were excluded from the experiment due to failure of acquisition of self-administration).
Following self-administration, rats were counterbalanced according to performance and assigned to 1 or 7 d withdrawal groups. For collection of tissue after 1 d of withdrawal (cocaine, n = 7; saline, n = 7), brains were harvested 24 h after the last day of self-administration testing. Rats were sacrificed by rapid decapitation, and brains were removed and sliced into 1-mm-thick sections using a brain matrix, and 2-mm-diameter tissue punches targeted at the NAc shell subregion were collected and rapidly frozen on dry ice. For animals undergoing 7 d of withdrawal (cocaine, n = 7; saline, n = 7), tissue was collected after the rats were returned to their home cages and left undisturbed for one week following the last day of cocaine-self administration.
For animals tested for acute exposure to cocaine, rats were sacrificed 1 h following the last self-administration session (cocaine, n = 5; saline, n = 6). In re-exposure to self-administration experiments, rats were returned to their colony rooms and left undisturbed for 7 d. Rats were subsequently retested for a single self-administration session (2 h self-administration) in an identical manner as in previous self-administration sessions, and sacrificed 24 h after the re-exposure test (saline, n = 6; cocaine, n = 6). Brains were harvested in a manner identical to those described above.
Pharmacological manipulation of Activin-receptor signaling in the NAc
Cocaine dose-response
Rats were first exposed to a 5 d cocaine self-administration period during which animals underwent self-administration training as described above (5 d; 2h/d; 1.0 mg/kg/inf cocaine). On day 6, animals were subsequently trained on a within-session dose-response procedure for 5 additional days that has been described previously with a few modifications21,26–28. Briefly, the 2 h self-administration period was divided into four 30 min components, each preceded by a 2 min time-out period. This arrangement allowed the assessment of a range of cocaine doses in a single session. The cocaine dose per injection was regulated by adjusting infusion volumes and pump durations. Rats were exposed to four doses of cocaine (0.03, 0.10, 0.30, and 1.00 mg/kg/inf) for 30 min. The order of doses tested was pseudo-randomized such that the same doses were never tested in the same order during training. Following each test session, catheters were flushed and rats were returned to the colony room. After 5 d within-session dose-response training (10 d total cocaine self-administration training), rats were exposed to a 7 d withdrawal. During this period, rats were implanted with bilateral guide cannulae (C235G-2.4; Plastics One Inc., Roanoke, VA) aimed at the NAc shell (all coordinates from Paxinos & Watson29; NAc measurements from bregma taken from surface of skull: AP: +1.8, ML: +1.2, DV: −6.5 mm). Animals were handled daily and sham injected during the recovery period in order to habituate them to the microinjection procedure.
Following the 7 d withdrawal period and recovery from cannula surgery, rats were counterbalanced according to self-administration performance and assigned to receive microinjections of an Activin receptor antagonist (SB-431542; Sigma-Aldrich, St. Louis, MO) (0.01 μM/μL dissolved in a mixture of DMSO and PBS (1:2 vol/vol) for a total of 1.0 μl/hemisphere30, n = 8), Activin A (R&D Systems Inc., Minneapolis, MN) (0.5 μg/hemisphere carrier-free dissolved in 0.1% bovine serum albumin [BSA] in PBS for a total of 1.0 μl/hemisphere5, n = 7) or vehicle; vehicles tested were statistically identical (data not shown: DMSO, n = 9; BSA, n = 9; 1.0 μl/hemisphere), and therefore subjects were collapsed into one control group: final n = 18). Microinjections were infused at a rate of 0.5 μL/min and injectors left in place for 10 min to allow for diffusion. Following microinjections, rats were placed in the operant chambers and retested on the within-session dose-response procedure described above. Brains were perfused following this experiment in order to verify cannula placement (< 10 % of animals tested for self-administration were excluded from the experiment due to anatomically incorrect cannula placements).
Drug-induced reinstatement
Following 10 d of cocaine (1.0 mg/kg/inf) self-administration, rats underwent a 7 d withdrawal period during which bilateral guide cannulae were implanted and handled daily as described above. Rats were returned to the experimental chambers for a within-session extinction protocol in which cocaine injections were withheld as previously described5,31. Rats were exposed to extinction sessions in the presence of the house light and the cues that during training had indicated drug availability. The animals were allowed to respond for eight to ten 1 h sessions (separated by 5 min intervals, during which the house light was extinguished) until their responses fell to less than 20 responses per session.
Following extinction, rats were counterbalanced according to self-administration and extinction performances and assigned to receive microinjections of Activin A (same as described above) or vehicle (n = 9 each)4. Following microinjection, rats were injected with 5 mg/kg cocaine (i.p.) and returned to the operant chambers and tested for drug-induced reinstatement. An additional set of rats were assigned to receive microinjections of SB-431542 (same as described above) or vehicle (n = 8 each)30 and were injected with 10 mg/kg cocaine (i.p.).
Food reinforcement
For these experiments, commercially available two-lever operant chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments, Inc., Allentown, PA) were used. Data were collected through an interface using Graphic State 3.03 software (Coulbourn Instruments, Inc., Whitehall, PA). Rats were trained to lever press for food reward (45 mg; BioServ Inc., Frenchtown, NJ). During the daily 1-h training sessions, rats could press the right lever or left lever (both active) under an FR1 schedule and earn up to 50 food pellets. The response requirement was gradually increased to FR10 over a period of 10 d. Rats that did not earn 50 food pellets under an FR10 schedule on day 10 were excluded from the study (n = 2). Following food training, rats were implanted with bilateral guide cannula aimed at the NAc or injected with HSV-GFP (n = 7), HSV-dnSmad3 (n = 7), or HSV-wtSmad3 (n = 8) and allowed 1 wk recovery from surgery before testing. In animals in microinjection experiments, rats received microinjections of SB-431542 (n = 7) or vehicle (DMSO in PBS, n = 7), or Activin A (n = 7) or vehicle (BSA in PBS, n = 7) prior to the test of food reinforcement.
Locomotor activity
Locomotor activity was recorded by an infrared motion-sensor system (AccuScan Instruments, Columbus, OH) fitted outside plastic cages (40 × 40 × 30 cm). The plastic cages contained a thin layer of corn cob bedding and were cleaned between each test session. The Fusion activity-monitoring system software monitors infrared beam breaks at a frequency of 0.01 s. The interruption of any beam not interrupted during the previous sample was interpreted as an activity score. The Versa Max animal activity monitoring software monitors the distance the animal travelled in 1 h. Naïve rats were implanted with bilateral guide cannula aimed at the NAc or injected with HSV-GFP (n = 7), HSV-dnSmad3 (n = 7), or HSV-wtSmad3 (n = 8) and allowed 1 wk recovery from surgery before locomotor activity was assessed. Locomotor activity was also assessed in rats receiving microinjections of SB-431542 (n = 7) or vehicle (DMSO in PBS, n = 7), or Activin A (n = 7) or vehicle (BSA in PBS, n = 7).
Generation of in vivo genetic tools to study the Smad3 pathway
Smad3 plasmids used to make the HSV vectors were generously gifted to us from Dr. Jean-Jacques Lebrun (McGill University). To further understand the mechanistic role of Smad3 signaling in addictive behaviors, we generated herpes simplex virus (HSV) vectors containing dominant negative-Smad3 (dnSmad3) in which serines in the SSXS motif at the c-terminus (which confer activation when phosphorylated and allow for translocation) were mutated to alanines (SAXA)32,33, and a wild-type Smad3 (wtSmad3) in a p1005 transcription cassette expressing green fluorescent protein (GFP) driven by a CMV promoter to allow for neuronal visualization. Such HSV vectors exhibit maximal expression 3–5 d post-injection34. The viruses were validated both in vitro and in vivo prior to use in behavioral experiments.
Alteration of Smad3 signaling in the NAc
Cocaine dose-response
Rats were trained to self-administer cocaine as described above for the microinjection within-session dose-response experiment. Rats were counterbalanced based on their performance, and during the 7 d withdrawal period, rats were assigned to receive bilateral injections of HSV-dnSmad3, HSV-wtSmad3 or HSV-GFP aimed at the NAc shell. HSV-GFP control animals were included for each replication of these experiments as an internal control for within-experiment validity. No behavioral differences were observed between HSV-GFP controls at any time between replicates, thus were collapsed across groups [Final sample sizes: HSV-dnSmad3 (n = 10), HSV-wtSmad3 (n = 11) or HSV-GFP (n = 18)]. Injectors were set at a 10° angle (measurements from bregma taken from surface of skull: AP: +1.7, ML: +2.45, DV: −6.7)35 and the HSV virus was manually infused at a rate of 0.2 μL/min for a total of 1.0 μL/hemisphere. Injectors were left in place for an additional 10 min to allow for diffusion. Rats were tested with the within-session cocaine dose-response procedure 3 d after viral injections, when maximal expression is observed. Brains were perfused following this experiment in order to verify viral targeting (< 10 % of animals tested for self-administration were excluded from the experiment due to anatomically incorrect cannula placements).
Drug-induced reinstatement
Rats were trained to self-administer cocaine as described above for the microinjection drug-induced reinstatement experiment. Rats were counterbalanced based on their self-administration and extinction performance, and during the 7 d withdrawal period after cocaine self-administration training, rats were assigned to receive bilateral injections of HSV-dnSmad3 (n = 9) or HSV-GFP (n = 10). Three days after these injections, rats were injected with 10 mg/kg cocaine (i.p.) and returned to the operant chambers and tested for drug-induced reinstatement for 1 h. In another set of animals, rats were assigned to receive injections of HSV-wtSmad3 (n = 10) or HSV-GFP (n = 11). Three days following these injections, rats were injected with 5 mg/kg cocaine (i.p.), and drug-induced reinstatement was tested.
RNA extraction and quantitative real-time (RT)-PCR for AcvR2a
NAc tissue punches were collected 1 or 7 d after the last cocaine administration and immediately stored at −80°C. RNA was isolated and purified from these samples using Trizol (Invitrogen of Thermo Fisher Scientific, Waltham, MA) and the RNeasy Micro Kit (Qiagen, Venlo, Limburg, the Netherlands) with a DNase step. RNA concentration was measured on a Nanodrop spectrophotometer (ND-100; Thermo Fisher Scientific, Waltham, MA) and 400 ng cDNA was then synthesized using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). mRNA expression changes were measured using quantitative RT-PCR with IQ SYBR Green Supermix (Bio-Rad Laboratories, Inc). Quantification of mRNA changes was conducted using an iQ5 system (Bio-Rad Laboratories, Inc.). Reactions were run in triplicate and analyzed using the ΔΔ CT method as described previously36,37 using GAPDH as a housekeeping gene.
Western blotting
NAc tissue punches from each rat were homogenized in 30 μL of homogenization buffer containing 320 mM sucrose, 5 mM HEPES buffer, 1% SDS, phosphatase inhibitor cocktails 2 and 3 (Sigma Aldrich, Saint Louis, MO; Catalog 2: P5726, 3: P0044), and protease inhibitors (Roche, Basel, Switzerland, Catalog 11-873-590-001). Protein concentrations were determined, and a total of 30 μg of protein was loaded onto 10% Tris-SDS polyacrylamide gels for electrophoresis separation. Proteins were transferred to nitrocellulose membranes, blocked with 5% non-fat milk in PBS, and incubated overnight at 4°C with primary antibodies diluted in Rockland Blocking Buffer (VWR International, Radnor, PA; Catalog RLMB-070-003): anti-rabbit AcvR2a (1:1500; Abcam, Cambridge, MA; ab134082), anti-rabbit p-Smad3 (1:500; Calbiochem of Millipore, Billerica, MA; Catalog PS1023), anti-rabbit Smad3 (1:400; Cell Signaling Technology Inc., Danvers, MA; Catalog 9523), and anti-mouse β-actin (1:10,000; Cell Signaling Technology Inc., Danvers, MA; Catalog 3700). After thorough washing with PBS containing 0.1% Tween-20, membranes were incubated with IRDye secondary antibodies (1:5000; LI-COR, Lincoln, NE; Catalog 926-68072,) dissolved in Rockland Blocking Buffer for 1 h at room temperature. The blots were imaged using the Odyssey Infrared Imaging system (LI-COR, Lincoln, NE) and quantified by densitometry using NIH Image J. The amount of protein loaded into each lane was normalized to β-actin.
Dendritic spine analysis
To study the role of Smad3 in the regulation of NAc shell medium spiny neuron morphology in vivo, we used methods previously described23. Rats injected with HSV constructs were sacrificed 4 h after cocaine-induced reinstatement tests via transcardial perfusion of 0.9% phospho-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were immersed in fixative overnight and then stored in PBS + 0.01% sodium azide. Brains were sectioned at 100 μm on a Vibratome (Leica Microsystems, Wetzlar, Germany) and blocked in 3% normal donkey serum with 0.3% Triton-X for 2 h at 4°C. Sections were then incubated overnight at room temperature in primary antibody (1:1000 anti-rabbit GFP; Molecular Probes of Thermo Fisher Scientific, Waltham, MA) diluted in PBS with 3% normal donkey serum and 0.3% Tween-20. Tissue sections were rinsed and incubated overnight at 4°C in anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA).
Immunofluorescence was imaged on an LSM 510 Meta confocal microscope (Carl Zeiss, Oberkochen, Germany) with a 63× oil-immersion objective. Images were acquired with a pinhole set at 1 arbitrary unit and a 1024 × 1024 frame size. Dendritic length was measured using Image J software, and spine numbers were counted. The average number of spines per 10 μm of dendrite was calculated. An average was obtained from 6–10 neurons per rat (n = 4–5 for each of four groups). MSN cells were located in the NAc shell. Only secondary dendrites that were at least 75 μm from cell body and able to be traced back to cell body were selected for analysis. Spine type analysis was carried out with the semi-automated software Neuron Studio (http://research.mssm.edu/cnic/tools-ns.html) that analyzes dendritic length, dendritic width, spine number, and spine head diameter in three dimensions, allowing for classification into major morphological subtypes associated with spine structure and function. Subsets of neurons were analyzed for spine head diameter (6–8 neurons/animal from 3–4 sections averaged per subject). All confocal acquisition and analyses of spines were conducted by investigators blind to the experimental conditions.
Chromatin immunoprecipitation (ChIP) followed by qPCR
Bilateral NAc punches were obtained from 1-mm-thick coronal brain sections 24 h after 7 d withdrawal from cocaine self-administration. Seven punches from every two animals were pooled for ChIP, and one punch was used for RNA isolation followed by qPCR (as described above using primers listed in Supplementary Table 1). ChIP was performed for Smad3 as described previously36, with minor modifications. Briefly, pooled NAc punches were fixed for 12 min in 1% formaldehyde and then quenched with 2 M glycine for 5 min. The chromatin was solubilized and extracted by cell and nuclear lysis. The chromatin was sheered using a Bioruptor 300 (Diagenode, Liège, Belgium) at 4°C at high sonication intensity for 30 s on/30 s off for 10 min, followed by 10 min of rest, which was repeated three times. Fragment size of 250–1000 bp was verified on a 2% agarose gel. Then, magnetic sheep anti-rabbit beads (Invitrogen, Waltham, MA) were incubated with anti-SMAD3 antibody (ab28379; Abcam, Cambridge, MA) at 4°C overnight on a rotator. Following washing of the magnetic bead/antibody complex, 70 μL (magnetic bead/antibody complex slurry) was incubated with the sheared chromatin sample for 16 h at 4°C. Five percent of each sample of sheared chromatin was used as an input control. Samples were washed with LiCl and Tris-EDTA buffers. Reverse cross-linking was performed at 65°C overnight, and proteins and RNA were removed using proteinase K (Invitrogen, Waltham, MA) and RNase (Roche, Basel, Switzerland) respectively. DNA was purified using a DNA purification kit (Qiagen, Venlo, Netherlands). Additionally, a normal IgG control was performed to test for nonspecific binding. Levels of specific Smad3-modifications at each gene promoter of interest were determined by qPCR (iQ5 system; Bio-Rad Laboratories, Inc., Hercules, CA). Specific primers were designed to amplify proximal promoter regions < 1000 bp long (listed in Supplementary Table 2). Input and immunoprecipitated DNA amplification reactions were run in triplicate with IQ SYBR Green (Bio-Rad Laboratories, Inc., Hercules, CA). Fold changes were calculated as cocaine relative to saline control (n = 5–7/group).
Statistical analyses
Statistical analyses were conducted using SPSS statistical software (IBM Corp, Armonk, NY). The primary dependent measures were: number of infusions for self-administration, the number of active responses during drug-induced reinstatement, spine density and spine head diameter, fold change (mRNA), relative density (protein), fold change (ChIP), rate of lever pressing (food reinforcement), number of food reinforcers earned (food reinforcement), and total distance traveled (cm: locomotor activity). Shapiro-Wilks test of normality and Bartlett’s tests of homogeneity of variance were conducted to test for normal distribution. In events that normal distribution could not be assumed, non-parametric tests were utilized. Performance during the self-administration was analyzed using a repeated measures two-factor within-subject analysis of variance (ANOVA), with drug (cocaine/saline) as the between-session variable, and time (day of testing) as the within-subject variable using Tukey’s post-hoc tests to determine the source of significance. Performance during the within-session dose response was analyzed using a two-factor repeated measures ANOVA, with virus (HSV-GFP, -dnSmad3, -wtSmad3) or microinjection (vehicle, Activin A, SB-431542) as the between-session variables, and dose as the within-subject variable, using Tukey’s post-hoc tests to determine the source of significance. Two-factor ANOVAs with drug (cocaine/saline) and virus (HSV-GFP, -dnSmad3, -wtSmad3) or microinjection (vehicle, Activin A, SB-431542) or withdrawal period (1 or 7 d) were conducted on tests of Western blot, and dendritic spine analysis. Follow-up Sidak’s or Tukey’s post-hoc tests were conducted where appropriate to determine the source of significance. Student’s t-tests were conducted on tests of drug-induced reinstatement, food reinforcement, locomotor activity and one-factor ANOVAs were conducted on tests of food reinforcement, locomotor activity, qPCR, and ChIP followed by Tukey’s or Fisher’s LSD post-hoc tests, which were corrected for multiple comparisons. Data distribution was assumed to be normal, but this was not formally tested. Significance was set at P < 0.05, and data are presented as the mean ± standard error of the mean. Full statistical values and tests are available in the supplementary methods checklist.
Supplementary Material
Acknowledgments
This work was supported by R01DA037257 (D.M.D.), NIAAA T-32-AA007583, and GM09545902. We would like to thank Danielle Adank for her technical assistance with the self-administration, and Drs. Jun-Xu Li, David Thorn, and Justin Siemian for their assistance in conducting the food reinforcement experiments. We thank Dr. Fraser Sim for his technical expertise in microscopy. We thank Dr. Jean-Jacques Lebrun (McGill University) for the gifted Smad3 constructs. We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo. The cocaine used in these experiments was gifted by NIDA.
Footnotes
The authors of this manuscript report no biomedical financial interests or potential conflicts of interest.
Author Contributions
A.M.G., Z-J. W., G.L.S., K.B., L.E.M., A.C., and J.A.M. performed behavioral experiments. A.M.G., Z-J.W., M.S.H., and G.L.S. performed all Western Blots, mRNA, and ChIP experiments. A.M.G. and D.D-W. conducted dendritic spine experiments. R.L.N. constructed and provided novel HSV constructs. A.M.G., K.C.D., and D.M.D. designed experiments, analyzed data, and wrote the manuscript. All the authors have read and approved the final version of the manuscript.
References
- 1.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 3.Ageta H, et al. Activin plays a key role in the maintenance of long-term memory and late-LTP. Learn Mem. 2010;17:176–185. doi: 10.1101/lm.16659010. [DOI] [PubMed] [Google Scholar]
- 4.Dow AL, Russell DS, Duman RS. Regulation of activin mRNA and Smad2 phosphorylation by antidepressant treatment in the rat brain: effects in behavioral models. J Neurosci. 2005;25:4908–4916. doi: 10.1523/JNEUROSCI.5155-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gancarz-Kausch AM, Adank DN, Dietz DM. Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Sci Rep. 2014;4:6876. doi: 10.1038/srep06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, et al. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. PNAS. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AC, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. The EMBO Journal. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman WM, et al. Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neuroscience. 2010;11:29. doi: 10.1186/1471-2202-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reissner KJ, et al. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–5658. doi: 10.1523/JNEUROSCI.3452-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen HW, et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 18.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Ann Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 20.Lobo MK, Nestler EJ, Covington HE., 3rd Potential utility of optogenetics in the study of depression. Biol Psychiatry. 2012;71:1068–1074. doi: 10.1016/j.biopsych.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scobie KN, et al. Essential role of poly(ADP-ribosyl)ation in cocaine action. PNAS. 2014;111:2005–2010. doi: 10.1073/pnas.1319703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gancarz-Kausch AM, et al. Transforming growth factor beta receptor 1 is increased following abstinence from cocaine self-administration, but not cocaine sensitization. PloS one. 2013;8:e83834. doi: 10.1371/journal.pone.0083834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz DM, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra R, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. FNMOL. 2013;6:13. doi: 10.3389/fnmol.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gancarz AM, Kausch MA, Lloyd DR, Richards JB. Between-session progressive ratio performance in rats responding for cocaine and water reinforcers. Psychopharmacology. 2012;222:215–223. doi: 10.1007/s00213-012-2637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantsch JR, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- 27.Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Elselvier; 2009. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Mizunoya W, Shibakusa T, Inoue K, Fushiki T. Transforming growth factor-beta in the brain regulates fat metabolism during endurance exercise. Am J Physiol Endocrinol Metab. 2006;291:E1151–1159. doi: 10.1152/ajpendo.00039.2006. [DOI] [PubMed] [Google Scholar]
- 31.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chipuk JE, et al. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277:1240–1248. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Song K, Sponseller TL, Danielpour D. Novel function of androgen receptor-associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem. 2005;280:5154–5162. doi: 10.1074/jbc.M411575200. [DOI] [PubMed] [Google Scholar]
- 34.Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. BioTechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- 35.Robison AJ, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson D, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.