Abstract
Background: Multiple sclerosis (MS) may negatively affect individuals' participation in physical activity (PA). We used accelerometers to determine PA level in individuals with MS with varying degrees of disability as measured by the Expanded Disability Status Scale (EDSS) during regular daily activities.
Methods: Participants wore an accelerometer from 8 a.m. to 9 p.m. for 7 consecutive days. Activity counts recorded during this period were analyzed in 1-minute epochs and categorized into one of four PA levels: light, moderate, hard, and very hard.
Results: The study cohort comprised 13 patients with MS and 12 controls. There were significant negative correlations for minutes spent in PA and EDSS measures on weekdays (r = −0.61), weekend (r = −0.64), and full week (r = −0.61) and number of steps taken on weekdays (r = −0.56), weekend (r = −0.80), and full-week average (r = −0.68). Significant positive correlations were found for minutes spent in light PA and EDSS score (r = 0.69). Significant negative correlations were found for minutes spent in moderate and hard PA and EDSS score. No significant difference was seen between the MS group and controls on any parameters (P > .05).
Conclusions: This study showed that accelerometers can be used to objectively quantify PA levels in individuals with MS with different disability levels. This cohort demonstrated that the amount of PA is inversely proportional to the degree of physical disability. Collected data revealed not only the amount but also the intensity of PA performed in real-life circumstances.
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system. It has the potential to negatively affect participation in physical activity (PA) owing to muscle weakness, imbalance, spasticity, fatigue, thermosensitivity, and fear of worsening of disease manifestations. Nearly 75% of individuals with MS report some degree of ambulatory difficulties.1
Physical activity is defined as any form of muscular activity or bodily movement produced by skeletal muscle contraction resulting in increased energy expenditure.2,3 More severe MS symptoms, including fatigue and thermosensitivity, have been associated with lower levels of PA participation.4–6 In the past, patients with MS were instructed not to exercise to avoid an increase in body temperature that might worsen symptoms and to conserve energy for activities of daily living. However, it has since been demonstrated that individuals with MS gain the same benefits from exercising as the healthy population and that PA reduces fatigue, increases quality of life, and improves muscular strength in patients with MS.7–9
Individuals with MS accumulate physical disability as a consequence of acute episodes of inflammation and demyelination in the central nervous system or a slower process of neurodegeneration. Treatment strategies are conditioned, in part, by the frequency of relapses, the types of neurologic deficits, the speed and degree of recovery, the changes in imaging biomarkers, and the documentation of worsening of physical or cognitive abilities. Clinicians use a variety of tools to assess these changes, but there is a need for the development of reliable, objective instruments that provide information to determine the efficacy, or lack thereof, of therapeutic interventions in trials and in clinical practice. Physical activity has been measured by self-report questionnaires, pedometers, and accelerometers. All three methods provide valid and reliable measures in the MS population.10,11 Accelerometers in particular provide an objective assessment of PA, with documentation of frequency, intensity, and duration of activity.12,13 Previous studies using accelerometers to measure PA in patients with MS have concentrated on activity counts and energy expenditure14 and on comparing the amount of activity with that of sedentary controls.15
The purpose of this pilot study was to objectively assess the PA level measured by accelerometers in individuals diagnosed as having MS and to determine its relationship with disability levels as measured by the Expanded Disability Status Scale (EDSS). The secondary objective was to compare activity levels between individuals with MS and controls. The hypothesis was that individuals with MS with higher EDSS scores would show less participation in PA compared with individuals with lower EDSS scores and that individuals with MS would have less PA participation than their healthy counterparts. Indirectly, we wanted to determine the feasibility of using this method in the MS population to consider its use in larger trials.
Methods
Participants
Thirteen patients diagnosed as having relapsing-remitting MS by their treating neurologist and 12 age-matched controls without a chronic disease condition participated in this cross-sectional study. The controls were a mixed convenience sample. Individuals in the MS group were to be relapse free for at least 3 months before the study. The age range for each group was 18 to 65 years.
The research protocol and procedures were all approved by the University of Utah institutional review board's ethics committee, and written informed consent was provided by participants before any assessments.
Measurement of PA
The ActiGraph GT1M (ActiGraph LLC, Pensacola, FL) accelerometer device was used to objectively collect the participants' PA levels.16 Data were collected from 8 a.m. to 9 p.m. for 7 consecutive days, according to standardized instructions,17 exceeding the minimum time deemed as a valid activity recording by Trost et al.18 Participants were instructed to remove the device while sleeping, swimming, and bathing. The GT1M model is biaxial, with an anteroposterior vector and a vertical vector,19 and is capable of detecting static (eg, force of gravity detected when stationary) and dynamic acceleration in units called counts, at a rate of 30 times per second.20 An embedded pedometer function measures the number of steps taken per day. This accelerometer is small (3.8 × 3.7 × 1.8 cm) and lightweight (40.2 g), is typically worn on the waist near the center of mass, and records activity counts per unit time or epoch.21 It has a battery life of up to 15 days and a memory capacity of 1 Mb.22,23 Study participants wore the device on an elastic band on the hip to capture measurements of PA at different intensities, energy expenditure, and steps taken. Energy expenditure was measured as metabolic equivalents (METs); 1 MET is defined as the amount of oxygen consumed while sitting at rest and is equal to 3.5 mL O2 per kg of body weight × min. It expresses the energy cost of PA as a multiple of the resting metabolic rate. This can be done by dividing the relative oxygen cost of the activity (mL O2/kg/min) by 3.5.24 The activity counts recorded during this period were analyzed in 1-minute epochs and categorized into one of four PA levels: light-intensity PA was defined as 1952 counts or less and is equivalent to an energy expenditure of 2.99 METs or less, moderate-intensity PA as 1953 to 5724 counts (3.0–5.99 METs), hard-intensity PA as 5725 to 9498 counts (6.0–8.99 METs), and very-hard-intensity PA as greater than 9498 counts (≥9.0 METs).25 The data were retrieved from the device by transferring it to a computer using a USB connection cable and were downloaded by specific software designed for the ActiGraph accelerometer for analysis and data processing. At least 10 hours of accelerometer data were required to consider 1 day of recording as valid. Accelerometer data are commonly expressed as a dimension-less unit—counts—that is translated into a quantitative estimate of caloric expenditure or a categorical measure of time spent in light-, moderate-, or vigorous-intensity activity.26
The EDSS27 is the most commonly used method to quantify disability in MS. It evaluates eight different functional systems: pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral functions, and ambulation. The scale ranges in score from 0 (normal) to 10 (death due to MS). A score of 0.5 to 5.5 refers to fully ambulatory individuals without any need of assistive devices, 6.0 to 6.5 represents need for assistive devices for walking, and 7.0 to 9.5 defines different degrees of restrictions, from wheelchair use to being totally dependent.27 The EDSS was scored by a single treating neurologist.
Statistical Analysis
All the statistical procedures were performed using SPSS for Windows, version 16.0 (SPSS Inc, Chicago, IL). Descriptive analyses for each group are reported as mean ± SE for the dependent variables. A Pearson product moment correlation coefficient was calculated to establish the correlation for minutes spent in PA and EDSS score, as well as PA according to METs and EDSS score. An independent t test was conducted to look at group differences (MS vs. controls) for PA. Statistical significance was defined as P ≤ .05 for all analyses.
Results
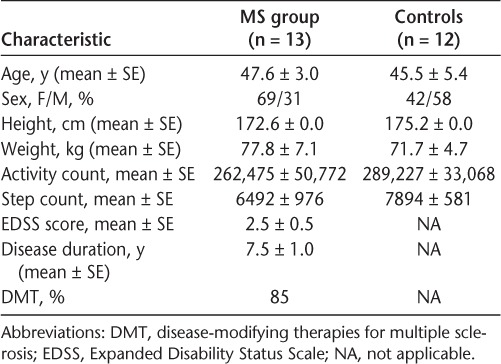
The characteristics of the participants are presented in Table 1. The sample consisted of 13 individuals with a definite diagnosis of relapsing-remitting MS (mean ± SE age, 47.6 ± 3.0 years) and 12 controls who were similar in age (mean ± SE age, 45.5 ± 5.4 years). All the participants wore the accelerometer as instructed without difficulties, and appropriate data were retrieved from the devices for the predetermined monitoring period. The MS group had EDSS scores ranging from 0.5 to 6.5, with a mean of 2.5.
Table 1.
Participant characteristics (N = 25)
There were significant negative correlations for minutes spent in PA and EDSS score for the MS group on weekdays (r = −0.61), weekend (r = −0.64), and full week (r = −0.61, P < .05), as well as for number of steps taken on weekdays (r = −0.56, P < .05), weekend (r = −0.80, P < .01), and full-week average (r = −0.68, P < .01).
A significant positive correlation was found for minutes spent in light PA (according to METs) and EDSS score (r = 0.69, P < .01) (Figure 1). However, for minutes spent in moderate PA, significant negative correlations were found between PA and EDSS score (r = −0.56, P < .05) (Figure 2). No significant relationship was found between minutes spent in very hard PA and EDSS score (r = −0.34, P > .05) (data not shown).
Figure 1.
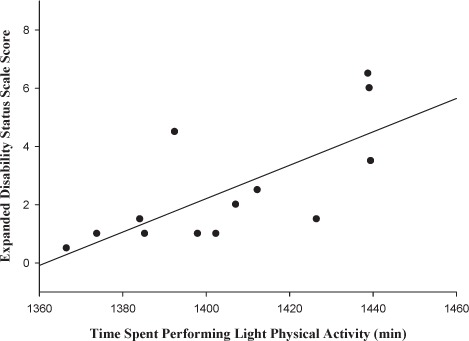
Relationship between Expanded Disability Status Scale score and time spent in light physical activity
Figure 2.
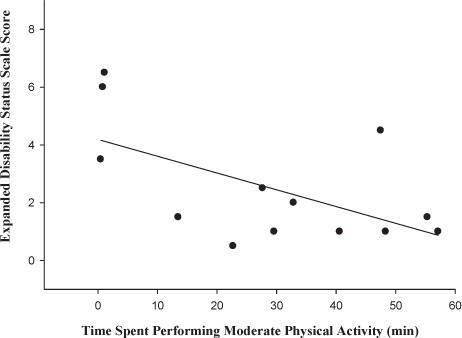
Relationship between Expanded Disability Status Scale score and time spent in moderate physical activity
Comparing the MS group with controls, there was no significant difference between the groups for any of the PA measures (P > .05). However, although it did not show statistical significance, the control group showed overall greater mean activity levels (289,227 vs. 262,475) and more steps taken (7894 vs. 6492) than the MS group on weekdays and on the weekend.
Discussion
Health benefits from regular PA in the general population are widely recognized, but they are less well characterized in the diseased population. However, research has proved that some individuals in the latter group, including those with MS, are able to achieve recommended daily PA levels28–30 and derive the same health benefits as the general population. Despite the positive results expected from PA, the MS population seems to be less physically active than people with or without other disabling diseases.31 Some individuals may believe that participating in PA32 worsens their disease because of the perceived intensification of symptoms induced by increased body temperature, although these symptoms are only transient, disappear promptly after cooling down, and do not represent disease worsening.33 Other contributors to decreased PA in MS include fatigue, spasticity, balance difficulties, poor movement coordination, sensory problems, and depression.34,35
Previous research has shown an inverse relationship between EDSS score and the amount of PA participation, where an increased level of disability as measured by the EDSS score corresponds to less time spent engaged in PA.36,37 However, some of these studies were conducted with self-reported EDSS scores,36,38 Patient-Determined Disease Steps scale scores,39 or PA levels.40 This study demonstrated that individuals diagnosed as having MS are able to spend time participating in PA of light intensity despite having increased disability as measured by EDSS scores.
Although some studies have used objective measures of PA, such as pedometers,41 wrist actigraphy,37 and accelerometers,41–45 there are few studies evaluating them in the diseased population. Those studies have focused on activity counts and energy expenditure,14 evaluating PA levels for fallers versus nonfallers,45 comparing PA levels with those of healthy sedentary controls,15 and examining the validity of self-reported measures of PA questionnaires after using an accelerometer and a pedometer for 7 consecutive days.41 Techniques to measure levels of PA in the MS population have more commonly included self-report measures such as the Goodin Leisure-Time Exercise Questionnaire,46 the 7-Day Physical Activity Recall Scale,47 the Exercise Self-Efficacy Scale,48 the International Physical Activity Questionnaire,49 and the Physical Activity Enjoyment Scale.50 Accelerometers provide objective numerical data on PA and eliminate the bias of self-report, which can be influenced by memory/recall deficits or desire to please the health-care provider.
This study documented accelerometer-determined PA levels in individuals diagnosed as having MS and in controls across 7 consecutive days and for at least 10 hours per day, the duration used in previous studies.16,51,52 In addition, the relationship between PA and degree of disability was also examined in the MS group. Participants were able to use the device as instructed, PA data were recorded without interruptions during the length of use, and the information could be downloaded and was available for analysis. The main finding was the linear relationship between light PA and degree of disability as measured by the EDSS. By capturing a full range of PA or inactivity measurements through objective monitoring with accelerometers in the diseased population, a comprehensive PA profile in the MS population can be properly ascertained.
Some of the findings in this study coincide with previous research. A negative relationship between accelerometer counts and EDSS scores was found in a 2008 study by Klassen et al.53 That study had only a 4-day data collection period, whereas the present study collected data for 7 consecutive days and used a different type of accelerometer. Collecting a full week's worth of data captures real-life PA with more fidelity by including leisure days. Negative correlation between accelerometer count and EDSS score was also found in a 2008 study by Motl et al,36 but this study used self-report EDSS scores instead of EDSS rating by a neurologist. A 1997 study by Ng and Kent-Braun15 used accelerometers and the 7-day recall questionnaire to examine PA in individuals with MS and healthy sedentary control subjects. They found that the activity level of the MS group was lower and that there was no significant correlation between activity level measured by accelerometer and EDSS score. Another study found that the average total daily activity count from the accelerometer had a significant negative correlation with the Patient-Determined Disease Steps scale, a self-report surrogate for the EDSS.54 The same study also showed a positive relationship between accelerometer count and the 6-Minute Walk test. The present results indicated that individuals with MS engage in PA despite their disability, with most of it falling in the light-intensity PA category. Although the control group had a higher activity intensity level than the MS group, this difference did not reach statistical significance. This finding is important because we would like to promote PA in diseased populations.
To our knowledge, this study is the first of its kind to look at activity count by epochs of light, moderate, heavy, and very heavy and compare it with level of disability by clinical measure of EDSS score as opposed to self-reported EDSS score.36 The study is not without limitations: the sample size is relatively small compared with other studies,16,51 and it includes only relapsing-remitting MS of relatively short disease duration and a low level of disability, thus excluding more debilitated individuals. It does, however, demonstrate the feasibility of using objective measurements of PA, such as accelerometers, to more accurately document real-life activity in individuals with MS, a practice that should be considered for outcome determination in interventional trials.
Conclusion
The findings of the present study provide evidence for the feasibility and usefulness of accelerometers in quantifying PA and categorizing its intensity during normal daily activities over a prolonged period in ambulatory individuals with MS of different disability levels. It showed a linear relationship between light PA measured by accelerometer and degree of disability measured by EDSS score in individuals with MS. Examining the relationship between PA by accelerometer count and level of disability in individuals with MS can have practical application as an outcome for clinical interventions and could be used in regular clinical practice as an objective measure of real-life PA. Additional studies are warranted to provide a more comprehensive understanding of the responses to long-term exercise in patients with MS and the potential implications for the disease.
PracticePoints.
Accelerometers can be used to quantify physical activity (PA) and to qualify its intensity during normal daily activities in individuals with MS.
The amount of PA is inversely proportional to the degree of physical disability.
Objective measures of PA should be considered as outcomes for interventions in trials and in clinical practice.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, Shepard RJ, Stephens T, editors. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- 3.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 4.Fjeldstad C, Brittain DR, Fjeldstad AS, Pardo G. Physical activity according to thermosensitivity and fatigue in individuals with multiple sclerosis. Med Sci Sports Exerc. 2010;10:108–115. [Google Scholar]
- 5.Motl RW, Arnett PA, Smith MM, Barwick FH, Ahlstrom B, Stover EJ. Worsening of symptoms is associated with lower physical activity levels in individuals with multiple sclerosis. Mult Scler. 2008;14:140–142. doi: 10.1177/1352458507079126. [DOI] [PubMed] [Google Scholar]
- 6.Motl RW, Snook EM, Schapiro RT. Symptoms and physical activity behavior in individuals with multiple sclerosis. Res Nurs Health. 2008;31:466–475. doi: 10.1002/nur.20274. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen A, Stenager E, Dalgas U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult Scler. 2011;17:1041–1054. doi: 10.1177/1352458511401120. [DOI] [PubMed] [Google Scholar]
- 8.Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008;14:129–135. doi: 10.1177/1352458507080464. [DOI] [PubMed] [Google Scholar]
- 9.Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Med. 1999;27:179–191. doi: 10.2165/00007256-199927030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Commun Health. 2007;30:144–150. doi: 10.1097/01.fch.0000264411.20766.0c. [DOI] [PubMed] [Google Scholar]
- 11.Motl RW, Zhu W, Park Y, McAuley E, Scott JA, Snook EM. Reliability of scores from physical activity monitors in adults with multiple sclerosis. Adapt Phys Activ Q. 2007;24:245–253. doi: 10.1123/apaq.24.3.245. [DOI] [PubMed] [Google Scholar]
- 12.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathie MJ, Coster AC, Lovell NH, Celler BG. Accelerometry: providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiol Meas. 2004;25:R1–R20. doi: 10.1088/0967-3334/25/2/r01. [DOI] [PubMed] [Google Scholar]
- 14.Motl RW, Snook EM, Agiovlasitis S, Suh Y. Calibration of accelerometer output for ambulatory adults with multiple sclerosis. Arch Phys Med Rehabil. 2009;90:1778–1784. doi: 10.1016/j.apmr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Ng AV, Kent-Braun JA. Quantitation of lower physical activity in persons with multiple sclerosis. Med Sci Sports Exerc. 1997;29:517–523. doi: 10.1097/00005768-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Hansen BH, Kolle E, Dyrstad SM, Holme I, Anderssen SA. Accelerometer-determined physical activity in adults and older people. Med Sci Sports Exerc. 2012;44:266–272. doi: 10.1249/MSS.0b013e31822cb354. [DOI] [PubMed] [Google Scholar]
- 17.Tudor-Locke C, Brashear MM, Johnson WD, Katzmarzyk PT. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Act. 2010;7:60. doi: 10.1186/1479-5868-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 19.Hanggi JM, Phillips LR, Rowlands AV. Validation of the GT3X ActiGraph in children and comparison with the GT1M ActiGraph. J Sci Med Sport. 2013;16:40–44. doi: 10.1016/j.jsams.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44:S86–S89. doi: 10.1249/MSS.0b013e3182399f5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandroff BM, Motl RW, Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev. 2012;49:467–476. doi: 10.1682/jrrd.2011.03.0063. [DOI] [PubMed] [Google Scholar]
- 22.John D, Tyo B, Bassett DR. Comparison of four ActiGraph accelerometers during walking and running. Med Sci Sports Exerc. 2010;42:368–374. doi: 10.1249/MSS.0b013e3181b3af49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettee Gabriel K, McClain JJ, High RR, Schmid KK, Whitfield GP, Ainsworth BE. Patterns of accelerometer-derived estimates of inactivity in middle-age women. Med Sci Sports Exerc. 2012;44:104–110. doi: 10.1249/MSS.0b013e318229056e. [DOI] [PubMed] [Google Scholar]
- 24.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine. ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription. Indianapolis, IN: American College of Sports Medicine; 2006. [Google Scholar]
- 26.Troiano RP. Translating accelerometer counts into energy expenditure: advancing the quest. J Appl Physiol. 2006;100:1107–1108. doi: 10.1152/japplphysiol.01577.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 28.Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011;35:26–33. doi: 10.1097/NPT.0b013e3182097190. [DOI] [PubMed] [Google Scholar]
- 29.Gronstedt H, Frandin K, Bergland A et al. Effects of individually tailored physical and daily activities in nursing home residents on activities of daily living, physical performance and physical activity level: a randomized controlled trial. Gerontology. 2013;59:220–229. doi: 10.1159/000345416. [DOI] [PubMed] [Google Scholar]
- 30.Weening-Dijksterhuis E, de Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: a comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90:156–168. doi: 10.1097/PHM.0b013e3181f703ef. [DOI] [PubMed] [Google Scholar]
- 31.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 32.van Diemen HA, van Dongen MM, Dammers JW, Polman CH. Increased visual impairment after exercise (Uhthoff's phenomenon) in multiple sclerosis: therapeutic possibilities. Eur Neurol. 1992;32:231–234. doi: 10.1159/000116830. [DOI] [PubMed] [Google Scholar]
- 33.Guthrie TC. Visual and motor changes in patients with multiple sclerosis: a result of induced changes in environmental temperature. Arch Neurol Psychiatry. 1951;65:437–451. doi: 10.1001/archneurpsyc.1951.02320040027002. [DOI] [PubMed] [Google Scholar]
- 34.Henze T, Rieckmann P, Toyka KV, Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society Symptomatic treatment of multiple sclerosis. Eur Neurol. 2006;56:78–105. doi: 10.1159/000095699. [DOI] [PubMed] [Google Scholar]
- 35.Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil. 2007;21:771–781. doi: 10.1177/0269215507077602. [DOI] [PubMed] [Google Scholar]
- 36.Motl RW, Snook EM, Wynn DR, Vollmer T. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis. 2008;196:492–495. doi: 10.1097/NMD.0b013e318177351b. [DOI] [PubMed] [Google Scholar]
- 37.Merkelbach S, Schulz H, Kolmel HW et al. Fatigue, sleepiness, and physical activity in patients with multiple sclerosis. J Neurol. 2011;258:74–79. doi: 10.1007/s00415-010-5684-3. [DOI] [PubMed] [Google Scholar]
- 38.Sandroff BM, Riskin BJ, Agiovlasitis S, Motl RW. Accelerometer cut-points derived during over-ground walking in persons with mild, moderate, and severe multiple sclerosis. J Neurol Sci. 2014;340:50–57. doi: 10.1016/j.jns.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Motl RW, Pilutti LA, Learmonth YC, Goldman MD, Brown T. Clinical importance of steps taken per day among persons with multiple sclerosis. PLoS One. 2013;8:e73247. doi: 10.1371/journal.pone.0073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:935–943. doi: 10.1016/j.apmr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 2006;28:1151–1156. doi: 10.1080/09638280600551476. [DOI] [PubMed] [Google Scholar]
- 42.Snook EM, Motl RW. Physical activity behaviors in individuals with multiple sclerosis: roles of overall and specific symptoms, and self-efficacy. J Pain Symptom Manage. 2008;36:46–53. doi: 10.1016/j.jpainsymman.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006;32:154–161. doi: 10.1207/s15324796abm3202_13. [DOI] [PubMed] [Google Scholar]
- 44.Spaniel F, Vohlidka P, Kozeny J et al. The Information Technology Aided Relapse Prevention Programme in Schizophrenia: an extension of a mirror-design follow-up. Int J Clin Pract. 2008;62:1943–1946. doi: 10.1111/j.1742-1241.2008.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sosnoff JJ, Sandroff BM, Pula JH, Morrison SM, Motl RW. Falls and physical activity in persons with multiple sclerosis. Mult Scler Int. 2012;2012:315620. doi: 10.1155/2012/315620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 47.Sallis JF, Haskell WL, Wood PD et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 48.McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. J Behav Med. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- 49.Craig CL, Marshall AL, Sjostrom M et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 50.Kendzierski DDK. Physical activity enjoyment scale: two validation studies. J Sport Exerc Psychol. 1991;13:50–64. [Google Scholar]
- 51.Lohne-Seiler H, Hansen BH, Kolle E, Anderssen SA. Accelerometer-determined physical activity and self-reported health in a population of older adults (65–85 years): a cross-sectional study. BMC Public Health. 2014;14:284. doi: 10.1186/1471-2458-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagstromer M, Troiano RP, Sjostrom M, Berrigan D. Levels and patterns of objectively assessed physical activity: a comparison between Sweden and the United States. Am J Epidemiol. 2010;171:1055–1064. doi: 10.1093/aje/kwq069. [DOI] [PubMed] [Google Scholar]
- 53.Klassen L, Schachter C, Scudds R. An exploratory study of two measures of free-living physical activity for people with multiple sclerosis. Clin Rehabil. 2008;22:260–271. doi: 10.1177/0269215507082740. [DOI] [PubMed] [Google Scholar]
- 54.Motl RW, Dlugonski D, Suh Y, Weikert M, Fernhall B, Goldman M. Accelerometry and its association with objective markers of walking limitations in ambulatory adults with multiple sclerosis. Arch Phys Med Rehabil. 2010;91:1942–1947. doi: 10.1016/j.apmr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


