Abstract
Background: Medication use is associated with falls in many populations, but the relationship between medications and falls in people with multiple sclerosis (MS) is not well understood.
Methods: The number and types of medications used by 248 ambulatory adults with MS in the United States (n = 53) and Australia (n = 195) were assessed. Participants completed fall diaries for 6 months. Associations between number and type of medications reported and falls, adjusting for age, disease severity, comorbidities, sex, and country, were evaluated using multiple logistic regression.
Results: Participants reported taking a median of three medications and two supplements. A total of 143 participants (58%) fell at least once in the 6 months, and 110 (44%) experienced one or more injurious falls. The adjusted relative odds of a fall or an injurious fall increased by 13% (P = .048) and 11% (P = .049), respectively, for each medication and by 43% (P = .015) and 55% (P = .001) for each neurologically active medication. Reported use of MS disease-modifying therapy was associated with 48% decreased odds of falling (P = .035) but not significantly decreased odds of injurious falls.
Conclusions: Reporting use of more medications and more neurologically active medications is associated with falls and injurious falls in people with MS. Close evaluation of the need for each medication, with associated minimization of neurologically active medications in patients with MS, may help prevent falls. Use of MS disease-modifying therapies may be associated with fewer falls. This relationship needs further evaluation.
People with multiple sclerosis (MS) have a high incidence of falls.1–5 More than 50% of people with MS fall in a 3- to 6-month period, and approximately 30% to 50% fall multiple times.4–6 Falls in people with MS are associated with injuries3,7,8 and adversely affect quality of life.9
Fall risk in MS is generally attributed to impairments in the wide range of functions affected by the disease, including balance, cognition, vision, muscle strength and tone, coordination, and sensation.10,11 Although there are limited interventions available to modify these endogenous fall risk factors, modifiable exogenous factors may also contribute to fall risk in MS. In older people, in whom fall risk is most well understood, the use of multiple medications,12 particularly psychotropics, has been found to significantly increase fall risk,13,14 and modifying medication prescription can prevent falls.15,16
Many patients with MS are prescribed and take multiple medications, including many neurologically active drugs. For example, they may be prescribed benzodiazepines for spasticity, stimulants for fatigue, and narcotics for pain. The only published study that included evaluation of the association between medication use and fall risk in MS reported that use of prescription medications increased the odds of falling by 12% for each prescription medication, but this study did not evaluate the effects of specific medication types on fall risk or the effects of medications on the risk of injurious falls.4
The purpose of this study was to examine the relationships between medications and falls, including injurious falls, in people with MS. The primary hypothesis was that the number of medications reported is significantly associated with prospectively ascertained falls and injurious falls after adjusting for appropriate covariates (age, disease severity, presence of comorbidities, sex, and country). We also evaluated the associations between medication type, as well as supplements, and falls and injurious falls.
Methods
Protocol Approvals and Participant Consents
The data used in the present analysis were obtained from previous prospective cohort studies of imbalance and falls in MS performed in the United States and Australia. Participants in these studies were recruited in 2010 and 2011. The US cohort was recruited from the outpatient MS specialty clinics at the Portland Veterans Affairs Medical Center and Oregon Health & Science University (both in Portland, OR) and from the surrounding areas. The Australian cohort was recruited from the outpatient physiotherapy clinic at the MS Australia Center in Lidcombe, Australia. The institutional review boards at all three institutions approved the protocol, and written informed consent was obtained from all the participants.
Study Design
This is an analysis of post hoc pooled data from two observational cohort studies, one in the United States and one in Australia. This analysis evaluated the relationship between reported medication use and subsequent falls. A randomized controlled trial of the association between multiple medication use and the adverse effect of falls is not feasible or ethical given the other potential risks associated with prescribing unneeded medications.
Participants
Data from two studies were pooled to increase sample size and enhance generalizability. The inclusion criteria for the US and Australian cohorts were age older than 18 years, a clinically confirmed diagnosis of MS (McDonald criteria 2005) of any subtype, being willing and intellectually able to understand and sign an informed consent form and to adhere to protocol requirements, ability to complete a written daily record of falls for 6 months, and being community dwelling. The exclusion criterion was inability to understand and follow directions in English. In addition to these criteria, the US cohort had an upper age limit of 50 years, required magnetic resonance imaging confirmation of the MS diagnosis, had mild-to-moderate MS-associated disability (Expanded Disability Status Scale [EDSS] score ≤6.0), and were excluded for conditions other than MS known to affect balance or gait, unhealed fractures or other conditions conveying risk of injury during balance testing, blindness, inability to walk more than 100 m, or a clinically significant MS relapse within 30 days before baseline testing. In addition to the criteria for both cohorts, the Australian cohort needed to be able to stand unsupported for at least 30 seconds and walk 10 m with or without an aid (MS disease step ≤5).17
Medications
Medications were reported by participants on self-report questionnaires completed on enrollment at baseline. MS disease-modifying therapies (DMTs) and antispasticity medications were listed on the questionnaire by name, with corresponding boxes to check off. Participants were asked to write in all other prescription medications, over-the-counter medications, and supplements (including vitamins and herbal and natural remedies) that they were taking. Medications written on the questionnaires in free-text fields were extracted computationally using the Python release 2.7.2 programming language and were then manually checked for errors. Medications were then classified using the first level of the Anatomical Therapeutic Chemical (ATC) classification system, which consists of 14 categories indicating the main anatomical system affected by the medications.18 These categories are as follows: 1) alimentary tract and metabolism, 2) anti-infectives for systemic use, 3) antineoplastic and immunomodulating agents, 4) blood and blood-forming organs, 5) cardiovascular system, 6) genitourinary system and sex hormones, 7) musculoskeletal system, 8) nervous system, 9) respiratory system, 10) systemic hormonal preparations (excluding sex hormones), 11) dermatologic agents, 12) sensory organs, 13) antiparasitic products, and 14) various. MS DMTs are not an ATC medication category, but they all fall into category 3, antineoplastic and immunomodulating agents.
Additional Baseline Measures
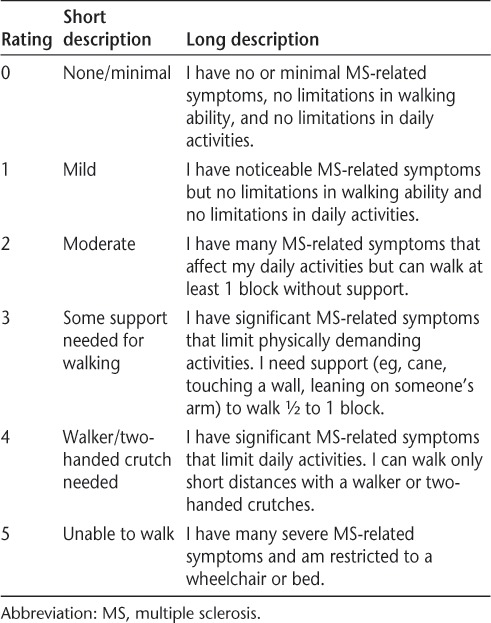
Demographic data (age and sex), years since MS diagnosis, MS subtype, and other medical conditions were obtained by medical record review and self-report questionnaires at baseline. MS-associated disability was determined using the self-reported MS Disease Severity Rating Scale.19 This scale allows participants to rate their disease severity from 0 (having minimal MS-related symptoms and no walking limitations) to 5 (having severe MS-related symptoms and being unable to walk) (Table 1). This scale was designed to provide patients with an easy-to-use measurement of their own condition by incorporating disability, symptom severity, and the impact of disease severity on daily activity into one rating scale. This scale was selected for this study owing to its ease of use compared with the self-rated EDSS. Scores on the MS Disease Severity Rating Scale correlate highly with neurologist-rated EDSS scores (r = 0.85).19
Table 1.
Self-rated MS Disease Severity Rating Scale completed by all the study participants19
Prospectively Counted Falls
Falls and injurious falls in the 6 months after baseline assessment were assessed prospectively by participants documenting their falls each day on monthly fall calendars and returning these calendars at the end of each month. Calendars and diaries are the current gold standard for collecting fall data prospectively.20 The calendar stated, “Please write in the number of falls you have each day. A fall is any unexpected event that results in you ending up on the ground, floor, or any lower surface.”20,21 Participants also documented each month whether they experienced any injuries, including bruises, cuts, grazes, fractures, dislocations, sprains, strains, pain, or other injuries, as a result of a fall and whether medical attention was sought. When medical attention was sought, participants documented the type of medical service used (eg, general practitioner visit, specialist doctor, emergency department visit, inpatient care, or other) and the number of visits required (or duration of admission).
Data Analysis
Data were analyzed to ensure that assumptions of normality were satisfied and were summarized using means and standard deviations or frequencies. Differences in demographic characteristics between those who fell or did not fall and between those who did or did not sustain an injurious fall were evaluated using independent-sample t tests for continuous variables and Fisher exact tests for categorical variables.
Associations between the number of medications taken and the odds of sustaining a fall or an injurious fall were evaluated by multiple logistic regression. The regression models included adjustments for age (categorized by 10-year increments), sex, presence of one or more comorbidities, MS disease severity, and country. The use of a walking aid and MS disease subtype were evaluated as potential confounding variables and were removed from the final regression model because they were determined not to be influential. Multiple logistic regression including the same covariates was used to evaluate the associations between the number of dietary supplements taken and falls.
To evaluate the associations between the use of nervous system and cardiovascular system medications, the number of medications in these categories was used in the regression models. For all other categories, because the number of medications used was too small to be used as a continuous variable, use or nonuse of the medication class was used in the regression models.
To evaluate whether specific types of nervous system medications were associated with falls, we further categorized the nervous system medications into the following eight subcategories, derived from subsequent levels of the ATC classification system: analgesics, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), antidepressants, antiepileptics, antipsychotics, benzodiazepines, sympathomimetics, and tricyclic antidepressants. Associations between the use or nonuse of medications in these subcategories and falls were also evaluated by multiple logistic regression.
The significance level was set at P < .05 for all the analyses. All the statistical analyses were performed using R version 2.14.0 software (http://www.R-project.org).
Results
Participant Characteristics
All the analyses are based on data from the 53 participants in the US cohort and 195 in the Australian cohort who returned at least three fall calendars and had complete demographic information. There were no statistically significant differences in demographic characteristics between the 248 participants with complete data and the 20 participants with insufficient data for inclusion.
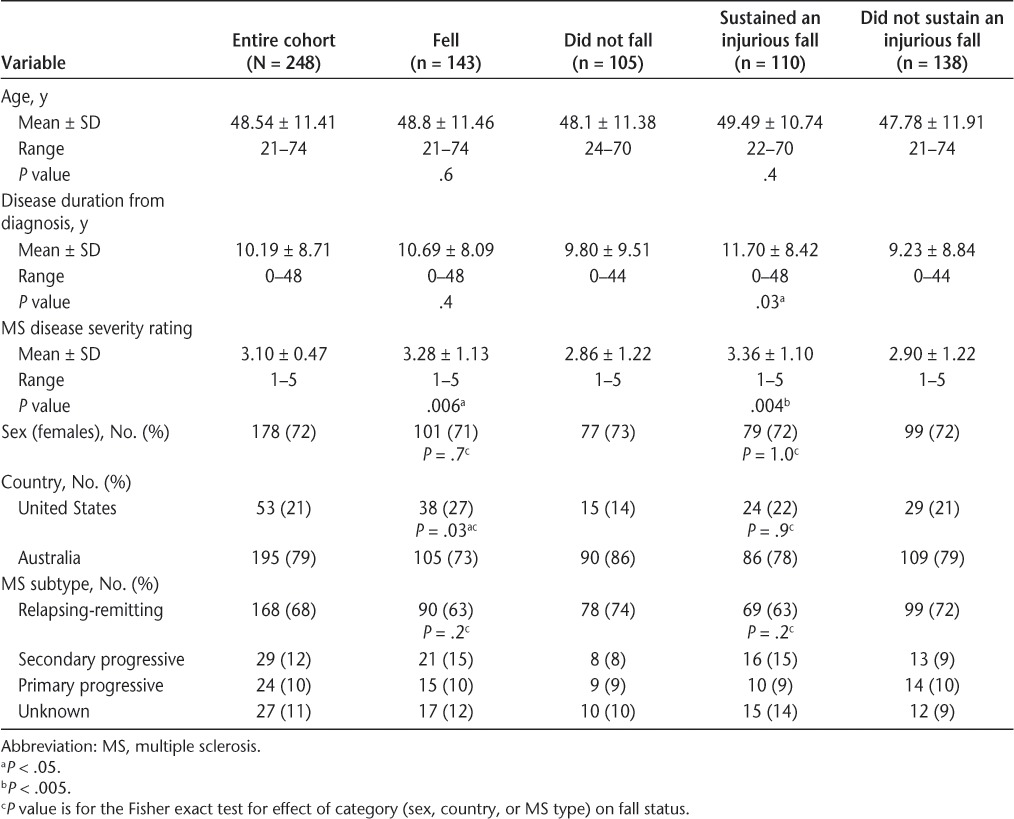
The demographic and MS disease characteristics of the study participants are summarized in Table 2. The participants' mean age was 48 years, 72% were female, and 68% had relapsing-remitting MS. This age, sex, and MS disease subtype distribution is typical for people with MS. The mean duration of disease was 10 years, and the mean disease severity rating was 3.1 (ie, some support needed for walking). Forty percent of the sample reported using a walking aid. A higher proportion of the US sample fell compared with the Australian sample. Those who fell and those who sustained an injurious fall had more severe disease than nonfallers, and those who sustained an injurious fall had statistically significantly longer duration of disease than those who did not sustain an injurious fall.
Table 2.
Demographic and sample characteristics for all 248 participants by fall status
Medication Use
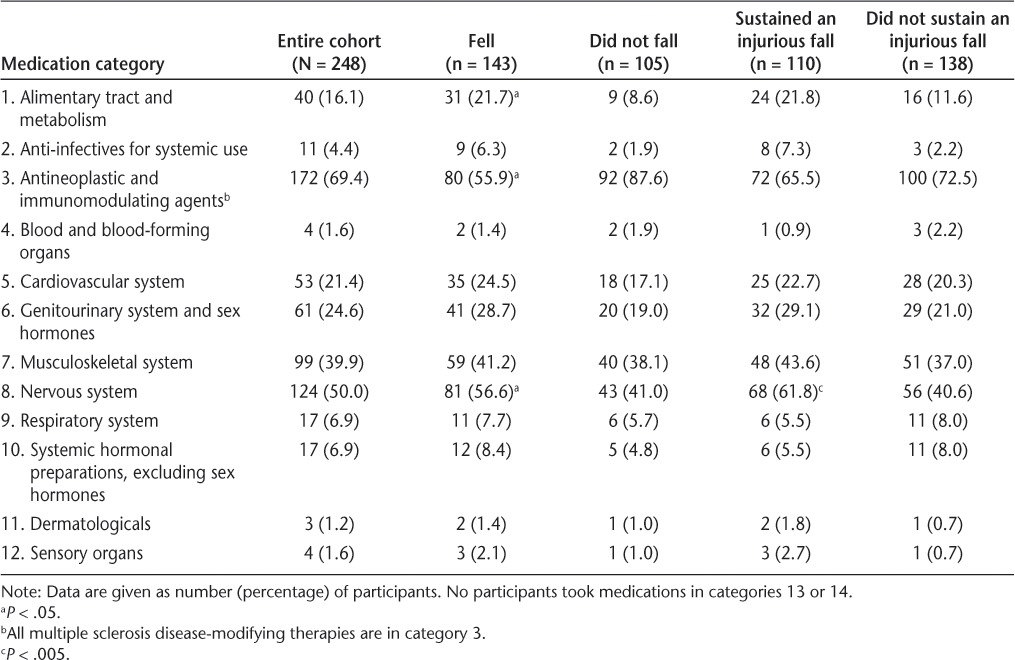
Participants reported taking 0 to 19 medications (median = 3), 0 to 11 supplements (median = 1), and medications from 12 of the 14 ATC categories. No participants took medications in category 13 (antiparasitic products) or category 14 (various). Participants took up to five cardiovascular system medications, up to eight nervous system medications, and up to three medications from the remaining ATC classes. A total of 172 participants (69%) reported taking MS DMTs; 81% of these had relapsing-remitting MS, 8% had secondary progressive MS, 5% had primary progressive MS, and 6% had an unknown MS disease subtype.
Falls Incidence
One hundred forty-three participants (58%) reported at least one fall during the 6 months, and 110 (44%) reported one or more injurious falls. The mean number of falls during the 6 months in those who fell was 9 (range, 1–180).
Number of Medications Reported and Falls
Reporting use of more medications was associated with a significantly increased risk of falling. The adjusted odds of falling increased by 13% for each medication reported (odds ratio [OR] = 1.13, 95% confidence interval [CI] = 1.00–1.28, P = .048). The association between the number of dietary supplements reported and falling was not significant (P = .143).
Types of Medications Reported and Falls
Reporting use of more nervous system medications was associated with a significantly increased risk of falling (Table 3). For each nervous system medication, the odds of falling increased by 43% (OR = 1.43, 95% CI = 1.09–1.93, P = .015). Use of any alimentary tract and metabolism medications was also associated with increased odds of falling (OR = 2.64, 95% CI = 1.20–7.23, P = .02). Use of any antineoplastic immunomodulating medications was associated with a 48% decreased odds of falling (OR = 0.52, 95% CI = 0.28–0.95, P = .035), and this association was entirely due to the use of MS DMTs.
Table 3.
Use of medications in each category for all 248 participants by fall status
Of the nervous system medications, the subcategory of SSRI and SNRI antidepressants was associated with a 96% increased odds of falling (OR = 1.96, 95% CI = 1.7–3.71, P = .03). No other medication category or subcategory was associated with increased odds of falling.
Medication Use and Injurious Falls
The associations between medication use and injurious falls were similar to the associations between medication use and falls (Table 3). The adjusted odds of sustaining an injurious fall increased by 11% for each medication taken (OR = 1.11, 95% CI = 1.00–1.25, P = .049), and there was no significant association between the number of dietary supplements taken and sustaining an injurious fall (P = .437).
The only level 1 category of medications associated with a significantly increased odds of sustaining an injurious fall was nervous system medications (OR = 1.55, 95% CI = 1.20–2.05, P = .001). Of the nervous system medications, the subcategory of SSRI and SNRI antidepressants was associated with a significantly increased odds of an injurious fall (OR = 2.87, 95% CI = 1.60–5.27, P = .0005). No medication category was associated with a decreased risk of injurious falls.
Discussion
This is the first study, to our knowledge, to evaluate the relationships between specific and multiple medication use with falls and injurious falls in people with MS. After adjusting for age, MS disease severity, comorbidities, sex, and country of residence, we found that more medications, more nervous system medications (particularly SSRI and SNRI antidepressants), and any alimentary tract and metabolism medications were all associated with prospectively ascertained falls and injurious falls. In contrast, use of MS DMTs was associated with lower fall risk.
The proportions of fallers and injurious fallers found in this study, 58% and 44%, respectively, for the entire sample are consistent with other studies of falls in people with MS.1–4,7,11,22–25 However, the US sample fell more (72% fallers) than has been found in previous studies and more than the Australian sample, despite on average being less disabled and younger than the Australian sample. The reasons for this difference are uncertain but may be related to younger age, which has been found to be associated with increased fall risk in MS,26 or to other unmeasured differences in the samples or in health-care delivery between countries.
The association between the use of more medications and falls is consistent with the recently published report of a statistically significant 12% increased odds of falls with the use of more medications in a cohort of people with MS in the United Kingdom4 and the well-documented association between the use of multiple medications and falls in older adults.27
The use of neurologically active medications and, specifically, antidepressants, irrespective of subgroup, is an established risk factor for falls in older people.13,28 This study indicates that the use of neurologically active medications, particularly SSRIs and SNRIs, is associated with falls in people with MS. In older adults, the newer SSRIs do not seem to be associated with less fall risk than the older tricyclic antidepressant medications.29 Despite their more favorable cardiovascular adverse effect profile, SSRIs may still cause orthostatic hypotension and syncope owing to their inhibition of calcium and sodium channels.30 It is also possible that the association between these types of medications and falls may not reflect that the medications are causing falls but rather that the underlying disorder or symptom they are treating contributes to fall risk. For example, depression, for which antidepressants are prescribed, is a strong risk factor for falls,31 and pain, for which many neurologically active medications may be prescribed, may also predispose a person to falling.
Alimentary tract and metabolism medications have not previously been associated with fall risk in people with MS or in other populations. This drug category includes medications used to treat diabetes, nausea, diarrhea, and constipation. It is possible that the association between these medications and falls in people with MS reflects the presence of spinal cord disease, as spinal cord lesions can cause constipation and may increase fall risk by causing lower-extremity weakness, spasticity, and sensory loss. However, because we did not have information on MS lesion locations and because too few participants were taking sufficient numbers of different subcategories of alimentary tract medications, we cannot further elucidate reasons for this association. This association may also reflect association between conditions treated by these medications, particularly diabetes, which is a known risk factor for falls. The use of dietary supplements was not associated with falls in this study, a finding that contrasts with a finding from the United Kingdom showing a decreased risk of falls in participants using over-the-counter medications (predominantly dietary supplements).4 This inconsistency between the studies may be due to differing use of dietary supplements in countries with different medical systems.
The finding of a reduction in falls associated with MS DMT use is surprising but encouraging. These medications have been shown to reduce MS relapse rates and to slow the accumulation of disability in people with relapsing MS.32–35 The association between MS DMT use and fewer falls observed was significant and remained so after adjusting for disease severity, and there was no association between MS disease subtype and falls. This suggests that MS DMTs have benefits for fall prevention across MS disease severity levels and disease subtypes. This apparent benefit may result from positive effects of MS DMTs on factors not well captured in the assessment of disease severity but that affect fall risk, such as cognition, fatigue, balance, and coordination. Alternatively, the association between MS DMT use and fewer falls may reflect other uncaptured features, such as access to medical care and socioeconomic factors, which could influence both MS DMT use and fall risk. Assessment of falls in a randomized controlled trial of MS DMTs is needed to definitively evaluate the effect of DMTs on fall risk.
Despite participants in this study taking various medications that reduce symptoms of MS, such as spasticity and urinary incontinence, associated with fall risk, no medication class other than MS DMTs was found to be associated with a reduced risk of falls. This is likely because use of these types of medications is as much an indicator of having the problem they are intended to treat as of effective control of the symptom.
This study has several strengths. It is one of the largest cohorts of prospectively counted falls in people with MS. This study also includes participants from more than one country and used a combined computerized and manual approach to optimize the accuracy and completeness of medication counts and categorization. These factors allowed us to analyze associations not only between the number of medications taken and the number and severity of falls but also between the specific types of medications taken and the number and severity of falls.
This study also has several limitations. Medication use and level of MS-related disability were determined only at baseline and only by patient self-report. Medication prescription or use was not verified through medical record review or by serum or urine testing at baseline or at the time of falls, and level of disability was not verified through clinician EDSS rating. In addition, this study did not distinguish medications taken on a scheduled basis from those taken only as needed and also did not evaluate the doses prescribed or taken. Patients' self-report of medication use may err toward underreporting or overreporting. Poor recall, particularly in people with potentially MS-associated cognitive impairment and who use multiple medications, seems most likely to result in underreporting of medication use. This would likely bias findings in the direction of the null. Overreporting of medication use is also possible, with patients not taking “as needed” medications at the time of a fall or not taking medications intended to be taken regularly owing to nonadherence or poor adherence. Furthermore, although we did capture self-reported use of dietary supplements, we did not ask about the use of other drugs, such as alcohol or marijuana, which may also affect fall risk. Because participants were primarily drawn from MS specialty centers, there may also have been a bias toward recruitment of participants with more severe MS and greater concerns about imbalance and falls than the general population of patients with MS.
Conclusion
The results of this study demonstrate an association between reporting using multiple medications at baseline, particularly neurologically active medications, and falls in the following 6 months. This finding may reflect the overall severity of ill health in patients taking multiple medications or the effect of disorders requiring neurologically active medications on fall risk. However, because these relationships were present even after adjusting for MS disease severity and the presence of comorbidities, it suggests that prescribers should consider the risk of falls and injurious falls in the risk-benefit assessment of prescribing medications for their patients with MS. Prescribers should also consider the association between a decreased risk of falls and the use of MS DMTs in patients with MS. Further research is needed to determine whether the observed associations between medication use and falls in people with MS reflect causality and whether interventions that safely and effectively reduce polypharmacy in patients with MS help prevent falls in this at-risk population.
PracticePoints.
The use of more medications and more neurologically active medications is associated with more falls and injurious falls in people with MS.
The use of MS disease-modifying therapies is associated with fewer falls.
Patients with MS may benefit from discussing the need for each medication, particularly neurologically active medications, with their health-care providers because this may help prevent falls.
Footnotes
Financial Disclosures: Dr. Cameron has received research support from the US Department of Veterans Affairs, Acorda Therapeutics, the Collins Medical Trust, and the National MS Society and honoraria for speaking and consulting from Acorda Therapeutics, Genzyme, and the Multiple Sclerosis Association of America. Dr. Bourdette has received research support from the National Institutes of Health, the US Department of Veterans Affairs, and the National MS Society and honoraria for speaking and consulting from Biogen Idec, Teva Neurosciences, and Elan Pharmaceuticals.
Funding/Support: Dr. Cameron was supported by a Career Development Award from the US Department of Veterans Affairs, Rehabilitation Research and Development Service (award E7244W). The Australian arm was supported by an Australian Multiple Sclerosis Research Australia grant. Drs. Hoang and Lord are supported by Australian National Health and Medical Research Council Research Fellowship Awards. Dr. Karstens was supported by National Library of Medicine training grant 5 T15 LM 7088-20.
References
- 1.Cattaneo D, De Nuzzo C, Fascia T, Macalli M, Pisoni I, Cardini R. Risk of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil. 2002;83:864–867. doi: 10.1053/apmr.2002.32825. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda PN, Shumway-Cook A, Bamer AM, Johnson SL, Amtmann D, Kraft GH. Falls in multiple sclerosis. PM R. 2011;3:624–632. doi: 10.1016/j.pmrj.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Cameron MH, Poel A, Haselkorn J, Linke A, Bourdette D. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehabil Res Dev. 2011;48:13–20. doi: 10.1682/jrrd.2009.12.0192. [DOI] [PubMed] [Google Scholar]
- 4.Gunn H, Creanor S, Haas B, Marsden J, Freeman J. Risk-factors for falls in multiple sclerosis: an observational study. Mult Scler. 2013;19:1913–1922. doi: 10.1177/1352458513488233. [DOI] [PubMed] [Google Scholar]
- 5.Gunn HJ, Newell P, Haas B, Marsden JF, Freeman JA. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysis. Phys Ther. 2013;93:504–513. doi: 10.2522/ptj.20120231. [DOI] [PubMed] [Google Scholar]
- 6.Hoang P, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil. 2014;95:480–486. doi: 10.1016/j.apmr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil. 2008;89:1031–1037. doi: 10.1016/j.apmr.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Bazelier MT, Van Staa T, Uitdehaag BMJ, Cooper C, Leufkens HGM, Vestergaard P. The risk of fracture in patients with multiple sclerosis: the UK general practice research. J Bone Miner Res. 2011;26:2271–2279. doi: 10.1002/jbmr.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler. 2007;13:1168–1175. doi: 10.1177/1352458507079260. [DOI] [PubMed] [Google Scholar]
- 10.Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010;10:407–412. doi: 10.1007/s11910-010-0128-0. [DOI] [PubMed] [Google Scholar]
- 11.Sosnoff JJ, Socie MJ, Boes MK et al. Mobility, balance and falls in persons with multiple sclerosis. PLoS One. 2011;6:e28021. doi: 10.1371/journal.pone.0028021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 13.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis, I: psychotropic drugs. J Am Geriatr Soc. 1999;47:30–39. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 14.Lord SR, Anstey KJ, Williams P, Ward JA. Psychoactive medication use, sensori-motor function and falls in older women. Br J Clin Pharmacol. 1995;39:227–234. doi: 10.1111/j.1365-2125.1995.tb04441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie LD, Robertson MC, Gillespie WJ et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;2:007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29:359–376. doi: 10.2165/11599460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255. doi: 10.1212/wnl.45.2.251. [DOI] [PubMed] [Google Scholar]
- 18.WHO Collaborating Centre for Drug Statistics Methodology. World Health Organization (WHO) guidelines for ATC classification and DDD assignment. http://www.whocc.no/atc_ddd_index. Updated 2013. Accessed August 23, 2013.
- 19.Shinto L, Yadav V, Morris C, Lapidus JA, Senders A, Bourdette D. Demographic and health-related factors associated with complementary and alternative medicine (CAM) use in multiple sclerosis. Mult Scler. 2006;12:94–100. doi: 10.1191/1352458506ms1230oa. [DOI] [PubMed] [Google Scholar]
- 20.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C, Prevention of Falls Network Europe and Outcomes Consensus Group Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 21.Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C, PROFANE-Group Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing. 2006;35:5–10. doi: 10.1093/ageing/afi218. [DOI] [PubMed] [Google Scholar]
- 22.Cameron MH, Nilsagard Y. Falls in people with MS: a transatlantic perspective. Paper presented at: 65th American Academy of Neurology annua. meeting; March 16–23, 2013; San Diego, CA. Abstract P04(106)
- 23.Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:1274–1279. doi: 10.1016/j.apmr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Gianni C, Prosperini L, Jonsdottir J, Cattaneo D. A systematic review of factors associated with accidental falls in people with multiple sclerosis: a meta-analytic approach. Clin Rehabil. 2014;28:704–716. doi: 10.1177/0269215513517575. [DOI] [PubMed] [Google Scholar]
- 25.Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil. 2009;23:259–269. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- 26.Nilsagard Y, Gunn HJ, Freeman J et al. Falls in people with MS: an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult Scler. 2015;21:92–100. doi: 10.1177/1352458514538884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartikainen S, Lonnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172–1181. doi: 10.1093/gerona/62.10.1172. [DOI] [PubMed] [Google Scholar]
- 28.Woolcott JC, Richardson KJ, Wiens MO et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Blackwell TL, Mangione CM et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–1637. doi: 10.1046/j.1532-5415.2002.50453.x. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Ungvari Z. Selective serotonin-reuptake inhibitor antidepressants increase the risk of falls and hip fractures in elderly people by inhibiting cardiovascular ion channels. Med Hypotheses. 2001;57:469–471. doi: 10.1054/mehy.2001.1366. [DOI] [PubMed] [Google Scholar]
- 31.Kvelde T, McVeigh C, Toson B et al. Depressive symptomatology as a risk factor for falls in older people: systematic review and meta-analysis. J Am Geriatr Soc. 2013;61:694–706. doi: 10.1111/jgs.12209. [DOI] [PubMed] [Google Scholar]
- 32.Kappos L, Antel J, Comi G et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 33.Kappos L, Gold R, Miller DH et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 34.Paty DW, Li DK, UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, II: MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 35.Polman CH, O'Connor PW, Havrdova E et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]


