Abstract
Background: This study estimated the risk of infection-related hospitalizations and death in patients with and without multiple sclerosis (MS).
Methods: We identified adults with MS in the US Department of Veterans Affairs (VA) system between 1999 and 2010. Each veteran with MS was matched, on age and sex, with up to four veterans without MS. Multivariable Cox proportional hazards regression models were performed to assess the influence of MS on the development of serious and fatal infections.
Results: The cohort included 7743 veterans with MS and 30,972 veterans without MS. Mean (SD) age was 53.8 (13.3) years, and 80.8% were male. The incidence per 1000 person-years of overall serious infections was 19.2 (95% confidence interval [CI], 17.6–20.8) for those with MS and 10.3 (95% CI, 9.8–10.9) for those without MS. Fatal infection incidence rates were 1.2 (95% CI, 0.8–1.7) for patients with MS and 0.5 (95% CI, 0.3–0.6) for patients without MS. Regression models showed that veterans with MS were at greater risk for overall serious (hazard ratio [HR] = 1.52, P < .01) and fatal (HR = 1.85, P = .03) infections and serious respiratory (HR = 1.31, P = .01), urinary tract (HR = 4.44, P < .01), and sepsis-related infections (HR = 2.56, P < .01).
Conclusions: This study provides evidence that VA patients with MS are more likely than those without MS to be hospitalized and die of infection.
Multiple sclerosis (MS) is the most common autoimmune inflammatory demyelinating disease of the central nervous system, affecting approximately 400,000 individuals in the United States.1 The Department of Veterans Affairs (VA) health-care system treats approximately 16,000 veterans with MS each year.2 MS is a chronic disease that results in an annual cost burden of $8500 to $54,000 per patient in the United States, much of it from complications secondary to MS.3,4
Two recent studies have demonstrated an increased risk of infection-related hospitalizations and infection-related mortality in patients with MS compared with those without MS in Sweden and the United Kingdom.5,6 The objective of this study was to examine the risk of hospitalization and death due to infections in patients with and without MS in a cohort of US veterans.
Methods
Study Design and Data Set
This study used a historical cohort design and data from the national VA health-care system, the largest integrated health-care system in the United States.7 The system collects data on utilization (pharmacy records and inpatient and outpatient encounters), clinical parameters (vital signs, laboratory test results, radiology reports, etc.), and demographics (age, sex, and race/ethnicity). We used data from the VA's Decision Support System, which includes pharmacy data, and Medical SAS data sets, including data from inpatient and outpatient medical encounters. Finally, we used unstructured data in the form of narrative text from clinical notes.
In addition to data from the VA, we also used data from the National Death Index (NDI), administered by the National Center for Health Statistics in the Centers for Disease Control and Prevention, to capture mortality. At the time of this study, NDI data were available to us only up to September 30, 2009.
Patients
We identified all patients nationwide in the VA system with a diagnosis of MS between January 1, 1999, and December 31, 2010. We defined a diagnosis of MS to be at least two instances of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 340 (multiple sclerosis), with the date of the first diagnosis defined as the index date. We then excluded patients having a diagnosis code of 341 (other demyelinating diseases of the central nervous system). In an attempt to limit the sample to regular users of the VA setting, we also excluded patients who did not have encounters in the VA system at least 180 days before the index date. Finally, we excluded patients with invalid death dates (eg, death dates that occurred before the index date).
For each included veteran with MS, we randomly matched four veterans without MS on corresponding year of birth and sex. To be included in this matching process, patients without MS were defined as VA patients with no occurrence of diagnosis code 340, 341, 323.6 (other causes of encephalitis, myelitis, and encephalomyelitis), or 277.86 (adrenoleukodystrophy). Matched non-MS patients were assigned the same index date as their MS counterparts.
Standard Protocol Approvals, Registrations, and Patient Consents
All relevant ethical safeguards have been met in relation to patient or subject protection. Approval for this study was obtained through the University of Utah's institutional review board and the VA's Office of Research and Development; therefore, this study was performed in accordance with the ethical standards contained in the 1964 Declaration of Helsinki and its later amendments.
Observation Period
We identified patient baseline characteristics and risk factors for infections on or 6 months before the index date. We followed patients starting the day after the index date and continuing until any of the following: 1) the first occurrence of the study-specified outcome as described later herein, 2) death, 3) the start date of a 12-month gap without a VA clinical encounter, or 4) the end of the study period (December 31, 2010, for serious infections; September 30, 2009, for fatal infections).
Outcomes
The primary outcome for this study was serious infection. An infection was considered serious if it was the admitting diagnosis for a VA hospital inpatient stay lasting more than 24 hours. The secondary outcome was infection-related death as identified in the NDI data. We identified overall serious (VA data) and fatal (NDI data) infections and those specific to opportunistic, respiratory, urinary tract, sepsis, and skin and soft-tissue infections using ICD-9-CM codes (Supplementary Table 1, published in the online version of this article at ijmsc.org).
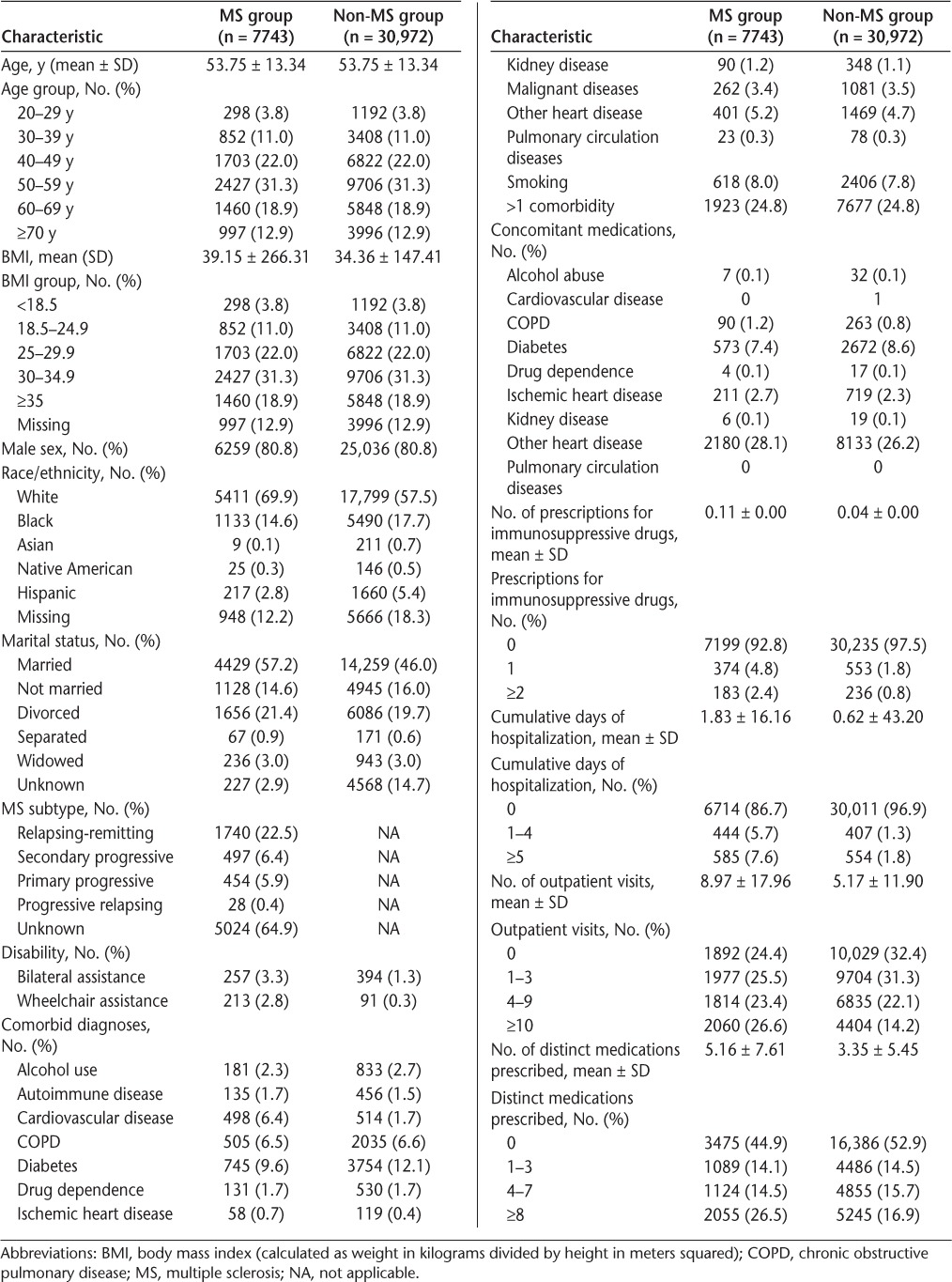
Table 1.
Descriptive statistics of MS and non-MS patients
Independent Variables
We used VA electronic medical record data and ICD-9-CM codes to identify demographic characteristics, disability status, comorbid conditions, treatments, and health-care utilization. The presence of these independent variables was captured in the 6 months before the index date for each patient. For variables whose definition relied on ICD-9-CM and Current Procedural Terminology procedure codes, we assumed that the condition was present if the patient had at least one code in the 6-month pre-index period. The definitions of these variables are in Supplementary Table 2. Patients missing data for a categorical or continuous variable were counted and described separately.
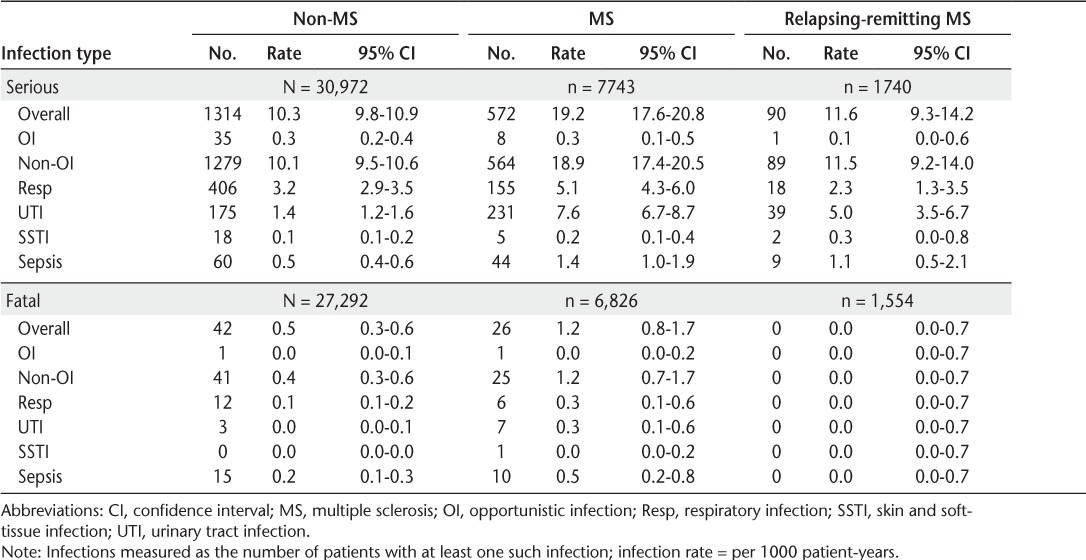
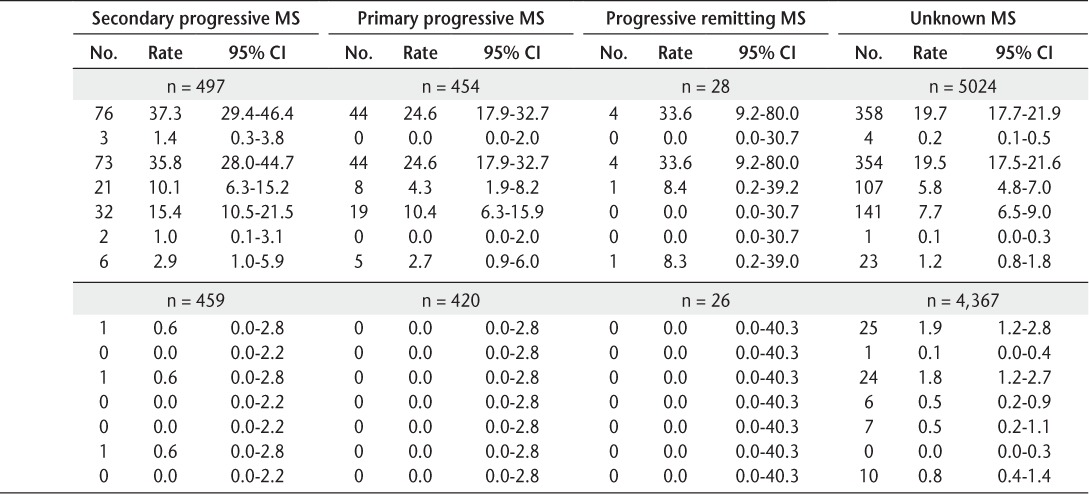
Table 2.
Infection rates in non-MS patients and patients with MS and by MS subtype
Identification of MS Subtype
MS can be categorized into four subtypes: relapsing-remitting (RRMS), secondary progressive (SPMS), primary progressive (PPMS), and progressive relapsing (PRMS). Because MS is wholly contained as a three-digit ICD-9-CM code without distinction of MS subtype, this information was extracted from patients' clinical notes using natural language processing (NLP). Clinical experts were interviewed for possible keywords and phrases denoting MS subtype. For each patient, electronic medical record clinical notes from the first MS diagnosis date onward were searched by NLP for these keywords and phrases. The presence of subtype-related keywords and phrases was analyzed by NLP in context to remove mentions that were negated (eg, “not relapsing remitting”) or unrelated to MS (eg, “RR” meaning respiratory rate rather than relapsing remitting). Each subtype mention in the patients' notes in the post-index period was captured, along with the date of each mention. One thousand mentions of MS subtype from the NLP results were validated, and all the records for 150 randomly selected patients were reviewed for missed mentions.
Patients with only one of the four subtypes during the entire post-index period were designated as having that subtype. It is common for patients with MS to progress from RRMS to SPMS. However, for patients for whom this progression occurred, we designated their baseline subtype as RRMS but allowed the subtype to change to SPMS over time as a time-varying covariate in the regression analysis. Patients with combinations of more than one subtype other than RRMS and SPMS were assigned the subtype with the mode value (ie, the subtype that was mentioned most often). Finally, patients with no true mode (ie, an equal number of documents mentioning two or more subtypes) and those with no mentions of a subtype in their notes were designated as having “unknown” subtype.
Analysis
Patient Characteristics
Descriptive statistics were calculated to characterize baseline demographics, disability, and comorbid diagnoses. Statistics were calculated separately for veterans with and without MS.
Infection Rates
We calculated the rates for each infection outcome separately for patients with and without MS overall and in categories of infection risk factors. For each infection rate, we constructed an exact 95% confidence interval (CI) using a method that relates the χ2 and Poisson distributions.8
Association Between MS and Infections
We estimated the impact of MS on the risk of serious infection using multivariable Cox proportional hazards regression models. These models were run separately for each infection outcome and with two different constructs of the key independent variable: MS. The first construct was a simple indicator for MS relative to no MS. The second construct was a time-varying categorical variable with the following categories: no MS (reference category), RRMS, SPMS, PPMS, PRMS, and unknown subtype. MS subtype was constructed as a time-varying independent variable, with patients potentially progressing from RRMS to SPMS.
To minimize bias, each regression model included control variables for demographic characteristics (age, body mass index, sex, race, and marital status); disability (wheelchair assistance and bilateral assistance); comorbid conditions (alcohol use, autoimmune diseases [rheumatoid arthritis, ulcerative colitis, Crohn's disease, systemic lupus erythematosus, Wegener's granulomatosis, scleroderma, Sjögren's disease, polymyositis, pemphigus, myasthenia gravis, and psoriasis], cardiovascular disease, chronic obstructive pulmonary disease [COPD], diabetes, drug dependence, ischemic heart disease, kidney disease, malignant disease, other heart disease, pulmonary circulation diseases, and smoking); medication exposures (treatment for alcohol abuse, COPD, diabetes, ischemic heart disease, other heart disease, and immunosuppressive drugs); and health-care utilization (cumulative days of hospitalization, number of outpatient visits, and number of distinct medications prescribed).
Results
Patient Characteristics
A total of 38,234 patients had at least one diagnosis for MS between January 1, 1999, and December 31, 2010 (Figure 1). The exclusion process resulted in 7743 veterans with MS matched on year of birth and sex to 30,972 veterans without MS, all included in the serious infection analyses.
Figure 1.
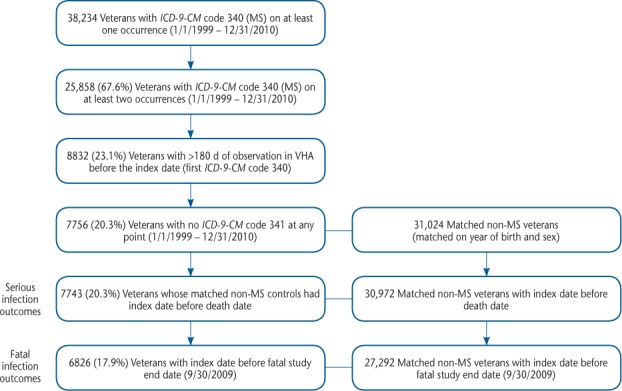
Patient attrition summary
ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MS, multiple sclerosis; VHA, Veterans Health Administration.
Baseline characteristics of the cohort are summarized in Table 1. The mean (SD) age of veterans in this study was 53.8 (13.3) years, and 80.8% were male. Race was identified in more than 80% of the cohort. Patients were predominantly white (60.0%) or black (17.1%). Only one-third of patients with MS had a subtype identified. The most common comorbidities were diabetes (MS, 9.6%; no MS, 12.1%), smoking (MS, 8.0%; no MS, 7.8%), and COPD (MS, 6.5%; no MS, 6.6%). Compared with patients without MS, those with MS had greater mean numbers of hospital days (1.8 vs. 0.6, P = .02), outpatient visits (9.0 vs. 5.2, P < .01), and medications prescribed (5.2 vs. 3.4, P < .01).
Infection Rates
The rates of serious and fatal infections for patients with and without MS are summarized in Table 2. The overall rate of serious infections in patients with MS (19.2 per 1000 person-years, 95% CI, 17.6–20.8) was nearly twice as high as that in those without MS (10.3 per 1000 person-years, 95% CI, 9.8–10.9). Most serious and fatal infections were nonopportunistic in patients with and without MS. The rates (95% CIs) of serious respiratory (5.1 [4.3–6.0] per 1000 person-years vs. 3.2 [2.9–3.5] per 1000 person-years), urinary tract (7.6 [6.7–8.7] per 1000 person-years vs. 1.4 [1.2–1.6] per 1000 person-years), and sepsis (1.4 [1.0–1.9] per 1000 person-years vs. 0.5 [0.4–0.6] per 1000 person-years) infections were much higher in patients with MS than in those without MS. Skin and soft-tissue infections were rare in patients with and without MS.
The fatal infection rate (95% CI) was also considerably higher in patients with MS (1.2 [0.8–1.7] per 1000 person-years) than in those without MS (0.5 [0.3–0.6] per 1000 person-years). Fatal respiratory infections (rates [95% CIs]: 0.3 [0.1–0.6] per 1000 person-years vs. 0.1 [0.1–0.2] per 1000 person-years) and sepsis infections (rates [95% CIs]: 0.5 [0.2–0.8 per 1000 person-years] vs. 0.2 [0.1–0.3] per 1000 person-years) were more common in patients with MS than in those without MS.
Overall, serious infection rates (95% CIs) ranged from 11.6 (9.3–14.2) per 1000 person-years for RRMS to 37.3 (29.4–46.4) per 1000 person-years for SPMS (Table 2). All but one of the fatal infections occurred in patients with MS for whom MS subtype was not identified.
Association Between MS and Infections
Veterans with MS were at 52.4% greater risk for infection than those without MS (P < .01) (Table 3). Additional risk factors that were significantly associated with an increased risk of serious infection included age 50 years and older; body mass index less than 18.5; marital status (divorced, not married, or widowed); Hispanic ethnicity; wheelchair assistance; diagnoses of COPD, diabetes, kidney disease, malignant diseases, and other heart diseases; history of COPD medication use; inpatient stays; and four or more outpatient visits in the 6 months before the index date.
Table 3.
Multivariable regression results for serious infections requiring a hospitalization
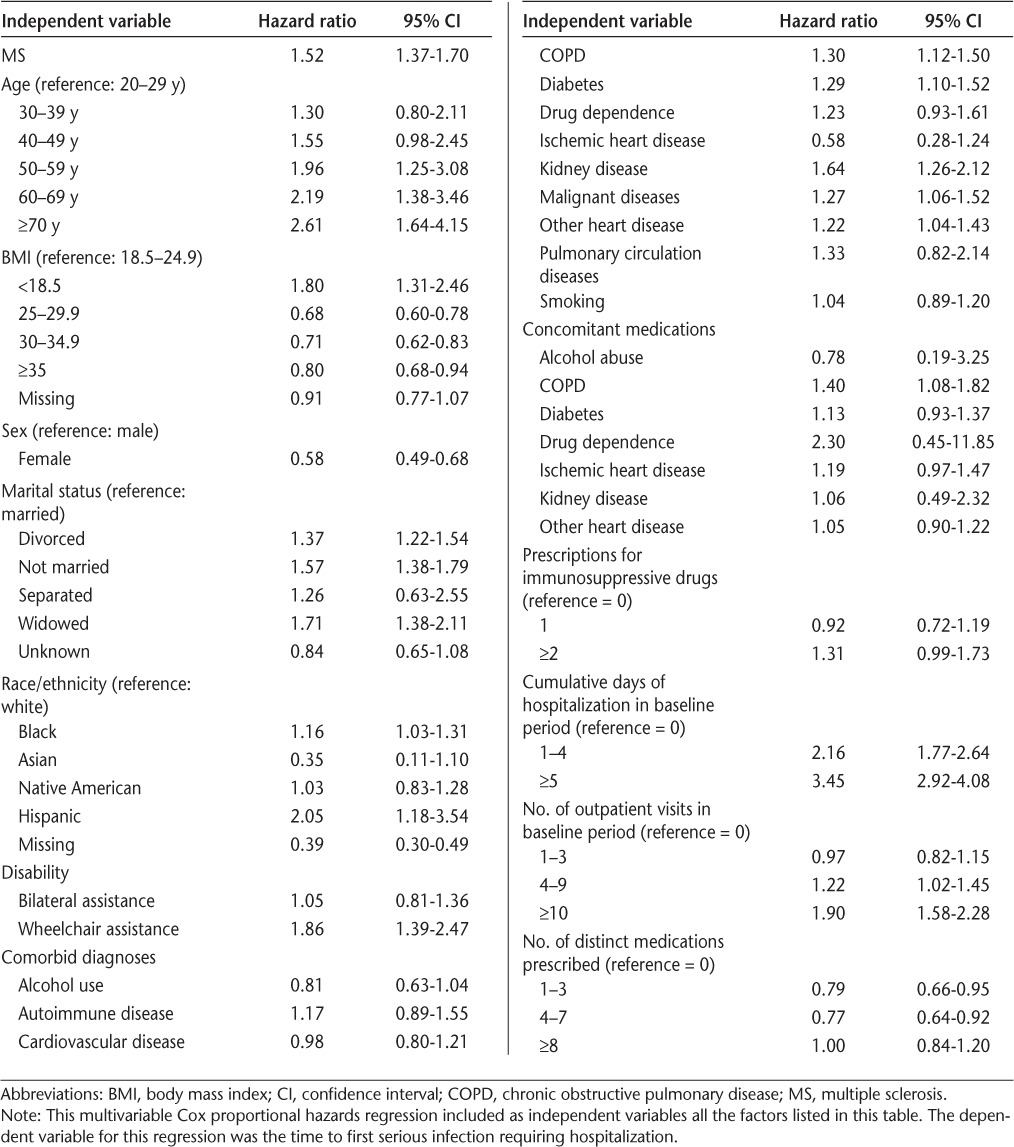
Table 4 shows the adjusted relationships between MS and different types of infections. This table shows results only for the MS variable, with other independent variables suppressed for ease of exposition. Having MS was significantly (all P ≤ .03) associated with an increased risk (hazard ratio [HR], 95% CI) of serious respiratory (1.31, 1.07–1.59), urinary tract (4.44, 3.59–5.51), and sepsis (2.56, 1.67–3.94) infections and fatal infections of any kind (1.85, 1.08–3.15).
Table 4.
Relationship between MS and different types of infections: results from multivariable Cox proportional hazards regressions
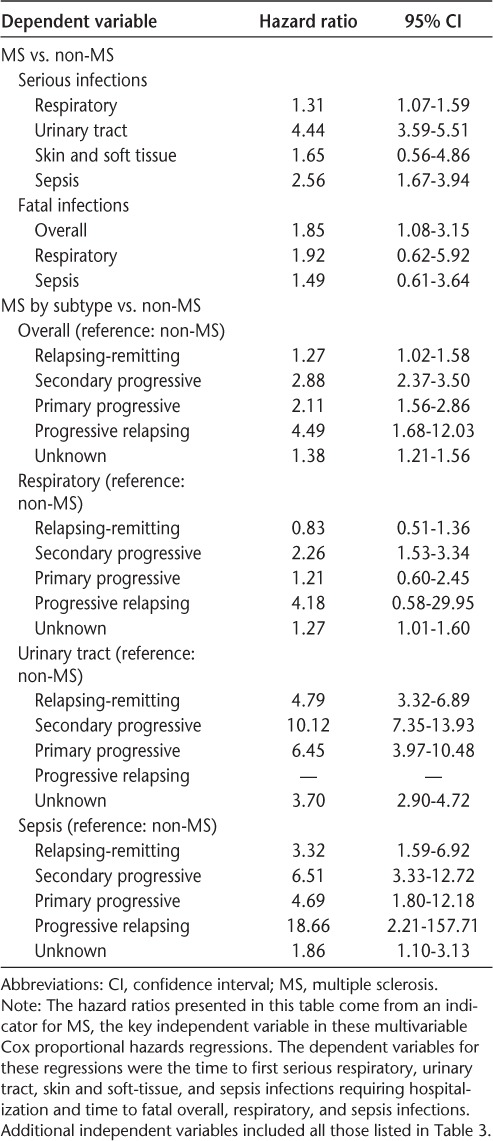
The adjusted relationships between MS subtype and different types of infections are also shown in Table 4. Patients with RRMS (HR = 1.27, 95% CI, 1.02–1.58), SPMS (2.88, 2.37–3.50), PPMS (2.11, 1.56–2.86), PRMS (4.49, 1.68–12.03), and unknown MS subtype (1.38, 1.21–1.56) had a significantly (all P ≤ .04) increased risk of serious infection compared with patients without MS. Compared with those without MS, the risk of serious respiratory infection was elevated for patients with SPMS and unknown subtype; the risk of urinary tract infections was elevated for patients with RRMS, SPMS, PRMS, and unknown MS subtype; and the risk of sepsis infections was elevated for patients with MS of all subtypes.
Discussion
The purpose of this study was to determine the rates of serious and fatal infections in US veterans with and without MS and to estimate the association between MS and the risk of infections. We found that, overall, veterans with MS were more than 50% more likely to have a serious infection than veterans without MS. In addition, patients with MS were significantly more likely than those without MS to have respiratory, urinary tract, and sepsis infections resulting in hospitalization, as well as fatal infections of all types. Although we found that veterans with each of the MS subtypes were at a significantly elevated risk for serious infection compared with those without MS, the magnitude of this increased risk was greatest in veterans with PRMS.
We know of only two other studies that have examined infection risk as an outcome of MS. The present results are similar in direction but substantially smaller in magnitude compared with those found in these previous studies. Similar to this study, Montgomery et al.6 identified patients with infection-related hospital admissions from an MS patient registry in Sweden and matched those to Swedish residents who did not have MS and used Poisson regression to obtain the relative risk of infection for patients with and without MS. Using a multivariable model that adjusted for baseline patient characteristics, the authors found that the relative risk of infection-related hospital admissions was 4.26 (95% CI, 4.13–4.40) and the relative risk of infection-related mortality was 5.19 (95% CI, 4.90–5.50). Using the same infection definitions (Supplementary Table 3) and regression methods as Montgomery et al., we found adjusted HRs of 1.32 (95% CI, 1.10–1.60) and 1.33 (95% CI, 1.10–1.63) for serious and fatal infections, respectively, in this VA cohort. One potential reason for the differences in effect size between this study and that by Montgomery et al. may be differences in data availability stemming from differences between the two health-care systems. Montgomery used hospitalization data from the Swedish National Inpatient Register, which had national coverage from 1987 onward.9 On the other hand, VA data do not include veterans' health-care encounters that occurred outside the VA system. Reports suggest that 28% to 47% of VA patients receive at least some health care in non-VA facilities.10–12 In addition, whereas our mortality records came from the NDI, Montgomery et al. used data from the Swedish Cause of Death Register, including deaths that occurred within 30 days of a hospitalization. Several studies have found these data to be unreliable for cause of death,13 including one that specifically examined postdischarge cause of death.14 Montgomery's use of these death data may have led to an overestimation of MS-related deaths. Lalmohamed et al.5 used the UK's General Practice Research Database to identify cause of death in patients with MS compared with those without MS. They found a 7.5-fold increase in the risk of acute respiratory infection–related deaths in patients with MS. The present results are smaller in size, in part, because Lalmohamed et al. identified both primary and secondary causes of death, whereas we were able to identify only primary causes of death through NDI data. In short, although differences in data and health-care systems may explain why our estimates differ in size compared with those of Montgomery et al. and Lalmohamed et al., all three studies support the conclusion that patients with MS are at greater risk for infection-related hospitalizations and mortality than patients without MS.
We identified veterans in this cohort as having MS if they had an ICD-9-CM code 340 on two or more separate encounter dates. An alternative electronic case-finding algorithm has been created in the VA that identifies a veteran as having MS if the veteran had a pharmacy record for a disease-modifying agent, evidence of MS-related service-connected disability through the Veterans Benefit Administration, or a mean of at least one inpatient or outpatient encounter each year during the time window of this study, in which the principal ICD-9-CM code was 340.15 As a sensitivity analysis, we implemented a revised version of this algorithm (we did not have access to Veterans Benefit Administration data) and found that 5375 of the 7743 patients in this study met the definition of MS with these new criteria. The covariate-adjusted risk of infection-related hospitalization in this group of veterans with MS compared with those without MS (HR = 1.55, 95% CI, 1.35–1.78) was similar to that in our original analysis, underscoring the robustness of the results.
The risk of infection has been reported to increase due to the immunosuppressive therapies used by patients with MS,16 although the magnitude of this increase is up for debate. Montgomery et al.6 did not control for therapy in their study; however, they suggest, based on the temporal variation of risk over 37 years of data, that therapy did not greatly influence their results. In contrast, the present multivariable regression analyses controlled for the number of immunosuppressive medications prescribed to each patient. This variable increased the risk of infection in the regression models, but this effect was not statistically significant. The findings from this study lend support to the suppositions of Montgomery et al., specifically, that the significantly elevated risk of serious infection in patients with MS may be more due to the disease itself than to immunosuppressive drug therapies.
There are several potential explanations as to why veterans with MS may be at greater risk for serious and fatal infections. For example, extensive literature has documented that vesicourethral and bladder dysfunctions, such as incomplete emptying, which are closely linked to risk of urinary tract infection,17 have an increased prevalence in patients with MS.18,19 In addition, patients with MS often develop respiratory problems as a result of reduced muscle strength, bulbar dysfunction, and ineffective clearance of secretions, which can lead to lower lung volumes and reduced respiratory function.20 With this increased stress on the respiratory system, it is not surprising that patients with MS would be at greater risk for respiratory infections.
A recent systematic review suggests that the annual health-care costs for MS range from $8528 to $54,244 per patient, placing it second to congestive heart failure among chronic diseases.3 A cursory review of estimates of the direct medical costs of hospitalizations for respiratory ($3900 [2012 US$]21), urinary tract ($5446 [2000 US$]22), and sepsis ($23,127 [2001 US$] for intensive care unit patients and $10,674 [2001 US$] for hospital ward patients)23 infections suggest that these serious infections lead to a substantial burden on the health-care system. Besides the obvious mortality and morbidity benefits, efforts to prevent these infections can have a substantial financial benefit for individuals with this costly condition.
This study is subject to some limitations. First, because the VA health-care system is not a completely closed system, some veterans may have received care outside of the VA. This may result in incomplete data that could lead to underreporting of infections and other data elements (such as wheelchair and bilateral assistance, alcohol use, and autoimmune disease). Unfortunately, this is a concern with most observational studies that use secondary data sources. Measurement error in the wheelchair and bilateral assistance variables is of particular concern because studies have found that patients with MS are much more likely to need mobility assistance than those without MS. One example of this is a recent study from Canada that showed that nearly 53% of patients with MS older than 55 years were unable to walk independently or without a cane outdoors compared with 9% of a comparison group of individuals without MS.24 Therefore, it is likely that the error of missing data on wheelchair and bilateral assistance is larger in patients with MS than in those without MS in the present study. However, it is not surprising that Healthcare Common Procedure Coding System codes for mobility assistance were problematic in this cohort because these codes have been found to poorly represent conditions in other VA studies.25 Another area in which this incomplete data capture was evident was in the characterization of MS subtypes. We were able to identify a subtype in only one-third of the patients with MS, leading us to believe that many of these patients receive treatment and MS-related care outside the VA. We attempted to minimize this problem by restricting the sample to veterans with a demonstrated pattern of receiving routine care in the VA system (ie, by excluding those without at least one medical encounter in the 180 days or more before the index diagnosis).
Second, to the extent that the unique characteristics of the VA population influence serious and fatal infections, these findings may not be generalizable to other patient populations. For example, the present cohort was primarily male because of the sex distribution in the VA population, despite the fact that the prevalence of MS is disproportionately higher in women in the general population. Finally, we attempted to identify incident cases of MS by restricting the sample to those with at least 6 months of observation time in the VA before their first MS diagnosis. However, some veterans may have been diagnosed as having MS before this date outside the VA.
In conclusion, we found that patients with MS were at greater risk for hospitalization and death due to infections compared with patients without MS. Future studies are needed to identify significant interactions between patient characteristics (eg, use of immunosuppressive drug treatments) and MS in which these relationships hold so as to enable targeted interventions to prevent infection in specific patient populations.
PracticePoints.
We found that veterans with MS were 52% more likely to have an infection that resulted in a hospitalization and 85% more likely to have a fatal infection than veterans without MS.
Similar results were found for specific types of infections and for MS subtypes.
Efforts to reduce infection-related complications of MS may improve patient health while lowering health-care costs.
Supplementary Material
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the George E. Wahlen Department of Veterans Affairs Medical Center, Salt Lake City, Utah. We thank Andrew Wilson, Stephen Agbor, and Rebekah Paredes for their contributions and assistance with this project and manuscript.
Footnotes
Note: Supplementary material for this article is available on IJMSC Online at ijmsc.org.
Financial Disclosures: Dr. Nelson is a consultant for Anolinx LLC; is funded by VA Health Services Research & Development grant CDA 11-210; and has received research support from the Centers for Disease Control and Prevention, the National Cancer Institute (grant KM1CA156723), and the VA Salt Lake City Center of Excellence in Musculoskeletal Care. Dr. DuVall has received research grant funding from Amgen Inc, Anolinx LLC, AstraZeneca Pharmaceuticals LP, F. Hoffmann-La Roche Ltd, Genentech Inc, Intermountain Healthcare, Merck & Co Inc, Mylan Specialty LP, Parexel International Corp, and Shire PLC. He has received federal funding from the Centers for Disease Control and Prevention, Department of Defense, Department of Veterans Affairs, National Heart, Lung, and Blood Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute of General Medical Sciences, National Institute of Standards and Technology, National Library of Medicine, National Science Foundation, and Patient Centered Outcomes Research Institute. He has academic appointments at the University of Utah and is employed by the University of Utah and the Department of Veterans Affairs. Dr. Kamauu is an owner of Anolinx LLC, which has received research funding from Genentech Inc, F. Hoffman-La Roche Ltd, Mylan Specialty LP, Mitsubishi, Novartis, Parexel, and Shire PLC. Ms. Knippenberg is an employee of the University of Utah. Drs. Schuerch and Foskett are full-time employees of F. Hoffmann-LaRoche, Basel, Switzerland. Dr. LaFleur served on a scientific advisory board for Genentech Pharmaceuticals, is funded by Agency for Healthcare Research and Quality grant HS018582, and has received research grants from Amgen Inc, Genentech Inc, Merck & Co Inc, and Novartis.
Funding/Support: The study was funded by Anolinx LLC through a contract with F. Hoffman-La Roche Ltd.
References
- 1.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19:S15–S20. [PubMed] [Google Scholar]
- 2.Cameron MH, Poel AJ, Haselkorn JK, Linke A, Bourdette D. Falls requiring medical attention among veterans with multiple sclerosis: a cohort study. J Rehabil Res Dev. 2011;48:13–20. doi: 10.1682/jrrd.2009.12.0192. [DOI] [PubMed] [Google Scholar]
- 3.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16:639–647. doi: 10.3111/13696998.2013.778268. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JD, Ghushchyan V, McQueen RB et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord. 2014;3:227–236. doi: 10.1016/j.msard.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Lalmohamed A, Bazelier MT, Van Staa TP et al. Causes of death in patients with multiple sclerosis and matched referent subjects: a population-based cohort study. Eur J Neurol. 2012;19:1007–1014. doi: 10.1111/j.1468-1331.2012.03668.x. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20:1153–1160. doi: 10.1111/ene.12130. [DOI] [PubMed] [Google Scholar]
- 7.Neily J, Mills PD, Young-Xu Y et al. Association between implementation of a medical team training program and surgical mortality. JAMA. 2010;304:1693–1700. doi: 10.1001/jama.2010.1506. [DOI] [PubMed] [Google Scholar]
- 8.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14:274–280. doi: 10.1046/j.1525-1497.1999.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes DM, Koelling K, Stroupe K et al. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45:214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 12.Liu CF, Bolkan C, Chan D, Yano EM, Rubenstein LV, Chaney EF. Dual use of VA and non-VA services among primary care patients with depression. J Gen Intern Med. 2009;24:305–311. doi: 10.1007/s11606-008-0867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appelros P, Terent A. Validation of the Swedish inpatient and cause-of-death registers in the context of stroke. Acta Neurol Scand. 2011;123:289–293. doi: 10.1111/j.1600-0404.2010.01402.x. [DOI] [PubMed] [Google Scholar]
- 14.Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29:495–502. [PubMed] [Google Scholar]
- 15.Culpepper WJ, II, Ehrmantraut M, Wallin MT, Flannery K, Bradham DD. Veterans Health Administration multiple sclerosis surveillance registry: the problem of case-finding from administrative databases. J Rehabil Res Dev. 2006;43:17–24. doi: 10.1682/jrrd.2004.09.0122. [DOI] [PubMed] [Google Scholar]
- 16.Winkelmann A, Loebermann M, Reisinger EC, Zettl UK. Multiple sclerosis treatment and infectious issues: update 2013. Clin Exp Neuroimmunol. 2014;175:425–438. doi: 10.1111/cei.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Lee SL, Im YJ, Han SW. Vesicoureteral reflux and bladder dysfunction. Transl Adrol Urol. 2012;1:153–159. doi: 10.3978/j.issn.2223-4683.2012.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Seze M, Ruffion A, Denys P, Joseph PA, Perrouin-Verbe B, GENULF The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007;13:915–928. doi: 10.1177/1352458506075651. [DOI] [PubMed] [Google Scholar]
- 19.Nortvedt MW, Riise T, Frugard J et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–112. doi: 10.1177/1352458506071210. [DOI] [PubMed] [Google Scholar]
- 20.Srour N, LeBlanc C, King J, McKim DA. Lung volume recruitment in multiple sclerosis. PLoS One. 2013;8:e56676. doi: 10.1371/journal.pone.0056676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polsky D, Bonafede M, Suaya JA. Comorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adults. BMC Health Serv Res. 2012;12:379. doi: 10.1186/1472-6963-12-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23:1123–1142. doi: 10.2165/00019053-200523110-00005. [DOI] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ploughman M, Beaulieu S, Harris C et al. The Canadian survey of health, lifestyle and ageing with multiple sclerosis: methodology and initial results. BMJ Open. 2014;4:e005718. doi: 10.1136/bmjopen-2014-005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson SD, Lu CC, Teng CC et al. The use of natural language processing of infusion notes to identify outpatient infusions. Pharmacoepidemiol Drug Saf. 2015;24:86–92. doi: 10.1002/pds.3720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


